Open common atrioventricular canal
Anatomically, this defect is the absence of a section of septa separating the right and left atria in their lower section, and the ventricles in their upper section. Instead of the normal fusion of the interatrial and interventricular septa, a large common opening is formed between all four chambers of the heart. The matter is further complicated by the fact that this is where the zone of attachment to the septum of the atrioventricular valves is located: in the left - the mitral valve, in the right - the tricuspid. nowhere to attach themselves
, and they fuse with each other, forming
valves common to both ventricles
.
Above them there remains a defect between the atria, and below them there is a defect between the ventricles. “full form”
occurs .
If the leaflets adhere to the interventricular septum, then there will be no interventricular defect underneath them. Only interatrial communication remains. Then they talk about “incomplete form”,
or primary atrial septal defect. It’s very simple to imagine this - draw a cross, and then erase the area where the vertical and horizontal lines connect. The vertical line is the interatrial (top) and interventricular (bottom) septa, the horizontal line is the valves, and the dotted line is their common valves: This is the full form. Now connect two horizontal lines with the bottom vertical - you get the letter "Y" with only one hole at the top - this is an incomplete form of the vice.
Unlike ventricular septal defects, the VVC will never close on its own.
Let's talk first about the incomplete form of the vice. It is called a primary atrial septal defect because it is only a large defect in the lower part of the atrial septum. But it is accompanied by another intracardiac disorder - “splitting
» the anterior leaflet of the mitral valve, the two halves of which do not fuse together (and a gap forms between them).
Mitral valve insufficiency
occurs , i.e. With each contraction, through this splitting, part of the blood from the left ventricle is thrown back into the left atrium. That is, in addition to the atrial septal defect, another reason appears that increases the residual blood volume in the cavities of the heart and the load on all its parts.
Complaints and clinical symptoms are very similar to those described for isolated atrial septal defects, but they develop earlier and at a faster rate. Usually, at the age of the first 3-6 months of life, there are already direct indications for surgery. Some patients come to surgeons as adults, but the likelihood that they will suffer from rhythm disturbances even after the defect is completely eliminated is quite high.
The essence of the operation is to close the defect using a patch (usually from the pericardium, i.e. the dense sac surrounding the heart) and suturing the split mitral valve leaflet. It is clear that this is an open-heart intervention with the help of artificial circulation. The operation is well developed and its risk is the same as when closing a conventional interatrial communication, i.e. practically insignificant.
With the full form of the vice
the situation is more complicated. There are several components: an atrial septal defect and a ventricular septal defect, merging into one huge hole, and one valve ring common to both ventricles, regulated by two large common leaflets. All parts of the heart work under enormous overload, constantly increasing the volume of blood. The small circle is especially crowded. The pressure in it naturally increases and, as with large VSDs, the danger of rapid development of irreversible changes in the pulmonary vessels is very high.
Clinically, the defect is severe. Symptoms of heart failure appear already in the first months of life, and the child’s condition requires constant drug support. The heart quickly increases in size - all four chambers are overloaded and have difficulty coping with the work. The child is very sick, eats and develops poorly, and constantly catches colds, often ending in pneumonia. The condition is not critical, but very dangerous. Surgery required
soon after a definitive diagnosis.
If there are conditions for surgical treatment, the defect can be eliminated simultaneously in the first months of life, and if there are no such conditions, treatment can be divided into two stages, i.e. First, narrow the pulmonary artery (as with VSD), and after a few months perform radical surgery.
The essence of correcting the defect is this. In an open-heart setting, the common valve leaflets are bisected to create two separate entrances to the right and left ventricles. The ventricular septal defect is then closed using a patch. The previously dissected parts of the common leaflets are sewn to this patch, thereby creating the right and left atrioventricular openings. Then, a separate patch is used to close the interatrial part of the former defect.
There is a lot of work here, and it takes quite a long time both for the operation itself and for using the heart-lung machine. At the same time, the capabilities of leading clinics specializing in the surgical treatment of defects in infants make it possible to perform this operation in infancy. Operations in the early stages are absolutely justified, because allow you to avoid many complications that are already difficult or impossible to correct. Today the operation is quite well developed and standard, and its results are good.
However... This is an eternal “however” in medicine... When a surgeon works on valve flaps, which, especially in small children, are the thinnest transparent petals, then any, even the thinnest material for sutures turns out to be too rough. In addition, the baby will grow, and the heart - and its valve openings - will enlarge as it grows. Therefore, the earlier the operation is performed, the more likely it is that someday his atrioventricular valves, especially the mitral valve, will work imperfectly, i.e. their insufficiency will appear. This can happen even after a perfectly performed first operation. At some point in adolescence or adulthood, the question may be raised about eliminating the insufficiency surgically: plastic surgery or valve replacement. But this, if it happens, will be much later. Here we want to emphasize that a child who has been successfully operated on for a full form of VVC should be regularly shown to a cardiologist and monitored the function of his valves, which may change over the years.
This does not mean at all that you need to protect your child from physical activity and convince him that he has a heart condition, under no circumstances. But he probably still shouldn’t engage in, for example, high-impact sports, which today involves stress beyond the limits of a healthy person’s capabilities. However, monitoring him by a competent pediatric cardiologist is the guarantee that everything will be done on time.
It should be added that, in contrast to previously described operations for patent ductus arteriosus, ventricular or atrial septal defect, coarctation, and incomplete AVK, surgery for complete AVK, especially in young children, is a particularly delicate and complex procedure that requires excellent preparation the entire surgical team and sufficient experience. Not long ago, it was associated with significant mortality even in the best specialized centers. Today this is not the case, and the results, both immediate and long-term, are excellent.
One of the founders of cardiac surgery, Dr. John Kirklin, once said: “In my opinion, this is the most beautiful operation that is performed for congenital heart defects.” And may she be not only the most beautiful for your child, but also the most successful.
How to get treatment at the Scientific Center named after. A.N. Bakuleva?
Online consultations
Publications in the media
Patent atrioventricular canal (AVC) is a group of congenital anomalies characterized by the presence of fused VSD and atrial septal defect (ASD) and impaired development of the AV valve apparatus. Statistical data • 5% of all congenital heart disease under the age of 1 year • 2–6% of all congenital heart disease, of which 70% are partially open AVK, 30% are complete and intermediate forms of open AVK • 25–30% are combined with Down syndrome.
Etiology: causes of congenital heart disease (see Tetralogy of Fallot).
Pathogenesis. The full form (common OAVC) is characterized by a primary ASD merging with a defect in the membranous part of the interventricular septum, and a highly located VSD; the AV valves have common leaflets for the left and right atrioventricular orifices. Partially open AVK is a combination of a primary ASD with splitting of the anterior mitral leaflet and/or tricuspid valve leaflet. Intermediate forms (oblique canals, or Gerbode defects) are characterized by blood flow from the left ventricle to the right atrium, which leads to overload of the right ventricle and dilation of the pulmonary artery trunk. Hemodynamic disorders consist of a combination of those with VSD and ASD (see Ventricular septal defect, Atrial septal defect).
Clinical picture
• Complaints: see Atrial septal defect, Mitral valve insufficiency.
• Objectively • Pallor of the skin •• Severe cyanosis in children over 4 years of age with the full form of open AVK •• The borders of the heart are expanded to the left and right •• Increased apical impulse •• Right ventricular impulse •• Systolic tremor •• Strengthening of the first sound above the apex of the heart and II tone above the pulmonary artery (with a partially open AVK absent) •• Pansystolic murmur over the entire region of the heart with a maximum in the third and/or fourth intercostal spaces, carried out in the interscapular and axillary region (with complete and intermediate forms) •• For incomplete forms Characteristic is a systolic murmur in the second intercostal space on the left (the murmur of an ASD) in combination with the murmur of mitral regurgitation at the apex of the heart.
Instrumental diagnostics • ECG : see Ventricular septal defect, Atrial septal defect, Mitral valve insufficiency • EchoCG: see Ventricular septal defect, Atrial septal defect, Mitral valve insufficiency • Chest X-ray: see Ventricular septal defect, Atrial septal defect partitions, Mitral valve insufficiency • Catheterization of the cardiac cavities: see Ventricular septal defect, Atrial septal defect, Mitral valve insufficiency • Left and right atriography and ventriculography, coronary angiography: see Ventricular septal defect, Atrial septal defect, Mitral valve insufficiency.
Drug treatment: prevention of infective endocarditis and treatment of heart failure.
Surgical treatment • Indications •• At an early age - variants of the defect refractory to conservative treatment, accompanied by clinical symptoms •• General open VVC •• At preschool age - open VVC, regardless of the presence of clinical symptoms (due to the high risk of infective endocarditis and the high frequency progression of heart failure at school age) • Contraindications: see Atrial septal defect • Methods of surgical treatment •• In case of incompletely open VVC - mitral valve plastic, ASD plastic with a patch of autopericardium or synthetic material •• If emergency correction of a general open VVC is necessary in children with body weight less than 3 kg, concomitant heart defects and with little clinical experience in radical correction of the defect at an early age - narrowing of the pulmonary trunk •• In other cases - simultaneous plastic surgery of VSD, ASD, mitral and tricuspid valves •• For intermediate forms of open AVK - plastic surgery VSD, if necessary, tricuspid valve annuloplasty.
Specific postoperative complications • Inadequate correction of the defect (most often mitral regurgitation) • AV block • Infective endocarditis.
Prognosis • Severe forms manifest themselves in the first months of life, with mild forms the general condition is not affected • Average life expectancy - 15 years • With incompletely open VKA, average life expectancy of non-operated patients - 20 years • Surgical mortality - less than 6.7% • In general open VVC and the natural course of the defect, 95% of patients die by the age of 5 • Postoperative mortality - up to 32% • Patients with intermediate forms of open VVC have a relatively favorable prognosis, but all require surgical treatment.
Synonyms: Defects of endocardial ridges; Atrioventricular communication; Persistent common atrioventricular orifice.
Abbreviations. AVK - atrioventricular canal.
ICD-10. Q21.2 Atrioventricular septal defect
Pediatrician tactics for critical congenital heart defects in newborns
In the structure of infant mortality, developmental anomalies occupy third place, and half of the mortality cases are determined by congenital heart defects (CHD). Among children who died from congenital heart disease and malformations of large vessels, 91% of patients were infants of the first year of life, of which 35% of deaths occurred in the early neonatal period (up to 6 days). About 70% of children die during the first month of life [3, 4].
The scale of the problem is emphasized by the high frequency of congenital heart disease: in different countries this figure varies from 0.6% to 2.4% per year in children born alive; taking into account intrauterine fetal death and early miscarriages, the overall frequency of congenital heart disease is 7.3% [1, 3].
Prenatal diagnosis. In order to reduce infant mortality, prenatal ultrasound screening is used, which makes it possible to detect most congenital heart diseases before the 24th week of gestation. If a defect is suspected, a targeted ultrasound of the fetus is performed using an expert-class device. The main goal is to prevent the birth of children with inoperable defects - hypoplastic left heart syndrome (HLHS), hypertrophic cardiomyopathy with signs of organic myocardial damage, multiple fetal malformations. The prenatal consultation should offer termination of pregnancy only if an incurable defect is accurately diagnosed [2, 4].
Classification. In the neonatal period (sometimes in the first days, hours or minutes after birth), defects called critical manifest themselves, since in 95–100% of cases they are accompanied by life-threatening conditions and determine early neonatal mortality. The group of critical defects includes transposition of the great vessels (TMS), TGV, atresia of the tricuspid valve or pulmonary artery with an intact interventricular septum (IVS), preductal coarctation of the aorta, common truncus arteriosus, single ventricle, double origin of the great vessels from the right ventricle and others [1 , 5].
Considering the high mortality rate of newborns and infants from congenital heart disease, a classification has been created for this age group of patients based on the definition of the leading clinical syndrome, the effectiveness of therapeutic tactics and determining the timing of surgical intervention [3, 4].
Syndromic classification of congenital heart disease in newborns and children of the first year of life (Sharykin A. S., 2005)
- CHD manifested by arterial hypoxemia (chronic hypoxemia, hypoxemic status) are “ductus-dependent” defects.
- CHD, predominantly manifested by heart failure (acute heart failure, congestive heart failure, cardiogenic shock).
- CHD manifested by heart rhythm disturbances (complete atrioventricular block, paroxysmal tachycardia).
These conditions can be combined, aggravating the severity of the condition of children; 50% of these children require surgical or therapeutic intervention in the first year of life.
Hemodynamics. Critical defects are characterized by ductus-dependent pulmonary or systemic circulation; they are united by a sudden sharp deterioration of an apparently healthy child at birth, associated with a decrease in blood flow through the ductus arteriosus. Ductus-dependent pulmonary circulation in TMS, atresia (or critical pulmonary artery stenosis) with an intact IVS provides blood flow through the duct into the pulmonary circulation, and when it is limited or stopped, severe arterial hypoxemia and acute hypoxia of organs and tissues develop.
Clinic of congenital heart disease with pulmonary ductus-dependent circulation
The anatomy of one of the most common critical defects - transposition of the great vessels - consists in the incorrect origin of the aorta - from the right and pulmonary arteries - from the left ventricle, which contributes to the separation of the circulatory circles: arterial blood circulates in the pulmonary circulation system, and venous blood circulates in the systemic circulation system.
The supply of oxygen to life-supporting organs is possible only if there are functioning fetal communications - ductus arteriosus, interatrial defect. This communication between the circulation does not provide compensation for hypoxemia. In order to compensate for the deficiency of peripheral circulation, the minute volume of blood flow increases, overload of the small circle occurs (this happens faster in the presence of a defect in the IVS), and pulmonary hypertension quickly develops. That is why, during the management of the patient, constant monitoring of symptoms of arterial hypoxemia and monitoring of clinical signs of heart failure (HF) is necessary -
The natural course of the vice is very severe. The child is born at term with normal body weight, but in the first hours after birth diffuse cyanosis of the skin appears, especially pronounced in the periphery - cyanosis of the face, hands, and feet. The condition of extreme severity is caused by severe arterial hypoxemia. Dyspnea and tachycardia appear 1–2 hours after clamping the umbilical cord. There is a progressive deterioration of the condition. The child is lethargic, lethargic, and easily cools down.
When fetal communications are closed, acute hypoxia leads to the development of multiple organ failure and death of the newborn within a few hours. As the child survives for several weeks, heart failure progresses. Severe malnutrition quickly develops. It should be noted that in the case of adequate observation and treatment tactics, as well as timely - up to a month - surgical correction of the defect in a child (since only during this period is radical correction possible by the method of arterial switching of the great vessels), physiological hemodynamics, growth and development rates are completely restored, physical and subsequently social adaptation. If the defect is corrected later, the outcomes are less favorable.
Diagnostic criteria for TMS include:
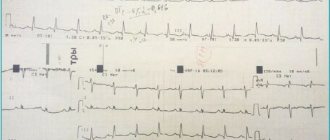
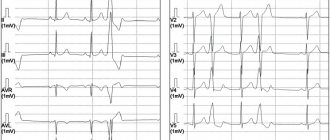
- Electrocardiographic signs of hypertrophy of the right atrium and right ventricle are a high P wave in the “right” leads - III, V1-3, deep S waves in the “left” - I, V5-6 and high R waves in leads III, V1-3.
- X-ray reveals cardiomegaly and an “ovoid” shape of the heart with a narrow vascular bundle as a result of the alignment of the contours of large vessels (photo).
- According to echocardiography, there is a parallel course of the outflow sections of the ventricles - the pulmonary artery and the aorta.
- The hyperoxide test is negative - when trying to supply 100% oxygen through a mask in patients with “blue” defects, after 10–15 minutes pO2 increases by no more than 10–15 mmHg. Art. (whereas in lung diseases the increase in pO2 is up to 100–150 mm Hg).
Scheme for examining a newborn child with suspected congenital heart disease:
- examination of the patient (with assessment of symptoms of hypoxemia and/or heart failure);
- assessment of pulsation in all extremities;
- auscultation of the heart and lungs (dynamic control of heart rate, breathing);
- measurement of blood pressure (BP) in all extremities (further dynamic control).
In addition, monitoring a child involves monitoring blood gases (pO2, pCO2), oxygen saturation (SatO2) using pulse oximetry and metabolic indicators - pH, BE. Gas exchange in the lungs is not impaired if PaO2 is in the range of 60–80 mmHg. Art., SaO2 - 96–98%. Arterial hypoxemia develops when PaO2 is less than 60 mm Hg. Art. and a hemoglobin saturation level of 85–75%.
Tasks of a pediatrician (neonatologist):
- ensure a reduction in the body's oxygen needs by creating thermal and physical comfort - incubator conditions, with an elevated position of the upper body;
- swaddling with the chest and arms free;
- limiting energy costs for physiological load (tube feeding);
- support of blood flow through the ductus arteriosus (infusion of fluids, prostaglandin E);
- correction of metabolic changes, if necessary, artificial ventilation of the lungs (ALV) without adding oxygen to the inhaled mixture, in a mode that excludes hyperventilation and with simultaneous infusion of the drug prostaglandin E (calculation of the drug dose is described below). When deciding whether to prescribe mechanical ventilation, it is necessary to take into account that oxygen has a vasoconstrictor effect on the ductus arteriosus, which makes oxygen therapy dangerous in this group of patients;
- if there is a threat of closure of ductus-dependent defects, the volume of infusions and feeding is increased to 110–120% of normal needs against the background of constant assessment of diuresis. It has been established that a 5% increase in body weight in a newborn in 1–2 days stabilizes the function of the ductus arteriosus.
Transportation to a cardiac surgery center is optimal during the first weeks and first month of life. It is first necessary to inform the cardiac surgery hospital about a patient with congenital heart disease with ductus-dependent circulation. The observation period until transfer and transportation to the center is carried out against the background of infusion of the drug prostaglandin E (Alprostan, Vazaprostan).
Clinical picture of congenital heart disease with systemic ductus-dependent circulation (the group of defects includes HFRS, severe coarctation of the aorta, interruption of the aortic arch). The most positive example of defects in this group is pronounced preductal coarctation, which occupies from 1% to 10% of critical congenital defects. With this defect, blood flow from the proximal part (below the origin of the ductus arteriosus) to the distal part is sharply limited or completely absent. The hemodynamic disorder, accordingly, lies in the fact that a small volume of blood enters the descending aorta (into the greater circle) only from the pulmonary artery through the ductus arteriosus. When the ductus arteriosus closes, hypoperfusion of organs and tissues and multiple organ failure acutely develop. Clinic: a full-term newborn with a sharp deterioration in the first few days of life - adynamia, cold extremities, a symptom of hypoperfusion of peripheral tissues (“white spot”), low filling pulse, high blood pressure in the arms and low or not determined in the legs, shortness of breath, tachycardia, oliguria with increasing azotemia, hepatomegaly with increased transaminases, necrotizing enterocolitis.
Let us consider the diagnosis and optimal therapeutic tactics for a patient with severe coarctation of the aorta using a specific clinical example.
Full-term newborn A. was delivered to the intensive care unit in serious condition: lethargic, does not suckle, pale skin, tachypnea 120 breaths per minute, breathing is symmetrical in all fields, no wheezing. Heart sounds are sonorous, 167 per minute, gentle systolic murmur in the third intercostal space to the left of the sternum, hepatomegaly (liver +5 cm from under the edge of the rib, dense). Diuresis is reduced, there is no peripheral edema. Blood pressure in the arms - 127/75 mm Hg. Art., pulsation in the femoral artery is not detected. SatO2 - 98%.
From the anamnesis: the condition suddenly worsened on the 14th day of life, when the child became lethargic, severe shortness of breath appeared, and was hospitalized by ambulance. A boy from a second, normal pregnancy, term birth with a weight of 3220 g, an Apgar score of 5 (9) points. He was discharged from the maternity hospital in satisfactory condition and was breastfed. Periodically there were episodes of anxiety and flatulence.
Upon admission, the child is intubated and undergoes mechanical ventilation with a low oxygen content in the inhaled mixture. An examination in the hospital revealed cardiomegaly (cardiothoracic index - 80%), depleted pulmonary pattern, and, according to electrocardiography, combined overload of both ventricles. Echocardiography revealed hypoplasia of the aorta below the origin of the left subclavian artery (and above the localization of the ductus arteriosus), in a place typical for the ductus arteriosus - punctate blood flow (closing ductus arteriosus). After 6 hours, the child’s condition worsened: oliguria developed, creatinine increased to 213 mmol/l, and transaminases increased by 4–5 times the laboratory norm. Humoral activity has not been established.
Rationale for diagnosis and tactics: given the clinical picture of severe respiratory and subsequently multiple organ failure in combination with cardiomegaly, systemic arterial hypertension, renal and liver failure, according to clinical data, coarctation of the aorta should have been suspected. A sudden deterioration in the child’s condition in the absence of signs of infection allows one to think about ductus-dependent systemic circulation; taking into account the imaging data of the heart and blood vessels, the diagnosis is made: “CHD, preductal coarctation of the aorta, arterial hypertension stage 2, secondary, multiple organ failure.”
From the moment the diagnosis is confirmed by echocardiography, it is necessary to begin therapy with Vazaprostan 0.02 (with an increase in dose to 0.05 mcg/kg/min) in order to restore blood flow through the ductus arteriosus. With this defect, the shunt is directed from the pulmonary artery to the descending aorta, and only this small portion of blood provides the entire systemic circulation.
Dose calculation and administration method. The child’s weight upon admission is 3220 g. 1 ampoule contains 20 mcg of Vazaprostan. In this case, drug administration began with a dose of 0.02 mcg/kg/min, that is, 0.02 ´ 3.2 = 0.064 mcg/kg/min was required. Over an hour, the dose of the drug was 0.064 ´ 60 = 3.8 mcg/hour. To administer the drug, 1 ampoule (20 mcg of Vazaprostan) was diluted in 20 ml of physiological solution (1 mcg in 1 ml). If there was no effect within two hours, the dose was increased to 0.04–0.05 mcg/kg/min: accordingly, the rate of drug administration increased to 7.6–9.5 ml/hour. In this case, therapy control was very indicative - after 6 hours of infusion, an improvement in the condition was noted, an increase in the blowing systolic murmur in the second intercostal space on the left, and an increase in the size of the flow through the ductus arteriosus according to echocardiography. Transferred to a maintenance dose of Vazaprostan - 0.01 and then 0.005 mcg/kg/min, which was maintained throughout the entire period of observation in the pediatric hospital and during transportation during transfer to the cardiac surgery hospital. In this clinical case, it was impossible to completely withdraw from mechanical ventilation (given the severity of respiratory failure); on the 5th day of hospitalization (19th day of life), the child was transferred to the clinic named after him during therapy. Meshalkin, where the defect was successfully corrected - the formation of an aortopulmonary shunt.
In cases of the development of heart failure in newborns with defects accompanied by massive discharge of blood into the pulmonary circulation, the same principle of monitoring key indicators and symptomatic therapy is applied:
- limiting fluid administration according to diuresis, in severe cases - up to 1/3 of the age norm (but limiting fluid to 50% of the daily physiological requirement is unacceptable);
- the presence of volume overload requires the use of diuretics (for edematous syndrome, preference is given to Furosemide/Lasix at a dose of 1–2 mg/kg; a combination with Veroshpiron is possible (1–3 mg/kg/day orally in 2–3 doses);
- Digoxin () is used to stop tachycardia (an economically unprofitable regimen for the myocardium and an ineffective volume for the peripheral circulation).
If symptoms of pulmonary circle overload appear (on auscultation - increase, splitting of the 2nd tone in the pulmonary artery, on EchoCG - increase in pressure in the pulmonary artery by more than 30 mm Hg after six days of life), as well as signs of impaired diastolic function of the heart, it is recommended to use drugs from the group of angiotensin-converting enzyme inhibitors (ACEIs). Capoten (captopril) is used at a dose of 0.5–1 mg/kg, subject to control of systemic blood pressure. The therapeutic effect of ACE inhibitors is associated with a decrease in peripheral vascular resistance and partial deposition of blood, resulting in a decrease in the volume of blood returned to the right side of the heart. Accordingly, the volume of the shunt and the load on the left chambers and vessels of the small circle are reduced. In addition, it is known that ACE inhibitors are an inhibitor of apoptosis stimulated by hypoxia, which explains the angio- and cardioprotective effect of the drugs.
Thus, pediatric tactics, including early diagnosis of critical congenital heart disease and therapy that controls intracardiac, central and peripheral hemodynamics, as well as, if possible, early coordination of actions with the cardiac surgery center can significantly improve the prognosis of patients and reduce infant mortality rates.
Literature
- Burakovsky V. A., Bukharin V. A., Podzolkov V. P. et al. Congenital heart defects. In the book. Cardiovascular surgery. Ed. V. I. Burakovsky, L. A. Bockeria. M.: Medicine, 1989; 345–382.
- Congenital heart defects. Directory for doctors. Ed. E. V. Krivosheeva, I. A. Kovaleva. Tomsk, 2009; 285.
- Sharykin A. S. Congenital heart defects. Guide for pediatricians, cardiologists, neonatologists. M.: Teremok Publishing House, 2005; 384.
- Sharykin A. S. Perinatal cardiology. Guide for pediatricians, cardiologists, neonatologists. M.: Teremok Publishing House, 2007; 347.
- Johnson Jr. WH, Moller JH Pediatric cardiology. Core handbooks in pediatrics. LIPPUNCOTT WILLIAMS & WILKINS, 2001; 326.
E. Yu. Emelyanchik *, Doctor of Medical Sciences, Professor D. B. Drobot *, Doctor of Medical Sciences, Professor E. P. Kirillova *, Candidate of Medical Sciences, Associate Professor V. A. Sakovich *, Doctor of Medical Sciences, Professor E. V. Basalova ** A. Yu. Cheremisina *
*GOU HPE Krasnoyarsk State Medical University named after. Professor V. F. Voino-Yasenetsky, ** Regional Clinical Children's Hospital, Krasnoyarsk
Contact information for authors for correspondence