| QT interval | |
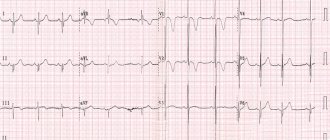
| Electrocardiogram showing the QT interval calculated by the tangent method | |
| ICD-10-PC | R94.31 |
| ICD-9-CM | 89,52 |
| MeSH | D004562 |
| MedlinePlus | 003868 |
| [edit in Wikidata] | |
QT interval
is a measurement made on an ECG used to evaluate certain electrical properties of the heart.
It is calculated as the time from the beginning of the Q wave
to the end
of the T wave
and approximately corresponds to the time elapsed from the moment the heart ventricles begin to contract until the end of relaxation. An abnormally long or abnormally short QT interval is associated with an increased risk of abnormal heart rhythm and sudden cardiac death. QT disturbances can be caused by genetic diseases such as long QT syndrome, certain medications such as sotalol or pitolisant, disturbances in the concentration of certain salts in the blood such as hypokalemia, or hormonal imbalances such as hypothyroidism.
Content
- 1 Measurement
- 2 Heart rate correction 2.1 Bazett formula
- 2.2 Fridericia formula
- 2.3 Saga Formula
- 2.4 Comparison of patches
- 3.1 Genetic causes
Dimension[edit]
Illustrations of Tangent and Threshold Methods of QT Interval Measurement
The QT interval is typically measured in lead II for evaluation of serial ECGs, with leads I and V5 being comparable alternatives to lead II. Leads III, aVL, and V1 are not routinely used to measure the QT interval. [1] Accurate measurement of the QT interval is subjective [2] because the end of the T wave is not always clearly defined and usually gradually merges with the baseline. The QT interval in an ECG complex can be measured manually using various methods, such as the threshold method, in which the end of the T wave is determined by the point at which the T wave component merges with the isoelectric baseline, or the tangent method, in which the end of the T wave is determined by the intersection of the tangent line , extrapolated from the T wave at the point of maximum descent to the isoelectric baseline. [3]
With the increasing availability of digital ECGs with simultaneous 12-lead recording, QT measurement can also be performed using the "superimposed median rhythm" method. In the beat median overlay method, the average ECG complex is constructed for each of the 12 leads. The 12 average contractions are superimposed and the QT interval is measured either from the earliest onset of the Q wave to the last offset of the T wave, or from the point of maximum convergence for the onset of the Q wave to the offset of the T wave.[4]
Heart rate correction[edit]
The QT interval varies with heart rate—as your heart rate increases, the QT interval shortens. These changes make it difficult to compare QT intervals measured at different heart rates. To account for this and thus improve the reliability of the QT measurement, the QT interval can be adjusted for heart rate using various mathematical formulas, a process that is often performed automatically by modern ECG recorders.
Bazett's formula[edit]
The most commonly used QT interval correction formula is Bazett's formula
, [5] named after the physiologist Henry Cuthbert Bazett (1885-1950), [6] calculating heart rate corrected for the QT interval (QTcB).
Bazett's formula is based on observations made in a 1920 study. Bazett's formula is often given in a form that returns QTc in the dubious unit of measurement, the square root of seconds. The mathematically correct form of Bazett's formula is:
Q T c B = Q T r r 1 s {\displaystyle QTc_{B}={QT \over {\sqrt {RR \over 1{\text{s)))))}
where QTc B is the heart rate-corrected QT interval and RR is the interval from the start of one QRS complex to the start of the next QRS complex. This mathematically correct formula returns QTc in the same units as QT, usually milliseconds. [7]
Some popular forms of this formula assume that QT is measured in milliseconds and RR is measured in seconds, often derived from heart rate (HR) as 60/HR. Therefore, the result will be expressed in seconds per square root of milliseconds. [8] However, reporting QTc using this formula creates “a requirement regarding the units in which the original QT and RR are measured.” [7]
In any case, Bazett's nonlinear QT correction formula is not generally considered accurate because it overcorrects at high heart rates and undercorrects at low heart rates. [9] Bazett's correction formula is one of the most suitable QT correction formulas for newborns. [10]
Fridericia formula[edit]
Fridericia [11] proposed an alternative correction formula using the cube root of RR.
Q T c F = Q T r r 1 s 3 {\displaystyle QTc_{F}={QT \over {\sqrt [{3}]{RR \over 1{\text{s)))))}
Saga Formula [edit]
The Framingham correction, also called the Saga formula, based on the Framingham Heart Study, which used long-term cohort data of more than 5000 subjects, is considered the best method [12]. [13]
Q T l c = 1000 ( Q T 1000 + 0.154 ( 1 - R R ) ) {\displaystyle QTlc=1000\left({\frac {QT}{1000))+0.154(1-RR)\right)}
Again, here's QT
and
QTlc
are in milliseconds, and
R
R is measured in seconds.
Comparison of fixes[edit]
A recent retrospective study suggests that the Fredericia method and the Framingham method may provide results that are most useful for stratifying 30-day and 1-year mortality risks. [12]
Upper limit of normal QT interval adjusted for heart rate according to Bazett's formula
, [5] using Friedericia's formula [11] and subtracting 0.02 s from QT for every 10 beats per minute increase in heart rate. [14] Up to 0.42 s (≤ 420 ms) is selected as the normal QTc for QT B and QT F in this diagram. [15]
Definitions of normal QTc range from equal to or less than 0.40 s (≤ 400 ms), [14] 0.41 s (≤ 410 ms), [16] 0.42 s (≤ 420 ms), [15] or 0.44 s (≤ 440 ms). RS). [17] For the risk of sudden cardiac death, the “borderline QTc” in men is 431–450 ms; and for women 451–470 ms. An “abnormal” QTc in men is a QTc greater than 450 ms; and for women - more than 470 ms. [18]
If the heart rate is not very high or low, the upper limits of the QT can be approximated by taking QT = QTc at a heart rate of 60 beats per minute (bpm) and subtracting 0.02 s from the QT for every 10 bpm . in heart rate. For example, with a normal QTc ≤ 0.42 s, one would expect the QT to be 0.42 s or less at a heart rate of 60 bpm. At a heart rate of 70 beats per minute, a QT can be approximately expected to be equal to or lower than 0.40 s. Likewise, for 80 bpm, the QT should be approximately equal to or lower than 0.38 s. [14]
QT interval value
First of all, this interval reflects the return of the ventricles from a state of excitation to a state of rest (ventricular repolarization). The normal value of the QT interval depends on the heart rate . When the rhythm frequency increases [shortening the RR interval (the interval between successive QRS complexes)], a shortening of the QT interval is characteristic; when the rhythm slows down (lengthening the RR interval), the QT interval is lengthened.
Anomalous intervals [edit]
Prolonged QTc causes premature action potentials during late phases of depolarization. This increases the risk of developing ventricular arrhythmias, including fatal ventricular fibrillation. [19] Higher rates of QTc prolongation are observed in women, older patients, high systolic blood pressure or heart rate, and short stature. [20] Prolonged QTc is also associated with ECG findings called Torsades de Pointes, which are known to degenerate into ventricular fibrillation, which is associated with higher mortality rates. There are many causes of QT prolongation; acquired causes are more common than genetic ones. [21]
Genetic reasons[edit]
Distribution of QT intervals among healthy men and women, as well as among individuals with congenital long QT syndrome
An abnormally long QT interval may be caused by long QT syndrome, whereas an abnormally short QT interval may be caused by short QT syndrome.
QTc length is associated with variations in the NOS1AP gene. [22] Jervell and Lange-Nielsen autosomal recessive syndrome is characterized by a long QTc interval in combination with sensorineural hearing loss.
Due to adverse reactions to medications[edit]
Main article: Drug-induced QT prolongation
QT prolongation may be associated with an adverse drug reaction. [23]
Antipsychotics
(especially first generation/"typical")
- haloperidol [24]
- Thioridazine [25]
- mesoridazine [25]
- chlorpromazine [25]
- ziprasidone
- sertindole [26]
DMARDs and antimalarials
- Hydroxychloroquine [27]
- chloroquine [27]
- quinine [27]
Antibiotics
- macrolides
- fluoroquinolones [28]
Other drugs
- methadone [29]
- vemurafenib
- pitolisant [30]
Some second-generation antihistamines, such as astemizole, have this effect. The mechanism of action of some antiarrhythmic drugs, such as amiodarone or sotalol, involves intentional pharmacological prolongation of the QT interval. In addition, high blood alcohol concentrations prolong the QT interval. [31] A possible interaction between selective serotonin reuptake inhibitors and thiazide diuretics is associated with QT prolongation. [32]
Due to pathological conditions [edit]
Hypothyroidism, a condition of low thyroid function, can cause prolongation of the QT interval on the electrocardiogram. Acute hypocalcemia causes prolongation of the QT interval, which can lead to ventricular arrhythmia.
A short QT may be associated with hypercalcemia. [33]
Use in drug approval studies[edit]
Since 2005, the FDA and European regulatory authorities have required that nearly all new molecular entities be evaluated in a rigorous QT study (TQT) or similar study to determine the drug's effect on the QT interval. [34] The TQT study serves to evaluate the potential susceptibility of a drug to arrhythmia. Typically, the QT interval was assessed using a personal reading device measuring approximately nine heartbeats per clinical time point. However, a significant portion of drug approvals after 2010 have involved a partially automated approach, combining automated software algorithms with expert human readers reviewing a portion of the heartbeats, allowing a significantly larger number of heartbeats to be assessed to improve accuracy and reduce costs. [35] In 2014, an industry consortium consisting of the FDA, iCardiac Technologies, and other organizations published the results of a seminal study showing how waivers of TQT studies can be obtained by evaluating early phase data. [36] As the pharmaceutical industry has gained experience conducting TQT studies, it has also become apparent that traditional QT correction formulas, such as QTcF, QTcB, and QTcLC, may not always be appropriate for evaluating drugs that affect autonomic tone. [37]
As a predictor of mortality[edit]
Electrocardiography is a safe and non-invasive tool that can be used to identify people at increased risk of death. In the general population, there was no convincing evidence that prolongation of the QTc interval is associated in isolation with increased mortality from cardiovascular disease. [38] However, several studies [ which?
] examined prolonged QT interval as a predictor of mortality in a subset of the population.
Rheumatoid arthritis[edit]
Rheumatoid arthritis is the most common inflammatory arthritis. [39] Research has linked rheumatoid arthritis to increased mortality from cardiovascular disease. [39] In a 2014 study [19] Panoulas et al. found that a 50 ms increase in the QTc interval increased the odds of all-cause mortality by 2.17 in patients with rheumatoid arthritis. Patients with the highest QTc interval (>424 ms) had higher mortality than patients with lower QTc intervals. The connection was lost when the calculations were adjusted for C-reactive protein levels. Researchers have suggested that inflammation prolongs the QTc interval and causes arrhythmias, which are associated with higher mortality rates. However, the mechanism by which C-reactive protein is associated with the QTc interval is still not understood.
Type 1 diabetes[edit]
Compared to the general population, type 1 diabetes may increase the risk of death, mainly due to an increased risk of cardiovascular disease. [20] [40] Almost half of patients with type 1 diabetes have a prolonged QTc interval (>440 ms). [20] Diabetes with a long QTc interval was associated with 29% mortality over 10 years compared with 19% with a normal QTc interval. [20] Antihypertensive drugs prolong the QTc interval but are not an independent predictor of mortality. [20]
Type 2 diabetes [edit]
QT interval dispersion (QTd) is the maximum QT interval minus the minimum QT interval and is related to ventricular repolarization. [41] A QTd greater than 80 ms is considered abnormally long. [42] Increased QTd is associated with mortality from type 2 diabetes. [42] QTd is a better predictor of cardiovascular death than QTc, which was not associated with mortality in type 2 diabetes. [42] A QTd greater than 80 ms has a relative risk of 1.26 for cardiovascular death compared with a normal QTd.
Problems in diagnosing long QT syndrome in newborns
For 30 years, long QT syndrome (LQT) has been actively studied, clinical data is being accumulated, genetic mutations and gene polymorphisms are being identified in various combinations that determine the heterogeneity of the phenotypic manifestations of channelopathy.
Presented by PJ Schwartz et al. In 1998, the results of electrocardiographic (ECG) screening in newborns, which identified a connection with sudden infant death [1–4], largely changed ideas about the monitoring of newborns and young children. A number of studies have shown that ECG screening can identify a risk group - newborns with bradycardia and/or changes in the electrocardiogram. In addition, the likelihood of idiopathic QTS has been found to increase as the repolarization period lengthens: thus, in newborns with a QTc interval duration of more than 470 ms, as well as their asymptomatic relatives, gene mutations are detected in 43% of cases, confirming the clinical diagnosis [1, 5, 6].
Newborns are the most difficult category of patients for timely detection of SUIQT. The reason for this is a wide range of perinatal conditions and diseases that have a negative impact on the functions of the central nervous system. On the one hand, these conditions mask disturbances of consciousness against the background of life-threatening conditions, on the other hand, they can initiate the early manifestation of the disease.
At the same time, it is known that pathology of the perinatal period can induce secondary disturbances in the electrical functions of the myocardium with the development of life-threatening conditions. Research on secondary SUIQT has found that it can be transient but have consequences as severe as genetically determined ion channel diseases. The involvement of myocardial damage, hypothermia, hypothyroidism, and hypocalcemia in the development of secondary disorders of the electrical functions of the heart and life-threatening arrhythmias has been revealed [7].
We present clinical cases of observation of newborn children born in March-April 2021 at the Perinatal Center of the Regional Clinical Children's Hospital, in order to draw the attention of neonatologists and pediatric cardiologists to the problem of timely diagnosis of SUIQT and the possibility of preventive therapy. The electrocardiogram in newborns was assessed according to the recommendations, the corrected QT interval was calculated using the Bazett formula [8, 9].
Clinical case 1. Patient N., born at 38–39 weeks of gestation, planned cesarean section (due to a scar on the uterus after similar operations in 2010, 2014) with a weight of 3470 g, Apgar score 8/9 points. Mom is 32 years old, chronic iron deficiency anemia, stage I; father is 37 years old, healthy. Previous pregnancies - the first in 2005 - urgent birth, the girl was developing according to her age, healthy; the second in 2009 - urgent delivery of a child weighing 4200 g (Apgar - 8-9 points) - the boy died on the seventh day of life; the third in 2010 - urgent birth of a child (breech presentation) weighing 3700 g - the boy died on the seventh day of life; the fourth in 2014 - urgent birth, birth weight 2300, the boy died on the fourth day. All cases of death of newborns in this family occurred at home and in the maternity hospital of the Republic of Tajikistan - without an identified cause, qualified as sudden infant death syndrome (SIDS).
At birth, the child has the clinical status of a full-term, functionally mature newborn. The condition is satisfactory, he actively suckles the breast, feeds on demand, and opens his eyes. Physiological reflexes are evoked, symmetrical, adequate response to examination. The skin is clean. The umbilical cord remains without signs of infiltration. Breathing is puerile, 40 per minute. Heart sounds are loud enough, with a heart rate of 136–144 per minute, blood pressure ranges from 57–84/39–55 mm Hg. Art. The abdomen is soft, the liver protrudes 2 cm from under the edge of the rib, the edge is elastic, the spleen is not palpable. Stool 3 times a day, yellow, mushy.
Taking into account the family history, it was decided to further examine the child in a hospital setting. From the fifth day of life he began to gain weight by 15.0–30.0 g per day. In the early neonatal period, jaundice was noted with hyperbilirubinemia up to 250 µmol/l on the second day and a decrease during phototherapy to 168 µmol/l. According to echocardiography, some expansion of the right parts of the heart was noted, functioning fetal communications - an aneurysm of the interatrial septum with a defect like the oval window of 5.0 × 6.0 mm and a ductus arteriosus measuring 2.0 × 2.0 mm with a left-to-right shunt, insufficiency tricuspid valve stage I, mean pulmonary artery pressure (MPAP) - 30 mm Hg. Art. Within 11 days, the MPAP value decreased to 20 mmHg. Art., closure of the ductus arteriosus is documented, but the interatrial defect remains the same size with a slight increase in the right sections.
According to electrocardiography, on the second day of life there is sinus rhythm with a heart rate of 143–156 per minute, the duration of the corrected QT interval is 520–616 ms, and incomplete blockade of the right bundle branch (Fig.). Analysis of Holter ECG monitoring during the day established sinus rhythm (average heart rate - 136 per minute, maximum - 176 per minute during the sanitary procedure), episodes of bradycardia below critical figures for the newborn period - 65 per minute during sleep, duration of the corrected QT interval during the entire observation time - 460–500 ms.
During 12 days of observation, no episodes of impaired consciousness or any other neurological symptoms were identified, and rhythm monitoring did not detect ventricular tachycardia. Electrolyte disturbances were also not detected: sodium - 141 mmol/l, potassium - 4.6 mmol/l, glucose - 4.6 mmol/l.
The assessment of symptoms according to the Schwartz criteria for this patient was as follows: duration of spontaneous QTc > 480 ms in a male patient - 3 points, cases of sudden unexplained death in the family - 0.5 points, a score of ≥ 3.5 points was obtained, that is, a high risk is noted idiopathic SUIQT.
Considering the unfavorable family history and the critical prolongation of the corrected QT interval, on the second day of life the child was prescribed bisoprolol at a dose of 0.3 mg (0.08 mg/kg per day) once per day under blood pressure control. During therapy, a decrease in blood pressure to 60–56/37 mm Hg was noted. Art. in the first two days of therapy, which required a correction of the drug administration regimen - a transfer to the administration of a daily dose in two doses. This led to stabilization of blood pressure at 65–72/40 mmHg. Art., the child was discharged under the supervision of a local pediatrician and pediatric cardiologist.
The child is currently 5 months old and is undergoing continuous maintenance therapy with a beta blocker, which is well tolerated. The condition is stable: adequate weight gain, intellectual development and normal rate of formation of motor skills; no life-threatening conditions have been recorded. At the age of 1 month, the QTc interval on the electrocardiogram is 463 ms, at 3 months - 457 ms, that is, positive clinical dynamics can be traced. Monitoring by a pediatrician and pediatric cardiologist continues, and he is under the supervision of an arrhythmologist at the Federal Center for Surgery in Krasnoyarsk. Venous blood was collected to isolate DNA and conduct molecular genetic research in the child, sibling and parents.
In addition to the dramatic family history, the clinical case under consideration is interesting due to the excessive prolongation of the corrected QT interval in the complete clinical “well-being” of the patient. The literature describes an association of QTc prolongation of more than 600 ms with death in the first week of life [10]. It is likely that in this patient, early initiation of beta-blocker therapy had a positive effect on the sympathetic regulation of heart rate, avoided the development of life-threatening heart rhythm disturbances and did not repeat the fate of the three previous siblings.
Clinical case 2. Patient V., from premature third birth at 35 weeks. A burdened medical, social and obstetric history - maternal smoking during pregnancy, a scar on the uterus after a cesarean section in 2009, 2001, the threat of uterine rupture along the scar, moderate thrombocytopenia without hemorrhagic syndrome, emergency cesarean section. Birth weight - 2170 g, body length - 49 cm, Apgar score - 7/8 points. At the 8th minute of life, she began to feel faint, pallor and acrocyanosis appeared, chest excursion was reduced, shortness of breath up to 60 per minute with retraction of the intercostal muscles, and wheezing. Symptom of "pale spot" - 3 seconds. Heart sounds are rhythmic, of sufficient volume, with a heart rate of 138 per minute. The abdomen is accessible to deep palpation, the liver protrudes 1.5 cm from under the edge of the costal arch. A diagnosis of transient tachypnea of the newborn was made, and the girl was transferred to the neonatal pathology department.
Over time, crepitating wheezing and increasing respiratory failure appeared; blood pressure was 57/26 mm Hg. Art., arterial hypoxemia (SaO2 - 87%, pH - 7.162, pCO2 - 62.6 mm Hg, pO2 - 59.8 mm Hg). According to echocardiography, functioning fetal communications are revealed - ductus arteriosus 2 mm and foramen ovale 3 mm, MPAP - 40 mm Hg. Art. The child's condition is classified as respiratory distress syndrome. Respiratory support was started (respirator SLE 5000, SIMV mode, parameters: pip 16 cm H2O, peep 5 cm H2O, MAP 9 cm H2O, FiO2 0.3, f 60 min, Tin 0.33 sec). A catheter (line, v. saphena) was installed for antibacterial therapy - ampicillin (0.11 g twice daily) and netromycin (100 mg/ml - 0.0098 ml once daily). Curosurf (80 mg/ml suspension 1.5 ml) 480 mg was administered once.
During the day, the condition stabilized: SaO2 - 95%, puerile breathing, carried out in all fields, heart rate - 158 per minute, blood pressure - 67/39 mm Hg. Art. She was transferred to independent breathing, fed through a tube, absorbed 7 ml on the third day of life, and antibacterial therapy was continued. The ECG documented sinus bradycardia with a heart rate of 106–120 per minute, the duration of the QTc interval was 482–540 ms. Over the next three days, the patient’s condition was stable, and the feeding volume was increased to 20 ml. On the fourth day of life - sudden clinical death. The episode of respiratory arrest and heartbeat occurred against the background of a stressful state - the child was worried, at 11.00 the catheter (line) was removed, at 11.13 - deep apnea, clinical death was recorded. A short-term episode of hypocalcemia (up to 0.5 mmol/l) and stress (catheter removal) were discussed as causes of cardiac electrical dysfunction and cardiac arrest. Family history of life-threatening conditions and sudden death is negative.
After 15 minutes of resuscitation measures (tracheal intubation, mechanical ventilation, drug therapy, chest compressions), heartbeats of more than 100 beats were recorded. per minute Intensive therapy was started aimed at treating post-resuscitation illness: mechanical ventilation in the normal ventilation mode, brain immobilization, inotropic stimulation, infusion therapy 120 ml/kg, antibacterial therapy as before. Against the background of massive drug sedation - coma 3, after withdrawal - the appearance of motor activity, gradual restoration of reflexes. Diagnosis at discharge: “Anoxic brain damage, severe. Periventricular leukomalacia in the stage of cyst formation. Long QT syndrome, probably secondary (due to transient hypocalcemia). Transient tachypnea of the newborn."
After 1 month, the ECG showed no prolongation of the QTc interval (416 ms). The child is observed by a neurologist, pediatrician, and pediatric cardiologist. According to the Schwartz criteria, the child has the following documented: prolongation of the QT interval more than 480 ms - 3 points, an episode of a rare rhythm - 0.5 points, clinical death due to a stressful state - 2 points, that is, in total - more than 3.5 points, which characterizes also a high probability of idiopathic SUIQT. Therefore, blood was drawn from the child and family members for molecular genetic testing.
The peculiarity of this clinical case is that, with equal probability, the causes of the clinical death of the child could be either a transient prolongation of the QTc interval against the background of hypocalcemia and the development of arrhythmia under the influence of pain stress, or a genetically determined disease of the ion channels of cardiomyocytes (mutation de novo).
Thus, the presented clinical cases demonstrate the alertness of pediatricians and neonatologists in the presence of a family history of sudden unexplained death of children, the efficiency and correctness of diagnosis of SUIQT, as well as the success of therapy. On the other hand, it is necessary to increase attention to the combination of such conditions as electrolyte imbalance, bradycardia, impaired repolarization on the electrocardiogram in newborns and to avoid creating stressful situations (including therapeutic and diagnostic manipulations) without correcting these symptoms.
Literature
- Shkolnikova M. A., Makarov L. M., Bereznitskaya V. V. et al. Life-threatening arrhythmias and sudden cardiac death in children // Bulletin of Arrhythmology. 2000; 18:57–58.
- Schwartz PJ, Stramba-Badiale M, Segantini A, Austoni P et al. Prolongation of the QT Interval and the Sudden Infant Death Syndrome // N Engl J Med. 1998; 338:1709–1714. DOI: 10.1056/NEJM199806113382401.
- Arrested M., Crotti L., Rognum TO, Insolia R., Pedrazzini M., Ferrandi C., Vege A., Wang DW, Rhodes TE, George AL Jr., Schwartz PJ Prevalence of long QT syndrome gene variants in sudden infant death syndrome // Circulation. 2007; 115:361–367.
- Schulze-Bahr E., Fenge H., Etzrodt D., Haverkamp W. et al. Long QT syndrome and life threatening arrhythmia in a newborn: molecular diagnosis and treatment response // Heart. 2004; 90 (1): 13–16.
- Lupoglazoff JM, Denjoy I., Villain E., Fressart V. et al. Long QT syndrome in neonates. Conduction disorders associated with HERG mutations and sinus bradycardia with KCNQ1 mutations // J Am Coll Cardiol. 2004; 43(5):826–830. DOI: 10.1016/j.jacc.2003.09.049.
- Schwartz PJ, Stramba-Badiale M, Crotti L, Pedrazzini M et al. Prevalence of the Congenital Long QT Syndrome // Circulation. 2009; 120(18):1761–1767. DOI: 10.1161/CIRCULATIONAHA.109.863209.
- Mu SC, Sung TC, Lin MI Transient Prolongation of QT Interval in a Neonate // Clin Neonatology. 1998; 5 (2): 27–29.
- Torres F., Hernández M., Garcia J., Marti-Almor J., Garcia-Algar O. et al. Newborn Electrocardiography as a Screening Method for Long-QT Syndrome // J Clin Exp Cardiolog. 2015; 6: 370. DOI:10.4172/2155–9880.1000370.
- Schwartz PJ, Garson A, Paul T, Stramba-Badiale M et al. Guidelines for the interpretation of the neonatal electrocardiogram A Task Force of the European Society of Cardiology // Eur Heart Journal. 2002; 23: 1329–1334 DOI: 10.1053/euhj.2002.3274.
- Perticone F., Ceravolo R., Mattiol PL Prolonged QT interval: A marker of sudden infant death syndrome? // Clin Cardiol. 1991; 14:417–421.
A. Yu. Cheremisina* E. V. Antsiferova**, Candidate of Medical Sciences N. A. Voronina** T. V. Stelmashuk*** E. Yu. Emelyanchik*, 1, Doctor of Medical Sciences, Professor S. Yu. Nikulina*, Doctor of Medical Sciences, Professor
* GBOU VPO Krasnoyarsk State Medical University named after. prof. V. F. Voino-Yasenetsky Ministry of Health of the Russian Federation, Krasnoyarsk ** KGBUZ KKKTSOMD, Krasnoyarsk *** KGBUZ KMDKB No. 1, Krasnoyarsk
1 Contact information
Links[edit]
- Panicker GK, Salvi V, Karnad DR, Chakraborty S, Manohar D, Lokhandwala Y, Kothari S (2014). "Drug-induced QT prolongation when the QT interval is measured in each of the 12 ECG leads in men and women with careful QT testing." J Electrocardiol
.
47
(2): 155–157. DOI: 10.1016/j.jelectrocard.2013.11.004. PMID 24388488. - Panicker GK, Karnad DR, Joshi R, Shetty S, Vyas N, Kothari S, Narula D (2009). "Z-score for assessing reader competence in a central ECG laboratory". Ann Noninvasive Electrocardiol
.
14
(1): 19–25. DOI: 10.1111/j.1542-474X.2008.00269.x. PMC 6932360. PMID 19149789. - Panicker GK, Karnad DR, Natekar M, Kothari S, Narula D, Lokhandwala Y (2009). "Intra- and interreader variability in QT interval measurement by tangent and threshold methods in a central electrocardiogram laboratory." J Electrocardiol
.
42
(4): 348–52. DOI: 10.1016/j.jelectrocard.2009.01.003. PMID 19261293. - Salvi V, Karnad DR, Panicker GK, Natekar M, Hingorani R, Kerkar V, Ramasamy A, de Vriesa M, Zumbrunnen T, Kothari S, Narula D (2011). "Comparison of 5 Methods for Measuring QT Interval on Electrocardiograms from a Rigorous QT/QTc Study: Impact on Assay Sensitivity and Categorical Outliers." J Electrocardiol
.
44
(2):96–104. DOI: 10.1016/j.jelectrocard.2010.11.010. PMID 21238976. - ^ ab Bazett HC. (1920). "Analysis of temporal relationships of electrocardiograms." Heart
(7): 353–370. - Rogen, A (March 2011). "Henry Cuthbert Bazett (1885–1950) - author of the QT interval correction formula." Stimulation Clin Electrophysiol
.
34
(3): 384–8. DOI: 10.1111/j.1540-8159.2010.02973.x. PMID 21091739. - ^ ab Molnar, Janos; Weiss, Jerry; Rosenthal, James (1 March 1995). "The Missing Second: What is the Correct Unit for the Bazett-Corrected QT Interval?" American Journal of Cardiology
.
75
(7):537–538. DOI: 10.1016/S0002-9149(99)80603-1. PMID 7864010. - Salvi V, Karnad DR, Panicker GK, Kothari S (2010). "Update on evaluating the effect of a new drug on cardiac repolarization in humans: challenges in early drug development". Br J Pharmacol
.
159
(1):34–48. DOI: 10.1111/j.1476-5381.2009.00427.x. PMC 2823350. PMID 19775279. } - Salvi V, Karnad DR, Panicker GK, Kothari S (2010). "Update on evaluating the effect of a new drug on cardiac repolarization in humans: challenges in early drug development". Br J Pharmacol
.
159
(1):34–48. DOI: 10.1111/j.1476-5381.2009.00427.x. PMC 2823350. PMID 19775279. - Stramba-Badiale M, Karnad DR, Goulene KM, Panicker GK, Dagradi F, Spazzolini C, Kothari S, Lokhandwala YY, Schwartz PJ (2018). "For neonatal ECG screening there is no reason to abandon the old Bazett correction". Eur J hearts
.
39
(31):2888–2895. DOI: 10.1093/eurheartj/ehy284. PMID 29860404. - ^ a b Fridericia L.S. (1920). "Duration of systole on the electrocardiogram of healthy people and patients with heart disease." Acta Medica Scandinavica
(53): 469–486. - ^ ab Vandenberk B, Vandael E, Robyns T, Vandenberghe J, Garweg C, Foulon V, Ector J, Willems R (2016-06-17), "Which QT correction formulas to use for QT monitoring?", Journal of the American Heart Association
,
5
(6), DOI: 10.1161/JAHA.116.003264, PMC 4937268, PMID 27317349 - Sagie A, Larson MG, Goldberg RJ, Bengston JR, Levy D (1992). "An Improved Method for Adjusting the QT Interval for Heart Rate (Framingham Heart Study)." Am J Cardiol
.
70
(7):797–801.
DOI: 10.1016/0002-9149 (92) 90562-D. PMID 1519533. [ full text needed
] - ^ abc Lesson III. Characteristics of a Normal ECG Frank G. Janowitz, MD. Professor of Medicine. University of Utah School of Medicine. Retrieved March 23, 2010
- ^ ab Dr. Dean Jenkins and Dr. Stephen Gerred. "Normal 12-lead adult ECG". ecglibrary.com
. Retrieved January 28, 2018 - Loyola University Chicago Stritch School of Medicine > Medicine I Matthew Fitz, MD Retrieved on Mars 23, 2010
- Image for Cardiovascular Physiology Concepts > Electrocardiogram (ECG, EKG) Richard E. Klabunde, Ph.D.
- medscape.com>QTc prolongation and risk of sudden cardiac death: is the debate over? February 3, 2006
- ^ ab Panaulas V.F., Toms T.E., Douglas K.M. and others (January 2014). "Lengthened QTc interval predicts all-cause mortality in patients with rheumatoid arthritis: an association driven by high inflammatory load". Rheumatology
.
53
(1): 131–7. DOI: 10.1093/rheumatology/ket338. PMID 24097136. - ^ abcde Rossing P, Breum L, Major-Pedersen A et al (March 2001). "Elongated QTc interval predicts mortality in patients with type 1 diabetes." Diabetes Medicine
.
18
(3): 199–205. DOI: 10.1046/j.1464-5491.2001.00446.x. PMID 11318840. - Van Noord, C; Eijgelsheim, M; Stricker, B. H. C. (2010). "QT prolongation associated with and without drugs". British Journal of Clinical Pharmacology
.
70
(1): 16–23. DOI: 10.1111/j.1365-2125.2010.03660.x. PMC 2909803. PMID 20642543. - Arking DE, Pfeufer A, Post W, et al (June 2006). "A common genetic variant of the NOS1 regulator NOS1AP modulates cardiac repolarization." Genetics of Nature
.
38
(6): 644–51. DOI: 10.1038/ng1790. PMID 16648850. - Leitch A, McGinness P, Wallbridge D (September 2007). "Calculate the QT interval in patients taking medications for dementia". BMJ (ed. Clinical Research)
.
335
(7619): 557. DOI: 10.1136/bmj.39020.710602.47. PMC 1976518. PMID 17855324. - "Information for Health Care Professionals: Haloperidol (marketed as Haldol, Haldol Decanoate, and Haldol Lactate)". Archived from the original on 2007-10-11. Retrieved September 18, 2007.
- ^ abc "Which psychotropics carry the greatest risk of QTc prolongation?" . www.mdedge.com
. Retrieved May 19, 2021. - Jump up
↑ Lewis R, Bagnall AM, Leitner M (2005).
"Sertindole for schizophrenia". Cochrane Database of Systematic Reviews
(3): CD001715. DOI: 10.1002/14651858.CD001715.pub2. PMC 7025766. PMID 16034864. - ^ abc Malloy, Terry (25 March 2021). "Guidance for patients at risk of drug-induced sudden cardiac death due to off-label treatment for COVID-19". Mayo Clinic News Network
. Retrieved May 19, 2021. - A, Briasulis; V, Agarwal; Wj, Pearce (2011). "QT Prolongation and Torsade De Pointes Caused by Fluoroquinolones: Uncommon Side Effects of Commonly Used Drugs." Cardiology
.
120
(2):103–10. DOI: 10.1159/000334441. PMID 22156660. - Haigney, Mark. "Cardiotoxicity of methadone" (PDF). Director of Cardiology
. Retrieved February 21, 2013. - https://wakix.com “WAKIX prolongs the QT interval; "Avoid use of WAKIX in patients with known QT prolongation or in combination with other drugs known to prolong the QT interval."
- Aasebø W, Erikssen J, Jonsbu J, K Stavem (April 2007). "ECG changes in patients with acute ethanol intoxication." Scandinavian Cardiovascular Journal
.
41
(2): 79–84. DOI: 10.1080/14017430601091698. PMID 17454831. - Tatonetti N.P., E.P., Daneshjou R, Altman RB (March 2012). "Data-Based Prediction of Drug Effects and Interactions". Translational Medicine Science
.
4
(125): 125ra31. DOI: 10.1126/scitranslmed.3003377. PMC 3382018. PMID 22422992. - Hypercalcemia [ full citation required
] - "Archive copy" (PDF). Archived from the original (PDF) on March 6, 2010. Retrieved December 9, 2009. CS1 maint: zipped copy as title (link)[ full link required
] - "iCardiac Takes an Automated Approach to Rigorous QT Study for Leading Pharmaceutical Company - Applied Clinical Trials". October 5, 2011. Archived from the original on October 5, 2011. Retrieved March 19, 2021.CS1 maint: bot: original URL status unknown (link)
- Or, Amy (2014-12-16). "Northwest-backed iCardiac expands its market with breakthrough". Wall Street Journal
. ISSN 0099-9660. Retrieved January 26, 2021. - "Garnett" (PDF). Retrieved June 6, 2014.
- Montanez A, Raskin JN, Hebert PR, Lam GA, Hennekens CH (May 2004). "Prolonged QTc interval and risks of all-cause and cardiovascular mortality and sudden death in the general population: a review and qualitative review of prospective cohort studies". Archives of Internal Medicine
.
164
(9):943–8. DOI: 10.1001/archinte.164.9.943. PMID 15136301. - ^ a b Solomon D.H., Carlson E.V., Rimm E.B. and others (March 2003). "Cardiovascular disease morbidity and mortality in women diagnosed with rheumatoid arthritis". Circulation
.
107
(9):1303–7. DOI: 10.1161/01.cir.0000054612.26458.b2. PMID 12628952. - Borch-Johnsen K, Andersen PK, Deckert T (August 1985). "The effect of proteinuria on relative mortality in type 1 diabetes mellitus (insulin dependent)". Diabetology
.
28
(8):590–6. DOI: 10.1007/bf00281993. PMID 4054448. - Okin PM, Devereux RB, Howard BV, Fabsitz RR, Lee ET, Welty TK (2000). "Assessing QT interval and QT dispersion to predict all-cause and cardiovascular mortality in American Indians: the Strong Heart Study". Circulation
.
101
(1):61–66. DOI: 10.1161/01.cir.101.1.61. PMID 10618305. - ^ a b c Giunti S., Gruden G., Fornengo P. et al. (March 2012). "Increased QT interval variance predicts 15-year cardiovascular mortality in patients with type 2 diabetes: the population-based Casale Monferrato study". Diabetes care
.
35
(3):581–3. DOI: 10.2337/dc11-1397. PMC 3322722. PMID 22301117.
Why is the disease called Long QT Syndrome?
Why is the disease called Long QT Syndrome?
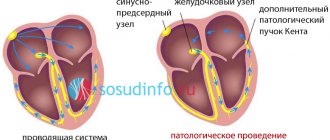
The name comes from the lengthening of a special indicator of the electrocardiogram - the QT interval. In addition, QT prolongation may be part of another disease, Romano-Ward syndrome or Jervell-Lange-Nielsen syndrome (see point 3).
What is the QT interval?
The duration of the QT interval is the time required to complete the processes of depolarization and repolarization of the myocardium. In this disease, the duration of repolarization is longer than normal, thus prolonging the interval. Its duration above 440 ms is considered prolongation. Electrophysiologically, prolongation of the QT interval is the result of overload of myocardial cells with positively charged ions during repolarization.
2.1 What is the corrected QT interval (QTc)?
The term "corrected" may be misunderstood. This does not mean that the measured interval may be incorrect, it means that the interval must be refined in relation to the heart rate. The reason is that the length of the QT interval depends on the heart rate. The concept of corrected QT interval can be compared to body mass index (Body Mass Index BMI).
What are boundary values?
A QTc interval longer than 440 ms is considered prolonged. The boundary values of the interval are close, but do not reach a diagnostically significant level. Values of 450-470 ms are regarded as borderline. The average QTc duration of patients diagnosed with Long QT Syndrome is approximately 490 ms. Values above 480 ms for women and 470 ms for men are a possible sign of long QT syndrome, in the absence of other factors that prolong this indicator - some medications, electrolyte disturbances, etc.
What is known from a medical point of view?
The disease can be either congenital, hereditary, or acquired. Congenital forms: The disease was first described in 1957. It exists in two variants. The autosomal dominant type is Romano-Ward syndrome (in honor of the doctors who first described the disease O. Connor Ward and C. Romano) and the autosomal recessive type Jervell-Lange-Nielsen (A. Jervell, F. Lange-Nielsen). Congenital forms are caused by genetic mutations. At least 9 such mutations are known, and there may be more. In recent years, 5 new genes have been discovered. The location of the sixth is known, but the gene itself has not yet been isolated. Jervell-Lange-Nielsen is associated with deafness. The syndrome is inherited in an autosomal recessive manner - both parents have one or more genes associated with the Syndrome, and the child receives both damaged genes - one from each parent. Statistically, each child born to such a couple has a 25% chance of receiving both damaged genes (homozygous status), a 50% chance of receiving only one copy of the gene (heterozygous status), and a 25% chance of receiving both normal genes and being healthy. When a child has both damaged genes, he is homozygous if both parents have damage in the same gene, and combined homozygous if one parent has damage in one gene and the other in the other. Jervell-Lange-Nielsen syndrome is a rare disease, since the likelihood of two parents meeting with long QT syndrome is low. The second form is Romano-Ward syndrome. The hearing is not damaged. It is inherited in an autosomal dominant manner - the patient has one damaged copy of the gene responsible for the development of the syndrome and one normal one. Each child born from a couple in which one parent has a damaged gene has a 50% chance of receiving a defective copy and thus having a defective gene. Long QT syndrome and 50% chance of getting a normal copy of the gene and being healthy. Acquired forms: Most often due to the use of certain medications. These medications are contraindicated in patients with long QT syndrome.
What are the symptoms?
Common manifestations of the disease are syncope (sudden loss of consciousness) or sudden death, developing against the background of physical activity or emotional stress. Onset most often occurs during prepuberty or adolescence, but can occur in infancy and middle age. Episodes of syncope are often misdiagnosed and considered to be syncope (vasavagal reaction) or an epileptic seizure. True epilepsy is rare in Long QT Syndrome, but the diagnosis of epilepsy is most often made in patients suffering from this disease. Sudden loss of consciousness during physical or psychoemotional stress should raise suspicion for the presence of Long QT Syndrome. A family history of unexplained loss of consciousness or sudden death at a young age should also raise suspicion. It is important that about a third of patients remain asymptomatic, but the absence of a clinical picture is not an exclusive factor. Every “unexplained” case of syncope or cardiac arrest at a young age should be considered as a consequence of this disease.
What is the therapy?
Beta blockers are the cornerstone of therapy for patients with long QT syndrome. This group is effective in approximately 90% of patients. However, recent studies (Association of Long QT Loci Syndrome and Cardiac Events Among Patients Treated With Beta Blockers: JAMA. 2004;292:1341-1344) show that patients with the LQT2 and LQT3 genotypes remain at high risk of cardiac events. New data on the genetics of the syndrome suggest that there is a subpopulation of patients who may be treated with other drugs instead of or in addition to beta blockers. For patients in whom conservative therapy has not been effective enough, implantation of a pacemaker or automatic defibrillator may be indicated. Another procedure (especially common in Europe) may be to surgically cut a specific nerve trunk, called left-sided sympathetic denervation. It is important to provide treatment to all patients. This is because fatal cardiac events such as sudden death cannot be predicted and often occur in asymptomatic patients (including children) as the first clinical manifestation of the disease.
Sports restrictions.
Competitions should be eliminated. In reality, there are very few data on how exercise affects adequately treated patients with long QT interval. It is known that patients of the LQT1 type have an increased risk of developing complications, in addition, a slight increase in risk is also typical for patients of the LQT2 type. This is especially noticeable in untreated patients.
Why is the disease called Long QT Syndrome?
The name comes from the lengthening of a special indicator of the electrocardiogram - the QT interval. In addition, QT prolongation may be part of another disease, Romano-Ward syndrome or Jervell-Lange-Nielsen syndrome (see point 3).
What is the QT interval?
The duration of the QT interval is the time required to complete the processes of depolarization and repolarization of the myocardium. In this disease, the duration of repolarization is longer than normal, thus prolonging the interval. Its duration above 440 ms is considered prolongation. Electrophysiologically, prolongation of the QT interval is the result of overload of myocardial cells with positively charged ions during repolarization.
2.1 What is the corrected QT interval (QTc)?
The term "corrected" may be misunderstood. This does not mean that the measured interval may be incorrect, it means that the interval must be refined in relation to the heart rate. The reason is that the length of the QT interval depends on the heart rate. The concept of corrected QT interval can be compared to body mass index (Body Mass Index BMI).