Thrombocytopenia
– a pathological condition characterized by a decrease in the number of platelets per unit volume of blood and the resulting increased bleeding. Platelets play an important role in stopping bleeding. The molecules of the outer membrane of this flat blood element are able to recognize damaged areas of blood vessels and integrate into the structure of the capillaries, turning into a kind of “patch”. A decrease in the concentration of platelets in the bloodstream leads to a large number of pinpoint hemorrhages.
Classification of the disease
Thrombocytopenia has its own classification, based on a number of factors, symptoms and manifestations. The mechanism of the initiation and development of thrombocytopenia may be associated with the characteristics of the life cycle of blood platelets:
- decreased platelet formation in the red bone marrow.
- increased destruction of platelets.
- redistribution of platelets in the hematopoietic system due to inflammatory processes in the human body.
According to the etiology, primary and secondary thrombocytopenia are distinguished. First type
manifests itself as an independent disease.
The second type
is as a consequence of pathological abnormalities. Thrombocytopenia can have an acute and chronic course. The acute condition lasts a short time (up to 6 months) and is characterized by a vivid clinical picture. A chronic condition may occur with a gradual increase in symptoms. The severity of the disease depends on the number of platelets in the blood and is conventionally divided into three degrees - mild, moderate and severe. Each of them has its own manifestations - from frequent nosebleeds to hemorrhagic rash (purpura) and extensive bleeding into internal organs.
Thrombocytopenia during pregnancy
Thrombocytopenia, or a reduced number of platelets in the blood, occurs in 7-12% of pregnancies. The average platelet count decreases during pregnancy (213,000/µL vs. 250,000/µL) and decreases as pregnancy progresses. The change in platelet count is associated with hemodilution, increased platelet consumption, and increased platelet aggregation caused by elevated thromboxane A2 levels. Thrombocytopenia can be defined as a platelet count less than 150,000/µL or a platelet count below the 2.5th percentile for pregnant women (116,000/µL).
Mild thrombocytopenia is 100,000-150,000/µL. Moderate thrombocytopenia is 50,000-100,000/µl. Severe thrombocytopenia is <50,000/µL. In a normal pregnancy, 7.6% of women will have mild thrombocytopenia during pregnancy, and 65% of these will not be associated with any pathology.
A retrospective study by Shin et al found that in pregnant women with aplastic anemia, obstetric complications were more common in patients with severe thrombocytopenia than in patients with mild thrombocytopenia. Increased platelet destruction includes gestational thrombocytopenia and immunological thrombocytopenia.
Non-immunologically associated thrombocytopenia may include the following: preeclampsia/eclampsia, HELLP syndrome, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, acute hepatosis of pregnancy, heparin-induced thrombocytopenia, vascular malformations, hypersplenic syndrome.
There may also be a decrease in platelet production in other pathological conditions, including vitamin B-12 and folate deficiency, as well as bone marrow suppression, which can be caused by the following reasons: drug exposure, aplastic anemia, paroxysmal nocturnal hemoglobinuria, infectious disease, bone marrow infiltration brain (hematological malignancy, non-hematological malignancy).
Splenic sequestration may be caused by the following: portal hypertension, liver disease, portal or hepatic vein thrombosis, myeloproliferative disorders, lymphoproliferative diseases, storage diseases (Gaucher disease), infection (malaria)
Immunological thrombocytopenia includes: immune thrombocytopenic purpura, systemic lupus erythematosus, antiphospholipid syndrome, collagenosis, drug thrombocytopenia, viral infections (HIV, Epstein-Barr virus), lymphoma.
The most common causes of thrombocytopenia in pregnant women: gestational thrombocytopenia (70%), preeclampsia (21%), immune thrombocytopenic purpura (3%), other (6%). The pathogenesis of gestational thrombocytopenia is unknown, but two main factors stand out: accelerated platelet activation occurs during placental circulation, and accelerated platelet consumption is associated with a decrease in platelet lifespan during pregnancy. No pathological significance for mother or fetus was noted. There is no risk of bleeding or complications of bleeding in the fetus. The platelet count returns to normal within 1-2 months after birth.
Causes of thrombocytopenia
Among the various reasons causing this serious disease, the main ones are:
Symptoms of thrombocytopenia
The clinical picture of thrombocytopenia depends on the cause of this pathology, as well as the severity of the disease. The main signs are multiple hemorrhages on the skin, as well as spontaneous bleeding - nasal, pulmonary, uterine. Other manifestations of the disease:
Thrombocytopenic purpura and pregnancy
Author : Lidiya Olegovna Buzyan , physician-hemostasiologist at the Nova Clinic network of reproductive and genetics centers.
A little less than a hundred years ago, Moshkowitz first described an unusual disease in a sixteen-year-old girl with anemia, small hemorrhages on the skin, which ultimately ended in the development of paralysis and coma. This disease is named after him - thrombotic thrombocytopenic purpura (Moschkowitz disease) .
In recent decades, thanks to active study and search for effective treatment methods, this rather rare (in the USA 3.7 cases per 1 million people) disease is much less likely to lead to death in patients. After plasma therapy was used in treatment, patient mortality decreased from 80% to 10%. However, the outcome directly depends on how quickly the correct diagnosis is made and specific treatment is started. Unfortunately, due to the low specificity of symptoms and rare cases of the disease in the practice of most ordinary doctors, it is not always possible to quickly understand the situation.
Moschkowitz's disease affects vital organs - the brain and kidneys. The damage occurs due to thrombosis of the microvessels feeding these organs. And massive thrombus formation, as a consequence, leads to the fact that the supply of platelets and red blood cells in the blood is depleted - they are retained and accumulate in thrombotic masses; therefore, a general blood test will show a decrease in platelets and hemoglobin (anemia and thrombocytopenia).
The causes of the development of the disease consist of a combination of hereditary predisposition and a triggering provoking factor. Congenital genetic defects lead to a decrease in the activity of a specific enzyme - ADAMTS-13, as a result of which, when exposed to a certain trigger mechanism, thrombus formation in such people is activated to a much greater extent than in others, small vessels become clogged and symptoms of kidney or brain damage appear.
The following factors can provoke the development of thrombotic purpura:
- pregnancy (according to various sources, from 12% to 30% of all cases of the disease occur during pregnancy);
- infectious diseases (activation of blood clots can be caused by viruses, bacterial toxins - in particular, streptococcal toxin for pneumonia, Shigella toxin for intestinal infections in children);
- vaccination;
- taking certain medications (oral contraceptives, antitumor drugs, immunosuppressants after organ and tissue transplantation, the thinning drug clopidogrel);
- surgical interventions;
- the presence of autoimmune diseases (in particular, systemic lupus erythematosus, antiphospholipid syndrome).
During pregnancy, the manifestations of Moschkowitz's disease can easily be confused with preeclampsia or eclampsia. Blood pressure may increase, protein appears in the urine, there may be headaches, convulsions, even the development of coma (in severe cases and without treatment). Brain damage can also manifest as strokes or ischemic attacks. About three-quarters of cases occur in the third trimester. A significant decrease in platelets in a blood test (usually less than 100 and even less than 60) in combination with anemia (hemoglobin below 100 g/l, and in 40% of patients below 65 g/l) is a reason to suspect the presence of Moschkowitz disease and, if appropriate clinical symptoms, immediately carry out additional diagnostics. Since anemia in thrombocytopenic purpura is a consequence of the destruction of red blood cells, the concentration of indirect bilirubin and the enzyme LDH may be increased in the blood. The Coombs test helps in diagnosis, the result of which confirms or excludes the presence of hemolysis (destruction of red blood cells).
A specific and confirmatory diagnostic method is to determine the activity of the ADAMTS-13 enzyme in the blood, but this method is very limited in availability, as is the genetic determination of mutations in the ADAMTS-13 gene.
If the diagnosis is confirmed or most likely, treatment begins immediately. For thrombotic purpura, this is, first of all, plasmapheresis, and if it is impossible, a transfusion of fresh frozen plasma. Usually several procedures are required, since after completion of treatment the pathological process is soon activated again. In 30-60% of patients, relapses of the disease are observed, then to prevent them, plasma transfusion is repeated every 2-3 weeks.
The course of pregnancy and the possibility of maintaining it depend on the severity of the disease. Of course, in case of life-threatening conditions, it is necessary first of all to save the woman’s life; delivery in this case is almost inevitable. In this case, the child can be born quite viable. At the same time, even the appearance of symptoms of the disease at a short stage of pregnancy, provided there is a good response to therapy, is not an absolute indication for its interruption.
For a mild form of the disease, a transfusion of fresh frozen plasma is sufficient; for moderate and severe forms, plasmapheresis is required. If the affected kidneys cannot cope with filtering urine, hemodialysis is added. When blood pressure rises, antihypertensive drugs are used, and for severe anemia, red blood cell transfusions are used. It is also necessary to monitor the condition of the blood coagulation system; if necessary, thinning drugs can be prescribed. Delivery is performed only if there is no effect from all possible therapeutic measures. Also, after childbirth, it may be necessary to remove the spleen to prevent relapses.
Cost of consultation for thrombocytopenia?
| Name of service | Price, rub.) |
| Initial appointment with a cardiologist | 2000 rub. |
| Repeated appointment with a cardiologist | 1500 rub. |
| Primary appointment with a general practitioner | 2000 rub. |
| Repeated appointment with a general practitioner | 1500 rub. |
| Prescription of treatment (drawing up an individual treatment regimen) | 1500 - 3000 rub. |
All our services and prices
Diagnosis and treatment
Increased bleeding
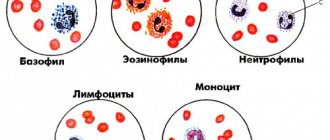
– a reason to immediately seek advice from a specialist (hematologist), who will take a detailed medical history and prescribe the necessary examinations. The main diagnostic method is a clinical blood test, which determines the quantitative composition of platelets. The patient may also be recommended a bone marrow puncture to assess the quality of this type of hematopoiesis, ultrasound of the spleen, chest x-ray, and tests for the presence of antibodies. During pregnancy, thrombocytopenia is diagnosed using a coagulogram (blood clotting test), which allows one to determine the quantitative composition of platelets in a woman’s blood.
A serious disease such as thrombocytopenia should be treated only under the supervision of a doctor. After receiving the results of all examinations, the specialist selects individual therapeutic measures for each patient. Severe hemorrhagic syndrome requires urgent hospitalization and emergency care. For severe bleeding, platelet transfusions are indicated, and angioprotectors and fibrinolysis inhibitors are prescribed. Avoid taking acetylsalicylic acid and anticoagulants. If there is a deficiency of vitamin B12 or folic acid, medications with a high content of these elements are prescribed. This significantly improves the general condition of the patient and the results of his tests. If immune thrombocytopenia is diagnosed, a course of prednisolone will be required. The dosage is determined by the individual characteristics of the patient and his body. If drug therapy is ineffective and repeated bleeding occurs, splenectomy (removal of the spleen) is indicated. For non-immune thrombocytopenia, symptomatic hemostatic therapy is recommended.
There is no special diet for thrombocytopenia, but you should adhere to the principles of a rational, balanced diet. You should introduce more vegetables, fruits, herbs, low-fat dairy products, vegetable oils, legumes, and seafood into your diet. Alcohol consumption is strictly prohibited. Undesirable foods: white rice, sugar, baked goods, marinades and pickles.
After consulting with your doctor, you can use traditional medicine - infusions and decoctions of medicinal herbs, sesame oil.
With severe COVID-19 in pregnant women, the number of thromboembolic complications increases significantly. This is due, on the one hand, to physiological changes in the hemostasis system towards hypercoagulation - a gradual, as the duration of pregnancy increases, an increase in the plasma level of fibrinogen, factors VII, von Willibrand, VIII, and the concentration of D-dimers; antithrombin III decreases slightly, fibrinolytic activity decreases.
On the other hand, SARS-CoV-2 infection also causes a number of pathophysiological reactions that significantly affect the hemostatic system. Thrombosis in patients with COVID-19 is triggered mainly by the extrinsic pathway of activation of the blood coagulation process (through the combination of tissue factor with factor VII).
Olga Svetlitskaya, Associate Professor of the Department of Anesthesiology and Reanimatology of BelMAPO, Candidate of Medical Sciences. Sciences. Damage to the alveolar-capillary barrier
The SARS-CoV-2 virus penetrates the lung tissue by binding to the ACE2 receptor, which is expressed in large quantities on the surface of type 2 pneumocytes and endothelial cells lining the capillary bed.
From the moment the virus enters and begins replication, the cell ceases to perform its inherent functions, begins to produce proteins characteristic of the virus, with the formation of new virions, and then is destroyed. Alveolar macrophages and monocytes circulating in the capillary bed of the lung tissue react to this. They begin to produce large quantities of interleukins IL-1, IL-6 and other pro-inflammatory cytokines.
The more virions multiply, the more type 2 pneumocytes and endothelial cells are destroyed, and the more serious the disruption of the alveolar-capillary barrier, which is clinically manifested by a decrease in saturation. The increase in the production of proinflammatory cytokines by macrophages and monocytes significantly affects the activation of the hemostatic system.
The interleukins produced, in particular IL-1 and IL-6, increase the synthesis of tissue factor on the surface of endothelial cells that are not yet damaged by SARS-CoV-2. In addition, IL-6 blocks the synthesis of tissue factor pathway inhibitor. The accumulation of tissue factor globally activates the process of blood coagulation throughout the vascular bed.
Massive platelet aggregation begins in the lumen of the pulmonary capillaries and the formation of clots. The narrower the lumen of the vessel, the less likely the formation of full-fledged blood clots in it; platelet sludge is more often observed in them. TNF-a (tumor necrosis factor alpha) blocks fibrinolysis.
Thus, with COVID-19, just as with septic conditions, two serious pathophysiological processes occur in parallel in the hemostatic system: global activation of thrombus formation against the background of suppression of fibrinolysis.
Difficulties of anticoagulant therapy for COVID-19
All patients, including pregnant women, should receive prophylactic and therapeutic doses of LMWH calculated for their actual body weight.
It is unacceptable to select the dose of heparin by eye, because this can lead to complications. All patients with COVID-10 upon admission should be weighed at the ED level and weight and height clearly recorded in the medical record.
What to do if it is not possible to determine the level of D-dimers?
Firstly, we must remember that the study of D-dimer levels, which is widely practiced among the general population of patients with COVID-19, in pregnant women has low clinical significance, since the spread of D-dimer levels in normal pregnancy is significant and has not yet been achieved establish reliable reference values for D-dimers during pregnancy. Therefore, the assessment of the level of D-dimers must be carried out in dynamics, paying attention not only to the absolute level, but also to the daily increase.
In situations where for some reason it is not possible to urgently determine the level of D-dimers, the level of LDH (lactate dehydrogenase) should be assessed. A dynamic increase in LDH, especially >1,000 U/L, in a patient with COVID-19 may be an indirect laboratory sign of pulmonary embolism and/or pulmonary infarction. This test is highly sensitive, but non-specific, however, since with COVID-19 the affected area is primarily the lungs, we can assume that the disaster occurs there.
Indirectly confirm the presence of PE: ECG (signs of overload of the right parts of the heart), ultrasound of the heart (expansion of the right parts, increase in the average value of PAP) and veins of the lower extremities (presence of blood clots).
The listed indirect signs allow one to suspect PE or a high risk of PE and promptly prescribe adequate doses of heparins.
What to do if you cannot reach your target RAPTT level?
First of all, you should check the correctness of the nomogram (calculation and correction of the bolus and the administered dose of unfractionated heparin to the actual body weight). If you are sure that you did everything correctly, but the RAPTT still did not reach the target values, determine the levels of antithrombin III and/or anti-Xa activity.
Antithrombin III (AT III) and what to do if it decreases
Heparin is a cofactor of AT III; it transforms AT III into an immediate-acting anticoagulant, enhancing its effects 1,000 times or more depending on the dose and speed at which it is administered. AT III deficiency reduces the ability of heparin to inhibit coagulation factors IIa and Xa.
The average normal AT III level is 80–120%. In pregnant women, it naturally gradually decreases and by the end of the 3rd trimester normally should not decrease to less than 65%. Practice shows that 60–65% is a completely manageable situation. Lower rates (55–50% and below) should alert a specialist.
In acute inflammation, the level of AT III always increases, but with the development of thrombosis, sepsis, disseminated intravascular coagulation syndrome, and the administration of heparins, it decreases. Therefore, a decrease in AT III levels in a patient with COVID-19 is a poor prognostic sign that indicates the development of serious complications and requires action.
In case of AT III deficiency, the decision is made individually.
There are three approaches for coagulation correction of AT III level:
1.Transfer of the patient to therapeutic doses of LMWH. In conditions of AT III deficiency, we focus on factor Xa binding, given the fact that the binding of 1 unit of Xa prevents the formation of 50 units of IIa (thrombin).
2. AT III replenishment with fresh frozen plasma (FFP) or AT III concentrate. Both options are not recommended for COVID-19. Transfusion of FFP in the setting of ARDS increases the risk of further lung damage due to the possible development of TRALI syndrome (acute injury to the lung parenchyma in response to transfusion of blood and its components). The administration of AT III concentrate (in cases where its level has not decreased less than 65%) can lead to increased bleeding, which is undesirable when treating pregnant women in critical condition.
3. Transfer of patients to direct thrombin inhibitors (independent of AT III) - direct factor IIa inhibitor (dabigatran) or direct factor Xa inhibitor (rivaroxaban). This method is used in the general group of patients and is not used in pregnant women.
Determination of Anti-Xa activity (IU/ml)
Currently, the ability to determine anti-Xa activity has appeared in many hospitals, and it is important to be able to correctly interpret the results ( see Table 1 for recommended levels ).
Blood sampling for this study is carried out 3–4 hours after subcutaneous administration of LMWH. It is best to do this after 4 hours, since it is at this time interval that the maximum level of concentration of administered LMWH in the blood plasma (anti-Xa activity) is observed.
Table 1. Classic recommended levels of anti-Xa activity Taking into account the fact that hospitalization of patients with covid pneumonia is generally associated with a high risk of pulmonary embolism, the level of anti-Xa activity should be maintained within the range of 0.5–1.0 IU/ml.
The principle of dose adjustment of LMWH based on anti-Xa activity is given in Table. 2 .
Table 2. Dose adjustment of LMWH for therapeutic concentrations of 0.5–1.0 IU/ml
What to do if D-dimer levels do not decrease?
One of the most frequently asked questions: we are doing everything correctly, but the level of D-dimers was and remains high. Hospitals fall into this trap when laboratories give a D-dimer response of “greater than some value,” for example, “>5,000 ng/ml.”
In this case, you need to contact the laboratory and, if possible, clarify the actual level of D-dimers at the time the patient was admitted to intensive care. It is quite possible that it was 7,000, 10,000 or even 20,000 ng/ml and is slowly decreasing with treatment.
Also, to correctly assess the situation, you should perform: monitoring the level of AT III and/or anti-Xa activity; Ultrasound of the heart with calculation of mean PAP; Ultrasound of the veins of the lower extremities (looking for blood clots).
As UFH is titrated, the blood clots gradually loosen and disintegrate, maintaining an elevated level of D-dimers. Therefore, if a pregnant woman has thrombosis of the veins of the lower extremities, it is necessary to periodically repeat an ultrasound and check the length of the blood clot. If it decreases, then everything is fine.
We must not forget that sometimes the source of elevated levels of D-dimers can be a hematoma that slowly goes away, sometimes over several weeks.
What should I do if my D-dimer levels start to rise again?
In a situation of sudden growth, it is necessary to carry out diagnostic measures:
- control of thrombus formation: ultrasound of the vessels of the lower extremities, ultrasound of the heart (dilation of the right cavities of the heart + average value of PAP);
- monitoring the severity of the inflammatory syndrome (the most accessible way is to determine C-reactive protein (CRP)).
An increase in the level of CRP over time indicates the acute phase of inflammation. Under the influence of IL-1, IL-6 and TNF-a, the synthesis of CRP increases after 6 hours, reaching its maximum concentration after 24–48 hours.
If during treatment there is a sharp rise in the level of D-dimers, in parallel the level of CRP begins to increase, correlating with an increase in the level of LDH, this indicates the fact of a catastrophe in the pulmonary vessels.
How to understand whether there is a pulmonary embolism in the absence of CT angiography?
An increased level of D-dimers by itself in this situation does not confirm PE, but the dynamics of this indicator is important. Clinical signs of pulmonary embolism should be assessed, in particular perfusion respiratory failure (decreased peripheral saturation <90%, compensatory tachypnea, tachycardia, hypotension, etc.).
Apply instrumental research methods available at this level of medical care: perform an ECG (overload of the right chambers of the heart), ultrasound of the heart (dilatation of the right chambers), measure the average value of PAP (with PE it can reach 35–60 or more mm Hg) , Ultrasound of the veins of the lower extremities (looking for blood clots).
What to do if the patient “bleeds” or a hematoma appears, and D-dimers are elevated?
In case of active bleeding, it is necessary to stop the administration of UFH and restore normocoagulation. If necessary, compensate for the deficiency of blood coagulation factors by transfusion of single-group fresh frozen plasma or a concentrate of prothrombin complex factors.
Further return to heparins is possible only if normocoagulation is achieved in the screening coagulogram and there are no signs of active bleeding.
The dosage of LMWH is determined individually, taking into account the cause of bleeding (diffuse bleeding, postoperative bleeding, etc.), platelet levels, dynamics and reasons for the increase in D-dimer levels.
read the material “COVID-19: features of the course and treatment in pregnant women” by following the link here.