Periventricular leukomalacia (PVL) underlies most motor disorders in patients with perinatal pathology of the nervous system, including cerebral palsy (CP) [1, 15, 17, 18]. As studies in recent years show [12, 13, 21], not every child developing in unfavorable intrauterine conditions develops PVL. Obviously, a decisive role in the implementation of a potentially pathogenic effect is played by the genetically determined individual reactivity of the child’s body, which determines the increased “susceptibility” of the structures of the periventricular region and other parts of the brain of the fetus and newborn to hypoxia. There is evidence in the literature [5, 6] that one of the triggers for the persistence of motor deficits in cerebral palsy is genomic instability, manifested by an increase in the level of red blood cells with micronuclei (EM) and lymphocytes with chromosome rearrangements. It is assumed that this phenomenon is based on the intensification of mutagenesis processes in the body of patients with cerebral palsy due to the enhanced generation of endomutagens and the weakening of antimutagenic genome protection systems. At the same time, both the mechanisms of genome destabilization themselves and the processes that support them remain unclear.
The most likely endomutagens in conditions of a chronic ischemic process in the brain may be products of free radical oxidation. With excessive formation, free radicals affect the cell, leading to various cytogenetic damages and subsequently to its death [6, 10, 14, 16, 20]. The role of reactive oxygen species and other free radicals is manifested primarily in oxidative damage to DNA, the carrier of hereditary information and the initial matrix for the synthesis of proteins in the body as a whole, which can cause a number of chromosomal aberrations and mutation of some genes in human cells [10]. Control over the excessive production of reactive oxygen species is carried out by the antioxidant system, which includes: superoxide dismutase (SOD), catalase (CAT), peroxidase, ceruloplasmin, glutathione peroxidase, glutathione transferase, etc. [2, 8].
In recent years, reports have appeared in the literature [5] about the presence of a high level of cytogenetic disorders in peripheral blood cells in patients with cerebral palsy. The mechanisms of formation of genome instability in cerebral palsy remain poorly understood. It is known that genomic instability can maintain the persistence of motor deficits in children with spastic forms of the disease, preventing successful rehabilitation therapy. Further studies of the pathogenetic links in the formation of cerebral palsy in children with PVL should clarify the mechanisms that maintain the severity of the disease and hinder the effectiveness of treatment measures. This will make it possible to purposefully influence the identified processes involved in the formation of gross motor disorders, to reasonably prescribe drug therapy and improve the quality of life of patients.
The purpose of this study is to study the relationship between the level of EOs and the intensity of free radical oxidation processes and the activity of antioxidant enzymes in children with the early residual stage of cerebral palsy.
Material and methods
51 children (24 boys and 27 girls aged 1-5 years) with spastic diplegia were examined. The children were hospitalized in the neurological department of the Children's Republican Clinical Hospital of the Ministry of Health of the Republic of Tatarstan.
The criterion for inclusion of patients in the study was the identification by neuroimaging - using neurosonography, magnetic resonance imaging (MRI) and X-ray computed tomography (CT) - of only PVL, without other pathology, and the absence of drug treatment and infectious process for 30 days.
The control group consisted of 20 healthy children. Both groups were comparable by gender and age.
A cytogenetic study of peripheral blood erythrocytes was carried out using a micronucleus test [19]. Blood sampling for the study was carried out on the 1st day of the patient’s admission to the hospital before the start of treatment. Peripheral blood smears were stained according to Romanovsky-Giemsa at pH 6.8 for 20 minutes, then washed well. For each patient, 20,000 red blood cells were examined, among which EMs were counted. The number of EMs was expressed as a percentage of the total number of erythrocytes examined.
The activity of SOD [7], CAT [9], and peroxidase [11] in blood hemolysate was studied using a biochemical method in the central research laboratory of the Kazan State Medical University using standard methods; lipid hydroperoxide [3] and malondialdehyde (MDA) in plasma [4].
Statistical processing of the results was carried out using the parametric Student t-test, non-parametric Mann-Whitney t-test and correlation analysis using the Origin 6.1 program.
Results and discussion
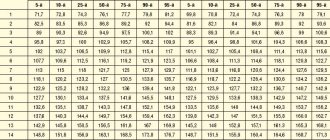
A cytogenetic study revealed an increased amount of EM in each examined child with cerebral palsy, which significantly (p<0.001) exceeded the rate of spontaneous mutagenesis (Table 1).
The detected cytogenetic abnormalities indicate genome destabilization. Genomic instability can occur under conditions of increased generation of endomutagens, leading to damage to the metabolic cycles of aneugenesis and, as a consequence, to an increase in the number of cells with cytogenetic rearrangements. The most likely generators of endomutagenesis in children with PVL resulting in cerebral palsy may be active free radical processes.
A study of the activity of antiradical defense enzymes (SOD, CAT, peroxidase) in the blood of patients with PVL with an outcome in cerebral palsy revealed their high level compared to the control group, indicating the activation of free radical processes. In children with PVL, significant activity of SOD, a key enzyme of antioxidant protection, was observed. Significant differences in the activity indicators of SOD (p<0.05) and CAT (p<0.05) were found in children with spastic diplegia compared to healthy ones (see Table 1). A tendency was revealed for the predominance of peroxidase activity in red blood cells in the examined children with cerebral palsy compared to controls, but no significant differences were obtained (p>0.05).
A study of the content of lipid peroxidation products (LPO) revealed an increase in both the primary (lipid hydroperoxide) and secondary (MDA) products that interact with thiobarbituric acid in the blood serum in patients with cerebral palsy. In the group of children with PVL, a significantly higher (p <0.002) MDA content was revealed compared to the control (see Table 1). The level of lipid hydroperoxide was increased, but no significant differences were found with the healthy group. This may be due to the fact that lipid hydroperoxide is an unstable product and quickly turns into a more stable and toxic product - MDA.
Based on the detected increase in the activity of antiradical defense enzymes and lipid peroxidation products (MDA), one can judge the course of severe oxidative stress in children with PVL and patients with cerebral palsy.
The obtained results of biochemical and cytogenetic studies indicate the presence of two simultaneously occurring pathological processes in children with PVL. On the one hand, this is an increase in the intensity of free radical oxidation processes, on the other hand, genome instability with a large number of cytogenetic rearrangements.
To assess the relationship between the cytogenetic status and the activity of free radical oxidation processes, a correlation analysis of these indicators in patients with PVL with the outcome in cerebral palsy was carried out. It turned out that both processes are interconnected with each other: SOD activity inversely correlates with the number of EM (r=–0.601), and the MDA content and CAT activity revealed a direct correlation with the number of red blood cells with rearrangements (r=0.67 and r=0.605, respectively ) (Fig. 1, 2).
Figure 1. Correlation curve between the amount of EM and SOD activity in children with PVL.
Figure 2. Correlation curve between the amount of EO and the level of MDA in children with PVL. The negative correlation of SOD activity with the level of cytogenetic disorders in children with PVL and patients with cerebral palsy may be associated with insufficient activity of antiradical enzymes for antimutagenic protection against the background of increased free radical oxidation processes. Under these conditions, the increased content of MDA exhibits a pronounced cytotoxic effect, causing destabilization of the genome.
The results of the analysis of the conducted studies indicate that in patients with PVL with an outcome in cerebral palsy, genome instability is formed against the background of activation of free radical processes. Pronounced processes of free radical oxidation provoke endomutagenesis and lead to an aneugenic effect in the cells of the body of patients with cerebral palsy. It is possible that pathological changes in the periventricular region can support and perhaps even initiate endomutagenesis in the body of children with PVL. The results obtained suggest that oxidative stress leads to destabilization of the cellular genome in children with PVL resulting in cerebral palsy and supports processes occurring (and possibly originating) in the periventricular region. These processes contribute to the aggravation of existing and the emergence of new pathological links in the patient’s metabolism. The above dictates the need to search for additional ways of pharmacological correction of oxidative stress and cytogenetic disorders in children with PVL.
Taking into account the actively occurring processes of free radical oxidation and endomutagenesis in the body of patients with PVL with an outcome in cerebral palsy, the further tactics of our study was an attempt to correct these disorders. For this purpose, a drug with both neurotropic and antioxidant effects was chosen - cortexin. As a result of randomization, 20 patients with PVL resulting in spastic diplegia were allocated, who received monotherapy with Cortexin for 20 days at a dose of 0.5 mg/kg per day. Blood sampling to study oxidative status and EO levels was carried out on the day of initiation of therapy (before treatment) and after 20 days of treatment.
A study of antioxidant status during therapy with Cortexin showed a significant decrease in the activity of antiradical defense enzymes compared with indicators before treatment: SOD - by 33.3% (p<0.05) and CAT - by 10% (p<0.05) (Table .2).
Peroxidase activity also decreased by 10% (p>0.05). A significant decrease in lipid peroxidation products after treatment with Cortexin was revealed: MDA activity - by 36.7% (p<0.05), lipid hydroperoxide - by 10.5% (p>0.05). The level of cytogenetic disorders during Cortexin therapy significantly (p<0.05) decreased by 40% compared to the values obtained before treatment, but still remained higher than in healthy children.
The study demonstrated the antiradical and antimutagenic effect of cortexin, manifested in a decrease in the activity of free radical oxidation and endomutagenesis processes in patients.
Thus, oxidative stress in children with PVL resulting in cerebral palsy is important both in the initiation of the pathological process and in its progression. PVL is formed against the background of a hypoxic process in the perinatal period, which triggers free radical oxidation. It is possible that oxidative stress contributes to the development of white matter necrosis in the periventricular areas of the brain. Subsequently, in the absence of adequate therapy that inhibits oxidative stress, astrocyte degeneration occurs with the proliferation of microglia and the accumulation of lipid-containing macrophages in necrotic tissue. Since the periventricular region is a matrix region for nerve cells, its necrotic changes lead to impaired differentiation of nerve cells and, consequently, to severe neurological consequences. The positive dynamics of free radical oxidation processes during Cortexin therapy indicates that the drug reduces LPO activity and slows down the processes of oxidative endomutagenesis in the body of children with PVL. The antiradical and antimutagenic properties of cortexin reduce the cytotoxic effect of free radicals on the cell genome, reducing genome instability in patients with PVL resulting in cerebral palsy and, accordingly, preventing the progression of the disease.
What diseases have symptoms similar to brain leukomalacia in a baby?
Multicystic encephalomalacia
- occurs in full-term children with severe asphyxia during childbirth;
- diffuse generalized brain damage;
— multiple cystic cavities of various sizes;
- cysts may have septa;
- usually occur in the cortex and nearby white matter;
- often in the frontal and occipital lobes.-
Vasculitis
- very rare;
— multiple lesions of the cortex and subcortical region;
- may be combined with hemorrhages;
— perfusion defects in the acute stage;
— sometimes areas of damage to the blood-brain barrier are identified.
Causes of brain leukomalacia in children
- Occurs in 1.5-6% of living newborns.
- Consequence of severe hypoxic-ischemic brain damage in premature newborns (less than 28 weeks)
- Causes include decreased blood oxygen and decreased cerebral perfusion
- Lesions occur primarily in the area of terminal microcirculation
- In premature newborns, the periventricular white matter is supplied with blood by vessels passing outside the ventricles (from the choroid plexus) and inward towards the ventricles (from the cortex of the lateral ventricles); a “zone of blood supply separation” occurs between the two systems
- Brain vessels at this stage of development do not have the ability to autoregulate. Lesions in periventricular leukomalacia are found near the anterior horns of the lateral ventricles, in the radial crown, in the central semi-oval, above the triangle of the lateral ventricles and in the parieto-occipital region
- Affects predominantly the internal and external capsules, motor cortex, corticospinal tracts, visual cortex and speech centers
- Cysts develop in necrotic areas; they may be separated from the ventricular system by a septum or may communicate with the ventricular system
- These lesions lead to the development of brain atrophy.
- Non-hemorrhagic form (2/3 cases): bilateral symmetry around the lateral ventricles.
- Hemorrhagic form (1/3 of cases): unilateral lesions with IV degree of hemorrhage.
Classification of the severity of periventricular leukomalacia Aicardi
I degree: periventricular leukomalacia in the area of the posterior horns.
II degree: periventricular leukomalacia in the area of the anterior and posterior horns.
III degree: periventricular leukomalacia along the lateral wall of the entire lateral ventricle.
IV degree: III degree of periventricular leukomalacia in combination with white matter cysts of the brain.