In cardiology, there is a concept of deviations that do not cause harm to human health, and therefore are considered by doctors as a variant of the norm. One such phenomenon is supraventricular crest syndrome. When faced with a diagnosis for the first time, patients show anxiety and look for ways to overcome the problem. Doctors agree that the phenomenon does not pose a threat to life and health, and therefore does not require treatment or correction. The syndrome occurs more often in children and is caused by individual characteristics of the heart structure.
Causes of the syndrome
Speaking about the etiology of the phenomenon, experts come to the conclusion that it is impossible to identify a number of reasons leading to its development. First of all, it should be noted that pectinate syndrome is not a disease. It is determined by the physiological characteristics of the structure of the human heart.
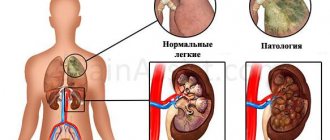
The main organ of the circulatory system consists of four chambers. Entering the heart, blood enters the right atrium, and then into the right ventricle. Passing through the valve and entering the lungs, where gas exchange occurs, the blood flow returns to the left atrium, then to the left ventricle and from there it goes to the main artery - the aorta.
The right ventricle, in which the so-called scallop is located, is the beginning of the pulmonary circulation. It is one of the four chambers of the heart. It is separated from the left atrium by the interventricular grooves, and from the atrium of the same name it is separated by the coronary groove.
The shape of this cardiac chamber is an irregular pyramid, consisting of three sides, with a flat rear and convex anterior and internal walls, one of which forms a septum with the left ventricle.
Being a hollow formation, this chamber consists of two sections, one of which (the anterior, so-called arterial cone) is narrow, and the second (posterior) is wider.
There is a valve between the atrium and the ventricle that prevents the backflow of blood.
In the right ventricle, as well as in the left, there are special structures that belong to the conduction system of the heart and are responsible for coordinating the work of its different parts. These formations include fibers, knots and bundles - it is the latter that form the so-called comb. Deviations in its work, which, however, are a variant of the norm, cause a phenomenon called supraventricular crest syndrome.
Sometimes the described condition is mistakenly called pancreatic scallop syndrome; it is diagnosed quite often in children and much less often in adult patients.
Etiology
The supraventricular crest is a bundle of muscle fibers of the heart, which is located in the right ventricle, between its posterior and anterior sections, and takes an active part in the so-called blood circulation. The right branch of the His bundle passes through it, and if its function is disrupted, they speak of the development of pancreatic crest syndrome.
Muscle fibers have the property of contracting. And since in children the heart beats much faster, an ECG reveals such changes. They are within normal limits and do not require third-party intervention, but only if the child has not previously had cardiac pathologies. If they were, then when diagnosing the syndrome, doctors also prescribe an ultrasound and after that it is determined whether the baby requires special treatment or not.
If, during the electrocardiogram, no noise was detected in the child and no complaints are received from him, then there is no need for therapy at all. As soon as the child’s body gets stronger and his heart begins to work like an adult’s, the signs of the syndrome on the ECG will disappear.
In people over 18 years of age, disturbances in the functioning of the supragastric scallop can provoke various pathologies:
- Heart disease.
- Ischemic disease.
- Angina pectoris.
- Arrhythmia, etc.
But it must also be said that in an adult, the presence of this syndrome does not have a negative effect on the functioning of the heart. Therefore, when making such a diagnosis, you simply need to periodically undergo an ECG. Treatment should be carried out only if an electrocardiogram reveals more serious disturbances in the functionality of the organ.
Characteristic signs
Scalloping syndrome has no pronounced external symptoms; internally it also does not cause any pain or discomfort. It is detected during a cardiogram.
In adults
The syndrome in adult patients (persons over eighteen years of age) is diagnosed extremely rarely. This is due to the fact that over the years, the cardiac structures become fully formed and function stably. Nevertheless, scallop syndrome is still recorded on the ECG in adults in rare cases. Apart from the fact that the phenomenon is recorded on thermal paper during the research process, it does not manifest itself in anything else.
In children
In a child or teenager, deviations of this kind are recorded quite often and cause unnecessary fears among parents. As in the case of adult patients, such conditions do not cause pain or persistent discomfort at rest. Some patients may experience the following symptoms:
- cardiopalmus;
- fast fatiguability;
- chest pain after significant physical activity.
If the syndrome is present, the child's ECG shows some deviations from the norm, which are its only confirmation.
Diagnosis and treatment
To make a decision on possible treatment methods for a particular disease, a specialist sends the patient for a comprehensive examination.
Diagnostics
Only one method of instrumental examination can provide a reliable picture when diagnosing the described condition - an electrocardiogram. The study is usually carried out in case of complaints about a concomitant disease or for preventive purposes. Thus, abnormalities in the functioning of the supraventricular structures are discovered by chance. During inspection and simple listening, violations of this kind are not recorded.
Since the syndrome is predominantly found in children, it makes sense to take a closer look at what ECG signs are used to conclude its presence.
- Shorter line length of PQ, QT intervals.
- Reduced width of the ventricular complex.
- Deviation of the electrical axis of the heart to the right.
- The difference between heartbeats is more than ten percent.
- Predominance of the right ventricle over the left.
- Change in the amplitude of the teeth (overestimation).
- Negative T waves in the V1-V3 pattern.
- Physiological alternation of the ventricular complex.
- Splitting of the QRS complex in lead V4.
- Deformation of the ventricular complex (on the recording it looks like jaggedness of the ascending limb of the S wave or as a narrow one, with a small amplitude r' in the V3R - V5R and V1 scheme).
The above features are a variant of the norm for children. Some of them are caused by the physiologically small (for a given age optimal) thickness of the chest wall, others by the location of the organ in the chest. When it comes to an adult patient with some of the above signs that appear on the cardiogram, there are individual characteristics of the functioning of the heart.
Since sometimes the syndrome can cause discomfort after heavy physical exertion, it makes sense to talk about differential diagnosis. So, often unpleasant sensations caused by a malfunction of the pancreas, stomach, and so on can be mistaken for heart pain.
Treatment
Since the condition in question is not a disease and does not pose a threat to human health and life, it does not require treatment or correction.
Suspicious patients, especially when it comes to the parents of a child who has ECG symptoms of supraventricular crest syndrome, often insist on treatment and medical care. In such cases, vitamin-mineral complexes and some other drugs may be prescribed to prevent diseases and strengthen the cardiovascular system.
Adult patients may be at risk of developing cardiovascular diseases in the following cases:
- hereditary predisposition;
- presence of bad habits (smoking, alcohol abuse);
- increased blood cholesterol levels;
- high blood pressure;
- sedentary lifestyle;
- excess weight.
In such cases, it is worth taking care of prevention, for which it is recommended to consult a cardiologist. You can take the following medications:
- In their pure form, vitamins are A, B1, B6, E, C, P, F, Q10.
- In their pure form, minerals are potassium, magnesium, calcium, phosphorus, selenium.
- "Ascorutin".
- "Asparkam."
- Other vitamin and mineral complexes, balanced in composition and prescribed if there are reasons for the development of diseases.
- Prevention includes lifestyle modification, proper nutrition, moderate physical activity and the absence of bad habits.
Syndrome of premature excitation of ventricles.
Heart rhythm disturbances are considered an important cardiac problem, since they often complicate the course and worsen the prognosis of many diseases and are one of the most common causes of sudden death.
Of particular interest to both clinicians and electrophysiologists is premature ventricular excitation syndrome (PVS), which in some cases, in the absence of clinical manifestations, can be an electrocardiographic finding, and in others it can be accompanied by life-threatening tachyarrhythmias.
Despite the successes achieved in the study of PPV, the issues of its diagnosis, patient management and treatment remain relevant today.
Definition. Classification
PPV (preexcitation syndrome, preexcitation syndrome) is an accelerated conduction of the excitation impulse from the atria to the ventricles along additional abnormal conduction pathways. As a result, part of the myocardium or all of the ventricular myocardium begins to be excited earlier than with the usual spread of excitation through the atrioventricular node, the His bundle and its branches.
According to the recommendations of the WHO expert group (1980), premature excitation of the ventricles, not accompanied by clinical symptoms, is called the “pre-excitation phenomenon,” and in the case when there are not only electrocardiographic signs of pre-excitation, but also tachyari paroxysms develop.
The anatomical substrate of PVS are bundles of specialized muscle fibers outside the conduction system of the heart, capable of conducting electrical impulses to different parts of the myocardium, causing their premature excitation and contraction.
Accessory atrioventricular connections are classified according to their location relative to the annulus fibrosus of the mitral or tricuspid valves, type of conduction (decremental type - increasing slowing of conduction along the accessory pathway in response to an increase in the frequency of stimulation - or non-decremental), and also according to their ability to antegrade, retrograde or combined implementation. Typically, accessory pathways have rapid, nondecremental conduction similar to that of normal tissue of the His–Purkinje conduction system and atrial and ventricular myocardium.
Currently, several types of abnormal pathways (tracts) are known:
- atrioventricular (Kenta), connecting the myocardium of the atria and ventricles, bypassing the atrioventricular node;
- atrionodal (James), located between the sinoatrial node and the lower part of the atrioventricular node;
- nodoventricular (Maheima), connecting the atrioventricular node (or the beginning of the His bundle) with the right side of the interventricular septum or the branches of the right bundle branch;
- atriofascicular (Breschenmash), connecting the right atrium with the common trunk of the His bundle.
There are also other additional conduction pathways, including “hidden” ones, capable of conducting an electrical impulse retrogradely from the ventricles to the atria. A small (5–10%) proportion of patients have multiple abnormal conduction pathways.
In clinical practice there are:
- Wolff–Parkinson–White syndrome (WPW syndrome), caused by the presence of bundles of Kent;
- Clerk-Levy-Christesco syndrome (CLC syndrome, shortened P-Q (R) interval syndrome), caused by the presence of the James bundle.
Electrocardiographic manifestations of PPV depend on the degree of preexcitation and the persistence of conduction along additional pathways. In this regard, the following variants of the syndrome are distinguished:
- manifest PPV (the ECG constantly shows signs of pre-excitation);
- intermittent (transient) PPV (on the ECG, signs of pre-excitation are transient);
- latent PPV (the ECG is normal under normal conditions, signs of pre-excitation appear only during a paroxysm of tachycardia or during provocation - physical activity, electrophysiological study (EPI), vagal or drug tests);
- hidden (changes are not detected on a standard ECG due to the conduction of excitation along additional pathways only in a retrograde manner).
Prevalence
According to various sources, the prevalence of PPV in the general population is approximately 0.15%. At the same time, paroxysms of tachyarrhythmias occur in every second patient (in 80–85% of cases – orthodromic tachycardia, 20–30% – atrial fibrillation (AF), 5–10% – atrial flutter and antidromic tachycardia). Hidden PPV is detected in 30–35% of patients.
PPV is a congenital anomaly, but can clinically manifest at any age, spontaneously or after any disease. Typically, this syndrome manifests itself at a young age. In most cases, patients do not have any other heart pathology. However, combinations of PPV with Ebstein's anomaly, cardiomyopathies, and mitral valve prolapse are described. There is an assumption that there is a relationship between PVS and connective tissue dysplasia.
In families of patients suffering from this syndrome, an autosomal dominant type of inheritance of additional pathways was identified in relatives of the 1st, 2nd, and 3rd degrees of kinship with various clinical and electrocardiographic manifestations.
The incidence of sudden death in patients with PPV is 0.15–0.6% per year. In almost half of the cases, cardiac arrest in persons with PPV is its first manifestation.
Studies of patients with PPV who have suffered cardiac arrest have retrospectively identified a number of criteria that can be used to identify individuals at increased risk of sudden death. These include the presence of the following signs:
- shortened RR interval – less than 250 ms during spontaneous or induced AF;
- history of symptomatic (hemodynamically significant) tachycardia;
- multiple additional paths;
- Ebstein's anomalies.
Story
An ECG with a shortened PQ interval and at the same time a widened QRS complex was first described by A. Cohn and F. Fraser in 1913. Isolated similar cases were subsequently described by some other authors, but for many years the cause of this ECG pattern was considered to be blockade of the branches of the His bundle.
In 1930, L. Wolff, J. Parkinson and P. White presented a report in which electrocardiographic changes of this type were considered as the cause of paroxysmal cardiac arrhythmias. This work provided the basis for conducting comprehensive studies aimed at elucidating the pathogenesis of these changes on the ECG, which were subsequently named Wolff-Parkinson-White syndrome.
Two years later, M. Holzman and D. Scherf suggested that the basis of WPW syndrome is the spread of the excitation impulse along additional atrioventricular pathways. In 1942, F. Wood provided the first histological confirmation of the presence of a muscular connection between the right atrium and the right ventricle, identified during autopsy of a 16-year-old patient with a history of episodes of paroxysmal tachycardia.
Despite these data, an active search for alternative mechanisms for the development of the syndrome continued until the 1970s, when EPI and surgical treatments confirmed the theory of accessory pathways.
Pathogenesis
The conduction of impulses from the atria to the ventricles during PPV occurs simultaneously along the normal conduction system of the heart and along the accessory pathway. In the conduction system at the level of the atrioventricular node, there is always some slowdown in the conduction of impulses, which is not typical for the anomalous tract. As a result, depolarization of a certain area of the ventricular myocardium begins prematurely even before the impulse propagates through the normal conduction system.
The degree of preexcitation depends on the ratio of conduction velocities in the normal conduction system of the heart, primarily in the atrioventricular node, and in the accessory conduction pathway. An increase in the conduction velocity along the accessory pathway or a slowdown in the conduction velocity through the atrioventricular node leads to an increase in the degree of ventricular preexcitation. In some cases, ventricular depolarization may be entirely due to the conduction of impulses along the accessory pathway. At the same time, when the conduction of impulses through the atrioventricular node accelerates or the conduction through the accessory pathway slows down, the degree of abnormal ventricular depolarization decreases.
The main clinical significance of additional conduction pathways is that they are often included in the loop of circular motion of the excitation wave (re-entry) and thus contribute to the occurrence of supraventricular paroxysmal tachyarrhythmias.
With PPV, orthodromic reciprocal supraventricular tachycardia most often occurs, in which the impulse is conducted antegrade through the atrioventricular node, and retrograde through the accessory pathway. Paroxysm of orthodromic supraventricular tachycardia is characterized by frequent (140–250 per 1 min), devoid of signs of preexcitation, normal (narrow) QRS complexes. In some cases, inverted P waves are observed after the QRS complex, indicating retrograde activation of the atria.
With antidromic supraventricular tachycardia, the impulse circulates in the opposite direction: antegrade - along the abnormal conduction pathway, retrograde - along the atrioventricular node. Paroxysm of antidromic supraventricular tachycardia in patients with PPV is manifested on the ECG by a frequent regular rhythm (150–200 per 1 min) with ventricular complexes of the type of maximally pronounced preexcitation (QRS = 0.11 s), after which inverted P waves are sometimes detected.
In 20–30% of patients with PPV, paroxysms of AF occur, in which, as a result of antegrade conduction of a large number of atrial impulses along the accessory pathway, the ventricular contraction frequency (VFR) can exceed 300 per minute.
Clinic
In many cases, PPV is asymptomatic and is detected only by electrocardiography. 50–60% of patients complain of palpitations, shortness of breath, chest pain or discomfort, fear and fainting. Paroxysms of AF become particularly dangerous in the case of PPV, since they are accompanied by a large heart rate, hemodynamic disturbances, and can often transform into ventricular fibrillation. In such cases, patients not only experience syncope, but also have a high risk of sudden death.
Independent risk factors for the development of AF in patients with PPV are age, male gender, and a history of syncope.
Diagnostics
The main method for diagnosing PPV is ECG.
In case of WPW syndrome against the background of sinus rhythm, a shortening of the PQ interval (<0.12 s) and a D-wave (flat slope in the first 30–50 ms) are detected on the ascending part of the R wave or the descending part of the Q wave, the QRS complex is usually widened (i0, 11 s). Deviation of the ST segment and T wave in the direction opposite to the D-wave and the main direction of the QRS complex is also characteristic.
Electrocardiographic signs of CLC syndrome are shortening of the PQ (R) interval, the duration of which does not exceed 0.11 s, the absence of an additional excitation wave - D-wave - in the QRS complex, the presence of unchanged (narrow) and undeformed QRS complexes (except in cases of concomitant blockade of the legs or branches of the His bundle).
With PPV, caused by the functioning of the Maheim beam, a normal PQ interval is determined in the presence of a D wave.
The simultaneous functioning of the James and Maheim beams leads to the appearance on the ECG of signs characteristic of WPW syndrome (shortening of the PQ (R) interval and the presence of a D-wave).
In connection with the spread in recent years of surgical methods for the treatment of patients with PPV (destruction of an abnormal bundle), methods for accurately determining its localization are constantly being improved.
On the ECG, the location of the Kent beam is usually determined by the direction of the initial moment vector of ventricular depolarization (the first 0.02–0.04 s), which corresponds to the time of formation of the abnormal D-wave. In those leads whose active electrodes are located directly above the area of the myocardium that is abnormally excited by the Kent beam, a negative D-wave is recorded. This indicates the spread of early abnormal excitation away from the active electrode of this lead.
Of particular practical interest are the capabilities of the spatial vector electrocardiography method, which makes it possible to accurately determine the localization of additional conduction pathways.
More detailed, compared to ECG data, information about the location of additional conduction pathways can be obtained using magnetocardiography.
However, the most reliable and accurate methods are intracardiac EPI, in particular endocardial (preoperative) and epicardial (intraoperative) mapping. In this case, using a complex technique, the area of the earliest activation (pre-excitation) of the ventricular myocardium is determined, which corresponds to the localization of the additional abnormal bundle.
Treatment
In patients with asymptomatic PPV, treatment is usually not required. Exceptions include individuals with a family history of sudden death, athletes, and those whose work involves danger to themselves and others (for example, divers and pilots).
In the presence of paroxysms of supraventricular tachycardia, treatment consists of stopping attacks and preventing them using various medicinal and non-medicinal methods. In this case, the nature of the arrhythmia (ortho-, antidromic tachycardia, AF), its subjective and objective tolerability, heart rate, as well as the presence of concomitant organic heart diseases are important.
With orthodromic reciprocal supraventricular tachycardia, the excitation impulse is conducted antegrade in the normal way, so its treatment should be aimed at suppressing conduction and blocking impulses in the atrioventricular node. For this purpose, reflex vagal tests are used, which are most effective when applied as early as possible.
The first-line drug for stopping orthodromic reciprocal supraventricular tachycardia is considered to be adenosine, the potential disadvantage of which is a transient increase in atrial excitability, which can provoke their extrasystole and fibrillation immediately after stopping the paroxysm of such tachycardia. Verapamil is considered to be another drug of choice for stopping orthodromic tachycardia in the absence of severe arterial hypotension and severe systolic heart failure. β-blockers are usually used as second-line drugs.
If these drugs are ineffective, procainamide is used to block conduction through the accessory atrioventricular pathway. Due to its safety and effectiveness, novocainamide is the drug of choice in the treatment of tachycardia with wide QRS complexes, when the diagnosis of orthodromic reciprocal supraventricular tachycardia is in doubt.
Reserve drugs are amiodarone, sotalol and class 1C antiarrhythmic drugs (AAPs): propafenone or flecainide.
In case of antidromic reciprocal supraventricular tachycardia, the impulse is conducted retrogradely through the atrioventricular node, therefore the use of verapamil, diltiazem, lidocaine and cardiac glycosides for its relief is contraindicated due to the ability of these drugs to accelerate antegrade conduction along the accessory pathway and thereby increase heart rate. The use of these drugs, as well as adenosine, can provoke the transition of antidromic supraventricular tachycardia to AF. The drug of choice for stopping such tachycardia is procainamide; if it is ineffective, amiodarone or class 1C AAP are used.
When paroxysmal AF occurs, the main goal of drug therapy is to control the ventricular rate and slow down conduction simultaneously along the accessory tract and the AV node. The drug of choice in such cases is also novocainamide. Intravenous administration of amiodarone and class 1C AAP is also highly effective.
It should be noted that the use of verapamil, digoxin and beta-blockers in AF for the purpose of controlling heart rate in persons with PPV is contraindicated due to their ability to increase the conduction velocity along the accessory pathway. This can transfer fibrillation from the atria to the ventricles.
To prevent paroxysms of supraventricular tachyarrhythmias caused by the presence of additional conduction pathways, class IA, IC and III AAPs are used, which have the property of slowing down conduction along abnormal pathways.
Non-drug methods for stopping attacks of supraventricular tachyarrhythmias include transthoracic depolarization and atrial (transesophageal or endocardial) pacing, and for their prevention - catheter or surgical ablation of accessory pathways.
In patients with PPV, electrical cardioversion is used for all forms of tachycardia, which are accompanied by severe hemodynamic disturbances, as well as when drug therapy is ineffective and in cases where it causes a deterioration in the patient's condition.
Radiofrequency catheter ablation of accessory tracts is currently the main method of radical treatment of PPV. Indications for its implementation are a high risk of sudden death (primarily the presence of AF paroxysms), ineffectiveness or poor tolerability of drug therapy and prevention of attacks of supraventricular tachycardia, as well as the patient’s reluctance to take AAP. If a short effective refractory period of the abnormal tract is detected in individuals with rare and mild paroxysms of arrhythmia, the question of the advisability of ablation in order to prevent sudden death is decided individually.
Before catheter ablation, EPI is performed, the purpose of which is to confirm the presence of an additional conduction pathway, determine its electrophysiological characteristics and role in the formation of tachyarrhythmia.
The effectiveness of radiofrequency catheter ablation is high (reaches 95%), and the mortality associated with the procedure does not exceed 0.2%. The most common serious complications of this treatment method are complete atrioventricular block and cardiac tamponade. Recurrences of conduction along the accessory pathway occur in approximately 5–8% of cases. Repeated radiofrequency ablation usually completely eliminates conduction along additional pathways.
Currently, the scope of surgical destruction of accessory tracts has significantly narrowed. For the same indications as catheter ablation, surgical treatment is resorted to in cases where the latter is impossible to perform for technical reasons or is unsuccessful, as well as when open-heart surgery is necessary due to concomitant pathology.
Literature
- Sychev O.S. Heart rhythm disturbances // Guide to cardiology / Ed. V.N. Kovalenko. – K.: Morion, 2008. – P. 1059-1248, 1215-1218.
- ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation // Circulation. – 2006. – No. 114. – P. 257-354.
- ACC/AHA/ESC Guidelines for the management of patients with supraventricular arrhythmias – executive summary // JACC. – 2003. – No. 8. – R. 1493-1531.
- Griffin B., Topol E. Manual of Cardiovascular Medicine. – The Lippincott Williams & Wilkins, 2004. – P. 1248.
- Hanon S., Shapiro M., Schweitzer P. Early history of the preexcitation syndrome // Europace. – 2005. – No. 7. – P. 28-33.
- Keating L., Morris A., Brady W.O. Electocardiographic Features of Wolff–Parkinson–White syndrome // Emerg. Med. J. – 2003. – No. 20. – R.491-493.
N.T. Vatutin, N.V. Kalinkina, E.V. Yeshchenko.
Donetsk State Medical University. M. Gorky;
Institute of Emergency and Reconstructive Surgery named after. VC. Gusak of the Academy of Medical Sciences of Ukraine.
Ukrkardio
Possible complications and prognosis
Since the phenomenon under consideration is not a pathology, it has no possible complications. One of the predictions for the development of the syndrome is incomplete blockade of the right bundle branch, but the described condition rarely develops into this form. If a blockade occurs, the process of conducting an electrical impulse is disrupted. As a rule, such a deviation is also considered as a variant of the norm, does not manifest itself and is recorded only during a cardiogram. If there are no concomitant diseases, this condition does not require treatment.
The prognosis for diagnosed supraventricular crest syndrome in the absence of other heart diseases is favorable.
( 1 ratings, average: 5.00 out of 5)
Pathogenesis
A child’s cardiogram has its own physiological and pathological characteristics. It is worth saying that changes in it depend on the age of the patient, when, as for an adult, there is a single norm. Among the “children’s” features we can list the shortening of the PQ and QT intervals, the GRS complex also narrows, and sometimes arrhythmia can be observed, while maintaining a positive P wave.
Pathophysiologists explain these phenomena by the fact that a child’s heart beats faster than an adult’s. Action potentials shift and layer on top of each other. Or the excitation does not have time to cover all cardiomyocytes before contraction - this is how artifacts appear on the ECG.
Supraventricular crest syndrome is one such physiological phenomenon. Its presence does not indicate any pathology, so doctors do not focus attention on it and over time the child outgrows it.