Methods of treating the disease
With Goodpasture's syndrome, inflammation of the renal and pulmonary capillaries of immune origin develops, resulting in glomerulonephritis and hemorrhagic pneumonitis. The disease is named after the American pathophysiologist who first described it a little over a hundred years ago. This syndrome is diagnosed predominantly in men, with a frequency of approximately 1:1000000. Lack of timely treatment is associated with an extremely negative prognosis and mortality for the majority of patients.
The causes of the disease have not been precisely clarified, but it has been associated with viral pathologies, including influenza and hepatitis A, long-term use of certain medications, inhalation of gasoline and varnish vapors, and smoking. There is also a hereditary predisposition. The influence of negative factors stimulates the production of autoantibodies to the membranes of the alveoli of the lungs and renal glomeruli. Studies have shown that the development of an autoimmune inflammatory process is largely influenced by the activation of T lymphocytes, proteolytic enzymes and free radicals, and the synthesis of cytokines such as interleukin-1 and platelet-derived growth factor.
There are malignant, moderate and slow variants of the pathology. Clinical signs of the malignant variant include pulmonary hemorrhage and acute renal failure. Moderate and slow variants are manifested by characteristic symptoms of lung damage (cough, shortness of breath, hemoptysis), low-grade fever, weakness, weight loss, and symptoms of renal pathologies (hematuria, swelling of the extremities, increased blood pressure).
Treatment tactics depend on the type of Goodpasture syndrome, the severity of symptoms and the general condition of the patient.
In the acute form of the malignant variant, intensive therapy is carried out:
- artificial pulmonary ventilation (ALV);
- oxygen inhalation;
- blood transfusion;
- hemodialysis;
- replenishment of lost fluid and normalization of water and electrolyte balance.
Patients are also prescribed:
- pulse therapy with methylprednisolone - the patient is briefly administered certain types of glucocorticosteroids in increased doses, which helps reduce the production of autoimmune antibodies and the frequency of developing complications;
- combined pulse therapy - the administration of a combination of corticosteroid hormonal drugs and cytostatic drugs to the patient also has an immunosuppressive effect, after the laboratory and radiological tests return to normal, the patient is transferred to a maintenance regimen of therapy;
- monoclonal antibodies are an innovative method based on the ability of drugs to attach to the CD20 protein expressed on the surface membrane of B-lymphocytes and inhibit their activity, thus renewing the population of lymphocyte cells and stopping tissue destruction by autoimmune antibodies;
- plasmapheresis is a safe way to purify plasma from circulating immune complexes by centrifugation; blood taken from the patient is placed in a centrifuge, where the plasma is separated from the red blood cell mass, which, in combination with plasma replacement solutions, is injected back into the patient’s bloodstream.
Therapy methods
Timely and adequate treatment helps slow the progression of Goodpasture syndrome. The main goal of treatment methods is to ensure maximum removal of BMK autoantibodies. The complex of therapeutic actions for the above-described pathology may include the following:
- Glucocorticoids (Prednisolone at a dose of 100 mg per day orally in combination with cytostatics at a dose of 1000 mg per day intravenously for three days in a row, then the patient can be transferred to a tabulated form of the medication).
- Immunosuppressive cytostatics (Imupran, Azathioprine or Cyclophosphamide 150-200 mg per day in combination with Prednisolone).
- Plasmaphoresis (helps remove low molecular weight products of nitrogen metabolism and autoantibodies from the blood).
- Blood transfusion.
- Oxygen therapy.
- Antithrombic drugs (Curantil, Dipyridamole 150-400 mg per day for 3-8 months).
- Vasodilators (Corinfar 40 mg/day).
- Anti-inflammatory non-steroidal drugs (Metindol, Indomethacin 25-30 mg daily 2-3 times a day).
- For anemia, it is advisable to prescribe iron supplements (Totema, Aktiferrin, Tardiferon, Hemohelper, Ferlatum, Maltofer).
- Hemodialysis.
- Kidney transplantation (for end-stage renal failure).
Treatment must be carried out until kidney function is completely stabilized and BMK antibodies disappear. With such therapy, the dangerous symptoms of the disease quickly stop.
Surgery
Data on kidney transplantation in patients with pulmonary-renal syndrome are sparse.
Experts recommend carrying out this procedure no earlier than 6 months after the disappearance of BMK antibodies in the blood.
All patients with a transplanted organ must carefully monitor the concentration of creatine in the blood and the titer of BMK antibodies over time.
Even such treatment does not protect against relapse of glomerulonephritis. The cause of the disease is not in the kidney itself, but in metabolic disorders.
Note. Traditional medicine recipes for the treatment of the above-described anomaly are not effective. Do not engage in self-diagnosis and self-medication; it is better to entrust your life to experienced, highly qualified specialists.
How is the disease diagnosed?
In Israeli clinics, examination of a patient, differential diagnosis and development of treatment tactics takes about three days.
- Day 1
- Day 2
- Day 3
At the initial consultation, the attending physician conducts a thorough examination of the patient, paying attention to pale skin, swelling of the face and a number of other external signs characteristic of Goodpasture syndrome.
When auscultating the lungs, wheezing is heard, the number and severity of which increases in the presence of hemoptysis. At the appointment, the specialist draws up a list of necessary diagnostic procedures. Carrying out the examinations specified in the list of appointments:
- general and biochemical blood tests - the occurrence of the inflammatory process is indicated by an increased number of leukocytes, a high ESR, anemia, biochemical analysis shows an excessive content of urea and creatinine;
- immunological tests - the most revealing is the detection of antibodies specific to the glomerular basement membrane (anti-GBM) using enzyme-linked immunosorbent assay (ELISA) and radioimmunoassay (RIA);
- sputum examination;
- X-ray of the lungs;
- kidney and lung biopsy;
— spirometry (determining the volume of external respiration);
— Ultrasound of the kidneys;
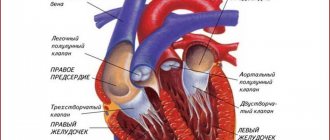
— electrocardiography (ECG);
— Ultrasound of the heart (EchoCG).
The research results are reviewed by a commission consisting of the attending physician and highly specialized specialists. After studying the data obtained, a diagnosis is made and treatment tactics are developed.
Publications in the media
Goodpasture syndrome is an autoimmune disease characterized by the presence of antibodies to type IV collagen with a-3 chain (Goodpasture Ag). This Ag is part of the basement membrane of the renal glomeruli and the alveolar membrane, therefore the main clinical manifestations of Goodpasture syndrome are pulmonary hemorrhage and progressive glomerulonephritis. Statistical data. Prevalence: 0.5 per 1,000,000. Age: 5–40 years. The predominant gender is male (6:1).
Etiology unknown. Risk factors include: smoking, respiratory infection, contact with volatile hydrocarbons.
Genetic aspects. An association with HLA DRw2 was revealed.
Pathomorphology • Kidneys •• Epithelial cell crescents •• Shrinkage of the renal glomeruli •• Interstitial inflammatory exudate • Lungs •• Intra-alveolar hemorrhages •• Macrophages loaded with hemosiderin •• Fibrosis of the interalveolar septa.
Clinical picture • Lung damage (precedes kidney damage by several weeks or months); varies from slight shortness of breath to repeated pulmonary hemorrhages with the development of respiratory failure • Rapidly progressive glomerulonephritis with the development of renal failure • Arterial hypertension (20%) • Influenza-like syndrome: fever, myalgia, arthralgia, weakness.
Laboratory data • CBC •• hypochromic IDA • OAM •• microhematuria •• proteinuria not reaching the nephrotic threshold • Antibodies against the basement membrane of the glomeruli of the kidneys (radioimmune method or indirect immunofluorescence method) • Perinuclear antineutrophil Abs.
Instrumental data • Kidney biopsy - deposits of immunoglobulins and complement in the basement membrane of the glomeruli, extracapillary crescents in 50% of the glomeruli • X-ray of the chest - volatile asymmetric cloud-like infiltrates; Lung destruction is not typical.
Differential diagnosis • Wegener's granulomatosis occurs with destruction of lung tissue and damage to the upper respiratory tract • Microscopic polyangiitis, in addition to pulmonary and renal syndromes, has others (multiple mononeuritis, skin changes) • Renal vein thrombosis and pulmonary embolism (acute development, lack of immunological activity) • Rapidly progressive glomerulonephritis, complicated by pulmonary edema.
TREATMENT
General tactics involve a combination of plasmapheresis, active immunosuppressive therapy and methods for correcting renal function. The diet corresponds to the diet for renal failure.
Drug treatment • GK •• Pulse therapy with methylprednisolone 30 mg/kg/day IV drip for 20–30 minutes for 3 days in a row •• Prednisolone after pulse therapy at a dose of 2 mg/kg orally until clinical effect with a gradual dose reduction : up to 1.75 mg/kg for 1 month, 1.5 mg/kg for 3 months, then each dose is prescribed for 6 months (1.25–1.0–0.75–0.5–0, 25 mg/kg), then each dose is prescribed for 12 months (0.125–0.0625 mg/kg). For patients over 60 years of age, the dose should be reduced by 25%.
• Cytotoxic immunosuppressants are used in combination with GCs •• Cyclophosphamide 2–3 mg/kg/day •• Azathioprine 1–2 mg/kg/day. Notes: the dose of the drug should be reduced by 50% when GFR decreases to 10 ml/min; control over the number of leukocytes and platelets in the blood is necessary.
Non-drug therapy • Plasmapheresis daily or every other day in combination with immunosuppressive therapy for 1-2 weeks • Hemodialysis - with the development of renal failure.
Surgical treatment • Kidney transplantation in end-stage chronic renal failure. Recurrence of the disease in the graft is not observed if Abs to the glomerular basement membrane were not detected in the blood before surgery.
The prognosis is unfavorable if at the time of diagnosis (before treatment) there are already signs of renal failure.
Synonym. Hereditary pulmonary-renal syndrome.
ICD-10 • M31.0 Hypersensitivity angiitis
Advantages of treatment in Israel
- Highly qualified specialists with extensive experience in treating rare autoimmune diseases.
- Equipping medical centers with modern equipment.
- Accurate diagnostics using modern techniques.
- Inclusion of progressive methods and the latest medications into a comprehensive treatment program.
- Reasonable prices.
Timely completion of a treatment course can significantly improve the prognosis and eliminate painful symptoms. Don’t waste time, contact the clinic of your choice and start treatment immediately.
- 5
- 4
- 3
- 2
- 1
(0 votes, average: 5 out of 5)
Goodpasture syndrome - what is it?
Goodpasture syndrome is rare: it affects one person per million of the population every year. The disease was named after the famous American doctor, pathophysiologist Ernest Goodpasture .
The doctor first described this pathology in 1919 during the influenza epidemic. Rheumatologists classify Goodpasture's syndrome as a systemic vasculitis.
This pathology is understood as a progressive disease of the lungs and kidneys of an autoimmune nature, which is characterized by the formation of antibodies to the basement membranes of the capillaries of the renal glomeruli and alveoli, manifested by multiple renal and pulmonary hemorrhages.
The mechanism of disease development is as follows:
- Under the influence of certain factors, human immunity changes, the structure of capillary membranes is modified.
- The body begins to perceive dehydration products as antigens.
- Antibodies are formed to the basic membranes of the kidneys, which react to antigens of the basic pulmonary membranes (class immunoglobulins G)
- Immune complexes begin to circulate freely in the vessels and are deposited on the walls of arterioles, capillaries, veins of the lungs and kidneys.
- Inflammation of the kidneys occurs, in which fibroblasts are activated and begin to produce connective tissue.
- The tissue grows, replacing the parenchyma.
- Proteolytic enzymes are released into the blood in excess, which destroy organ tissue.
Usually people aged 20 to 30 and 50 to 60 years old are affected. It has been scientifically proven that women are less susceptible to this syndrome than men.
Treatment
Without treatment, Goodpasture syndrome quickly leads to the death of the patient, mainly from renal failure, less often from pulmonary hemorrhage.
There are no dietary restrictions, except in cases of renal failure.
Almost all patients are prescribed complex therapy, which includes prednisolone, cyclophosphamide and plasmapheresis sessions. This treatment is aimed at removing autoantibodies and suppressing the inflammatory process in the kidneys. It is effective in 80% of patients.
Plasmapheresis is necessary to remove autoimmune components
If the level of creatinine in the blood exceeds 600 µmol/l, the effectiveness of treatment is significantly reduced, and in this case, therapy is supplemented with regular lifelong hemodialysis sessions. Without this, the prognosis of the disease is unfavorable.
Under the influence of therapy, antibodies to the basement membrane disappear within 1 - 1.5 years. The disease goes into remission. However, 2–10% of patients subsequently develop an exacerbation. It occurs within the first 10 years after recovery and is characterized by pulmonary hemorrhage or kidney damage. Treatment is carried out in the same way as at the onset of the disease.
After antibodies to the basement membrane are not detected in the blood for six months, a kidney transplant is considered. Relapse of the disease in the transplanted organ develops in 1-10% of cases.
Mortality during the first episode of the disease reaches 40%. The earlier treatment is started, the greater the patient’s chances of recovery. When the pathology recurs, the patient is already familiar with its symptoms, treatment begins earlier, and therefore the prognosis is better.
The average 5-year survival rate for this disease is 80%. Less than 30% of surviving patients require regular hemodialysis sessions. However, the average life expectancy is only about 6 years, and older age and pulmonary hemorrhage are factors that reduce it.
Thus, survival with Goodpasture syndrome depends almost entirely on the timing of diagnosis and initiation of therapy. The sooner a patient sees a doctor, the sooner he is in the hands of a specialist rheumatologist or nephrologist, undergoes a kidney biopsy procedure and begins full treatment, the greater the likelihood of recovery.
Symptoms
At the onset of the disease, nonspecific symptoms of Goodpasture syndrome appear:
- malaise, unexplained weakness, fatigue;
- increased body temperature for no clear reason;
- pain in various joints without swelling or redness;
- weight loss.
Already at this stage, even in the absence of pulmonary bleeding, the patient develops anemia.
Nonspecific symptoms in Goodpasture syndrome are usually less pronounced than in other rheumatological diseases. Often the patient does not see a doctor, and the disease begins to progress rapidly.
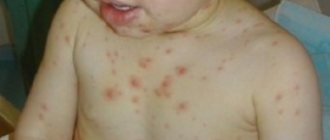
In almost 70% of patients, the first symptom of the disease is hemoptysis. This is especially true for smokers. Now doctors are noticing a decrease in the frequency of this symptom, which may be due to a decrease in the prevalence of this bad habit.
In addition to hemoptysis, symptoms include shortness of breath and cough, and later pulmonary hemorrhage develops.
Pulmonary hemorrhage does not necessarily occur against the background of hemoptysis and does not depend on its severity. In many patients it develops suddenly and causes death within a few hours. This condition is accompanied by severe shortness of breath and bluish skin. It may be complicated by pulmonary edema or pneumonia.
A few months after the onset of the first pulmonary symptoms, a patient with Goodpasture syndrome develops rapidly progressive glomerulonephritis, which causes renal failure. This condition also often leads to death within the next two years.
The main symptoms of kidney damage:
- weakness, lack of appetite, pale skin;
- headache and lower back pain;
- various pains in the heart area;
- increased blood pressure;
- widespread swelling;
- blurred vision;
- decrease in urine volume.
These symptoms of Goodpasture syndrome fully manifest themselves within 4 to 6 weeks after their onset. Therefore, such glomerulonephritis is called rapidly progressive.
Development of the disease
Goodpasture syndrome is an autoimmune disease caused by the formation of antibodies to the body's own cells. Antibodies formed to the glomerular basement membrane participate in its development. They are aimed at binding to a certain (fourth) type of collagen, and to one of the sections of its molecule - the non-collagen domain of the 3rd chain.
The structure of type IV collagen
There are different types of collagen. The 4th type is a network of connected spirals, each of which consists of 3 threads. It is a specific fragment of such a biopolymer that pathological autoantibodies are aimed at. This fragment is called Goodpasture antigen, and does not cause any pathological reactions in healthy people.
Goodpasture antigen is present in large quantities in the renal glomeruli, alveoli, as well as in the walls of the capillaries of the retina, cochlea of the inner ear, and choroid plexuses of the brain.
Antibodies bind to the Goodpasture antigen, which causes activation of the complement system - special immune proteins. A cascade protein reaction consistently unfolds, as a result of which inflammatory cells - leukocytes - are attracted to the site of contact of antibodies with antigens.
Leukocytes infiltrate the affected tissues and destroy them. In response to a complex immune response, there is ultimately an increase in the number of epithelial cells that are deposited on the basement membrane in the form of microscopic crescents. As a result, kidney function is significantly impaired, and they cannot fully eliminate toxic products. The same reaction in the pulmonary vessels leads to their damage and blood entering the cavity of the alveoli.
Causes
The cause of Goodpasture syndrome is unknown. Therefore, measures to prevent it have not been developed.
Some factors have been identified that may be related to the occurrence of the disease:
- viral infections, such as influenza A2c;
- prolonged contact with gasoline and organic solvents;
- smoking;
- shock wave lithotripsy (“crushing stones”) in the ureter for urolithiasis;
- genetic characteristics - the presence of certain genes of the HLA system in a person.