QT prolongation
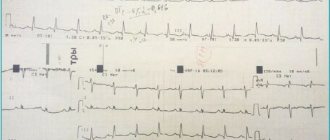
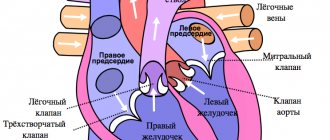
Long QT syndrome is a combination of a prolonged QT interval on a standard ECG and life-threatening polymorphic ventricular tachycardias (torsade de pointes). Paroxysms of ventricular tachycardia of the “pirouette” type are clinically manifested by episodes of loss of consciousness and often end in ventricular fibrillation, which is the direct cause of sudden death.
The duration of the QT interval depends on the heart rate and gender of the patient. Therefore, they use not the absolute, but the corrected value of the QT interval (QTc), which is calculated using the Bazett formula:
QTc = QT/√RR
where: RR is the distance between adjacent R waves on the ECG in sec. ;
K = 0.37 for men and K = 0.40 for women.
QT interval prolongation is diagnosed if the QTc duration exceeds 0.44 s.
It has been established that both congenital and acquired forms of QT interval prolongation are predictors of fatal rhythm disturbances, which, in turn, lead to sudden death of patients.
In recent years, much attention has been paid to the study of the variability (dispersion) of the QT interval - a marker of the inhomogeneity of repolarization processes, since increased dispersion of the QT interval is also a predictor of the development of a number of serious rhythm disturbances, including sudden death. QT interval dispersion is the difference between the maximum and minimum values of the QT interval measured in 12 standard ECG leads: D QT = QTmax – QTmin.
Thus, there is no consensus on the upper limit of normal values for the dispersion of the corrected QT interval. According to some authors, a predictor of ventricular tachyarrhythmia is a QTcd of more than 45; other researchers suggest that a QTcd of 70 ms and even 125 ms be considered the upper limit of normal.
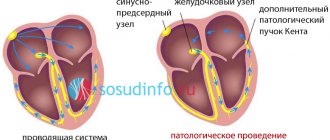
There are two most studied pathogenetic mechanisms of arrhythmias in long QT interval syndrome. The first is the mechanism of “intracardiac disturbances” of myocardial repolarization, namely, the increased sensitivity of the myocardium to the arrhythmogenic effect of catecholamines. The second pathophysiological mechanism is an imbalance of sympathetic innervation (decreased right-sided sympathetic innervation due to weakness or underdevelopment of the right stellate ganglion). This concept is supported by animal models (QT prolongation after right stellectomy) and the results of left stellectomy in the treatment of refractory forms of QT prolongation.
The incidence of QT interval prolongation in individuals with mitral and/or tricuspid valve prolapse reaches 33%. According to most researchers, mitral valve prolapse is one of the manifestations of congenital connective tissue dysplasia. Other manifestations of “connective tissue weakness” include increased skin extensibility, asthenic body type, funnel chest deformity, scoliosis, flat feet, joint hypermobility syndrome, myopia, varicose veins, hernias. A number of researchers have identified a relationship between increased variability of the QT interval and the depth of prolapse and/or the presence of structural changes (myxomatous degeneration) of the mitral valve leaflets. One of the main reasons for the formation of prolongation of the QT interval in people with mitral valve prolapse is genetically predetermined or acquired magnesium deficiency
Acquired prolongation of the QT interval can occur with atherosclerotic or post-infarction cardiosclerosis, with cardiomyopathy, against the background and after suffering myo- or pericarditis. An increase in QT interval dispersion (more than 47 ms) may also be a predictor of the development of arrhythmogenic syncope in patients with aortic heart defects.
Prolongation of the QT interval can also be observed with sinus bradycardia, atrioventricular block, chronic cerebrovascular insufficiency and brain tumors. Acute cases of prolongation of the QT interval can also occur with injuries (chest, craniocerebral).
Autonomic neuropathy also increases the QT interval and its dispersion, so these syndromes occur in patients with diabetes mellitus types I and II.
Prolongation of the QT interval can occur with electrolyte imbalance with hypokalemia, hypocalcemia, hypomagnesemia. Such conditions arise under the influence of many reasons, for example, with long-term use of diuretics, especially loop diuretics (furosemide). The development of ventricular tachycardia of the “pirouette” type is described against the background of prolongation of the QT interval with a fatal outcome in women who were on a low-protein diet to reduce body weight.
QT prolongation is well known in acute myocardial ischemia and myocardial infarction. A persistent (more than 5 days) increase in the QT interval, especially when combined with early ventricular extrasystoles, has an unfavorable prognosis. These patients showed a significant (5–6 times) increased risk of sudden death.
Hypersympathicotonia undoubtedly plays a role in the pathogenesis of QT prolongation in acute myocardial infarction, which is why many authors explain the high effectiveness of b-blockers in these patients. In addition, the development of this syndrome is also based on electrolyte disturbances, in particular magnesium deficiency. The results of many studies indicate that up to 90% of patients with acute myocardial infarction have magnesium deficiency. An inverse correlation between the level of magnesium in the blood (serum and erythrocytes) and the QT interval and its dispersion in patients with acute myocardial infarction was also revealed.
In patients with idiopathic mitral valve prolapse, treatment should begin with the use of oral magnesium preparations (Magnerot 2 tablets 3 times a day for at least 6 months), since tissue magnesium deficiency is considered one of the main pathophysiological mechanisms of the formation of QT interval prolongation syndrome, and “weakness” of connective tissue. In these individuals, after treatment with magnesium preparations, not only does the QT interval normalize, but also the depth of prolapse of the mitral valve leaflets, the frequency of ventricular extrasystoles, and the severity of clinical manifestations (vegetative dystonia syndrome, hemorrhagic symptoms, etc.) decrease. If treatment with oral magnesium supplements after 6 months has not had a complete effect, the addition of b-blockers is indicated.
Another important cause of prolongation of the QT interval is the use of special medications; one of the drugs most often used in clinical practice is Amiodarone (Cordarone).
Amiodarone belongs to class III antiarrhythmic drugs (class of repolarization inhibitors) and has a unique mechanism of antiarrhythmic action, since in addition to the properties of class III antiarrhythmics (potassium channel blockade), it has the effects of class I antiarrhythmics (sodium channel blockade), class IV antiarrhythmics (calcium channel blockade ) and non-competitive beta-blocking action. In addition to the antiarrhythmic effect, it has antianginal, coronary dilation, alpha and beta adrenergic blocking effects.
Antiarrhythmic properties: - increasing the duration of the 3rd phase of the action potential of cardiomyocytes, mainly due to blocking the ion current in potassium channels (the effect of a class III antiarrhythmic according to the Williams classification); - decreased automatism of the sinus node, leading to a decrease in heart rate; - non-competitive blockade of alpha and beta adrenergic receptors;
Description - slowing of sinoatrial, atrial and atrioventricular conduction, more pronounced with tachycardia; — no changes in ventricular conductivity; - an increase in refractory periods and a decrease in the excitability of the myocardium of the atria and ventricles, as well as an increase in the refractory period of the atrioventricular node; - slowing down conduction and increasing the duration of the refractory period in additional atrioventricular conduction bundles.
Other effects: - absence of negative inotropic effect when taken orally; - reduction of oxygen consumption by the myocardium due to a moderate decrease in peripheral resistance and heart rate; - increase in coronary blood flow due to a direct effect on the smooth muscles of the coronary arteries; - maintaining cardiac output by reducing pressure in the aorta and reducing peripheral resistance; - influence on the exchange of thyroid hormones: inhibition of the conversion of T3 to T4 (blockade of thyroxine-5-deiodinase) and blocking the uptake of these hormones by cardiocytes and hepatocytes, leading to a weakening of the stimulating effect of thyroid hormones on the myocardium. Therapeutic effects are observed on average a week after starting to take the drug (from several days to two weeks). After stopping its use, amiodarone is detected in the blood plasma for 9 months. The possibility of maintaining the pharmacodynamic effect of amiodarone for 10-30 days after its discontinuation should be taken into account.
Each dose of amiodarone (200 mg) contains 75 mg of iodine.
Indications for use
Relapse Prevention
- Life-threatening ventricular arrhythmias, including ventricular tachycardia and ventricular fibrillation (treatment should be started in the hospital with careful cardiac monitoring).
- Supraventricular paroxysmal tachycardia: - documented attacks of recurrent sustained supraventricular paroxysmal tachycardia in patients with organic heart diseases; - documented attacks of recurrent sustained supraventricular paroxysmal tachycardia in patients without organic heart disease, when antiarrhythmic drugs of other classes are not effective or there are contraindications to their use; - documented attacks of recurrent sustained supraventricular paroxysmal tachycardia in patients with Wolff-Parkinson-White syndrome.
- Atrial fibrillation (atrial fibrillation) and atrial flutter
Prevention of sudden arrhythmic death in high-risk patients
- Patients after a recent myocardial infarction with more than 10 ventricular extrasystoles per hour, clinical manifestations of chronic heart failure and a reduced left ventricular ejection fraction (less than 40%). Amiodarone may be used in the treatment of arrhythmias in patients with coronary artery disease and/or left ventricular dysfunction
For patients with chronic heart failure, amiodarone is the only antiarrhythmic drug approved for use. This is due to the fact that other drugs in this category of patients either increase the risk of sudden cardiac death or depress hemodynamics.
In the presence of coronary heart disease, the drug of choice is sotalol, which, as is known, is 1/3 a beta-blocker. But given its ineffectiveness, we again have only amiodarone at our disposal. As for patients with arterial hypertension, from among them, in turn, there are patients with severe and unexpressed left ventricular hypertrophy. If the hypertrophy is small (in the 2001 Guidelines, the thickness of the left ventricular wall is less than 14 mm), the drug of choice is propafenone, but if it is ineffective, as always, amiodarone (along with sotalol). Finally, with severe left ventricular hypertrophy, as with chronic heart failure, amiodarone is the only possible drug.
Complications and prognosis
Of the complications of this syndrome, of course, it should be noted sudden cardiac death caused by ventricular tachycardia, which turned into ventricular fibrillation followed by asystole (cardiac arrest).
According to studies, the prognosis of this syndrome without treatment is unfavorable, since long QT interval syndrome causes the development of sudden cardiac death in 30% of all cases. That is why this syndrome requires the close attention of cardiologists and arrhythmologists, since in the absence of effect from drug therapy, the only method that can prolong the life of a child with a congenital form of the syndrome is pacemaker implantation. When it is installed, the prognosis for life and health becomes favorable, since life expectancy reliably increases and its quality also improves.
Acquired form
Previously, it was believed that the occurrence of acquired LQT syndrome is associated with a disruption in the functioning of ion channels, which is caused not by a mutation, but by the influence of some external or internal factors. This statement is true, but it has been proven that a genetic defect contributes to the development of the pathological process. At the same time, it is difficult to distinguish the acquired syndrome from congenital pathology, since they have much in common. Typically, this pathology goes undetected for a long time and manifests itself under unfavorable conditions, for example under stress or physical exertion. Factors that contribute to prolongation of the QT interval include:
- taking medications (we’ll look at which ones below);
- electrolyte disturbances (lack of potassium, sodium, magnesium);
- heart rhythm disturbances;
- cardiac ischemia;
- myocarditis;
- hypertrophic cardiomyopathy;
- diseases of the nervous system (trauma, infection, tumor);
- changes in hormonal status (diabetes mellitus, pathology of the thyroid gland or adrenal glands);
- alcoholism;
- fasting, etc.
Of particular danger is the exposure of a susceptible organism to several risk factors.
Diagnostic principles
Diagnosis of the syndrome is based on clinical data and electrocardiography results. Holter monitoring provides additional information to the doctor.
Taking into account the fact that it is not always easy to make a diagnosis, major and minor diagnostic criteria have been developed. The latter include:
- lack of hearing from birth;
- variability of the T wave in different leads (on the electrocardiogram);
- disruption of the processes of repolarization of the ventricular myocardium;
- low heart rate.
Among the major criteria are:
- prolongation of the corrected QT interval more than 450 ms at rest;
- episodes of loss of consciousness;
- cases of illness in the family.
The diagnosis is considered reliable if two major or one major and two minor criteria are present.
Content
- 1 Measurement
- 2 Heart rate correction 2.1 Bazett formula
- 2.2 Fridericia formula
- 2.3 Saga Formula
- 2.4 Comparison of patches
- 3.1 Genetic causes