The cause of fibrosis and how to diagnose it
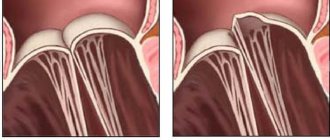
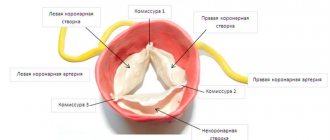
An important role in the functioning of the valvular apparatus of the heart is played by the valves, which are represented by loose connective tissue consisting of dense collagen and continuously extending into the chordae tendineae (according to Wikipedia).
Under a number of circumstances, the number of blood vessels supplying the structure of the valves is reduced. As a result, elastic fibers are replaced by dense fibrous tissue, which is characterized by sufficient strength. Having lost lability, the valves lose the ability to provide physiological hemodynamics. Most often, the mitral valve is affected by pathology, less often – the aortic valve. Classification of pathology:
- focal. There is moderate damage to the structure of the valve apparatus;
- diffuse. The affected area covers the leaflets and subvalvular space;
- cystic. It is characteristic of an advanced stage and is considered a separate pathology with the formation of cavity formations.
Fibrosis of the aortic and mitral valve leaflets is explained by the following reasons:
- age-related changes with loss of natural collagen potential;
- rheumatic attack (especially repeated), for example, after oropharyngeal infections. The damage is attributed to antibody formation and cross-reactivity between group A streptococcal carbohydrates and heart valve glycoprotein. According to research, almost every five-year-old child has a history of a throat infection. It is possible to develop chronic rheumatic heart disease with damage in the form of post-inflammatory marginal fibrosis;
- Marfan syndrome, dysplasia, in which the anatomical and functional features of the connective tissue are genetically abnormal;
- atherosclerosis of the aorta. Calcification of plaques and subsequent thickening of its wall;
- foci of necrosis (with a heart attack) or inflammation (with myocarditis) near the valve ring.
Diagnosing fibrosis is not very difficult. Initially, clinical blood and urine tests are prescribed to detect the possible presence of inflammation. Biochemical research indicates changes in the levels of cholesterol, sugar, uric acid, total protein, and creatinine.
Ultrasound of the heart reveals the degree of narrowing, valve insufficiency, evaluates the contractile function of the myocardium, blood volume during systole.
X-ray examination reveals myocardial hypertrophy, pulmonary congestion, and calcification of valve fragments.
CT, MRI, CAG are performed if surgical intervention for prosthetic structures is necessary.
Common diagnostic methods and methods
Thanks to modern medicine, the diagnosis of the described disease is carried out with high accuracy and does not take much time. In addition, such an examination is absolutely easy.
During a visual examination, a qualified specialist will undoubtedly pay attention to the pale tint of the epidermis, the cyanosis of the patient’s lips and legs.
Next, more precise methods are used (by the way, the thickness of the dampers over 6 mm is considered a significant deviation). In order to identify compaction of the anterior (posterior) valve of the mitral valve, the attending cardiologist usually prescribes the following procedures:
- Echocardiography (EchoCG). Provides information about the extent of valve damage and the stage of the pathology.
- Electrocardiogram (ECG). Indicates hypertrophy of the cardiac zones.
- Obtaining an x-ray of the chest area. It will help determine the presence of congestion in the lungs.
- Auscultation. Detects heart murmurs.
- MRI. Allows you to detect the slightest neoplasms in the valve apparatus of a characteristic organ.
At the discretion of the doctor, the patient may additionally be required to donate blood (urine) for clinical or biochemical analysis.
How does this process affect the patient’s quality of life?
The symptoms of the disease depend on which specific valve is affected by fibrosis, although some signs of pathologies may coincide. Most often, the patient does not show any complaints for a long time, his condition remains satisfactory. Deterioration in well-being is typical for an advanced process and the formation of complications.
If the mitral valve leaflets are sealed, it is noted:
fatigue during routine physical activity and sports;- shortness of breath at rest;
- episodes of heart rhythm disturbances in the form of extrasystole or atrial fibrillation;
- chest pain;
- periodic swelling.
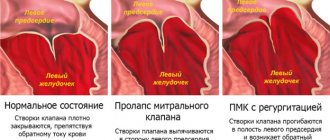
If the thickening of the mitral valve leaflets continues to progress in the absence or ineffectiveness of treatment, MVP occurs with varying degrees of regurgitation or without.
Fibrosis of the aortic valve walls causes:
- progressive shortness of breath;
- pain in the heart during exercise;
- dizziness and fainting when playing sports;
- heartbeat disturbances.
Sometimes the life of a patient with cusp fibrosis is complicated by episodes of hemoptysis and asthmatic attacks due to hemodynamic disturbances.
For young patients, issues related to pregnancy and military service come to the fore. The first is decided individually depending on the stage of the fibrotic process, the presence of stenosis and hemodynamic disturbances. A woman carrying a child is observed, in addition to an obstetrician-gynecologist, by a cardiologist. Childbirth takes place by caesarean section.
The army and the opportunity to engage in professional sports depend on the decision of the medical commission. It is taken into account whether the pathology led to a pronounced defect and the presence of concomitant diseases.
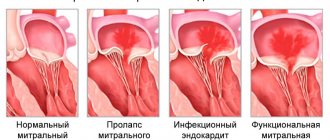
Mitral valve prolapse: clinical variants, modern concepts
Despite more than half a century of history of studying mitral valve prolapse (MVP), a number of issues related to diagnosis, determination of prognosis, and tactics for managing patients with this pathology continue to cause difficulties for practitioners. This is largely due to the morphological and clinical heterogeneity of this condition, suggesting the existence of different variants of mitral prolapse.
MVP is a syndrome inherent in various nosological forms and each time requiring nosological verification. The list of diseases manifested by MVP is extensive, which is explained by the complexity of the structure of the heart valve apparatus and the variety of mechanisms of mitral prolapse. MVP may be based on changes in the fibrous ring, mitral valve leaflets and chordae attached to them, dysfunction of the papillary muscles, and impaired contractility of the left ventricular myocardium.
In clinical practice, MVP can appear in five forms:
- primary MVP;
- secondary MVP as a consequence of myocardial diseases;
- secondary MVP as a manifestation of hereditary monogenic syndromes;
- mitral prolapse as a variant of the norm or a manifestation of a minor anomaly of cardiac development;
- MVP as an “echocardiographic disease”.
MVP as an “echocardiographic disease”
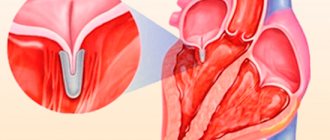
MVP as an “echocardiographic disease” is a situation of erroneous diagnosis of mitral prolapse. At the dawn of the introduction of two-dimensional echocardiography into clinical practice, MVP was diagnosed in 5–15% and even 35% of people examined. Such significant overdiagnosis was associated with a misconception about the flat configuration of the mitral valve. Ultrasound series from the late 1980s. demonstrated the three-dimensional (saddle-shaped) shape of the mitral valve ring and made assessment from a parasternal longitudinal position mandatory. The modern definition of MVP interprets it as systolic bulging of one or both mitral valve leaflets at least 2 mm above the plane of the mitral annulus with mandatory registration along the long axis of the heart. As can be seen, the definition clearly specifies both the anatomical criteria and the technical aspects of the research procedure.
The transition to unified criteria for ultrasound diagnostics and the publication of the results of the Framingham study made it possible to eliminate contradictions in views on the prevalence of MVP, which turned out to be significantly lower than previously thought. Of the 3491 participants in the Framingham Study who underwent 2D echocardiography using consensus diagnostic criteria, 47 (1.3%) had classic (with mitral leaflet thickening) and 37 (1.1%) nonclassical MVP, with an overall incidence of 2.4% [ 1]. Similar results were obtained based on the Russian population.
Secondary MVP in myocardial diseases
Among other reasons, MVP can develop against the background of coronary pathology, rheumatism, cardiomyopathy, myocarditis, myocardial dystrophy - conditions that cause impaired contractility of the left ventricular myocardium of a local or diffuse nature, dysfunction or separation of the papillary muscles. Such variants of mitral prolapse are considered secondary, since they are part of the structure of the clinical picture of the corresponding disease. The presence of secondary MVP in the absence of significant mitral regurgitation has little effect on the symptoms of the underlying disease. The exception is cases of acute mitral regurgitation that develops as a result of avulsion of the papillary muscle during myocardial infarction or blunt cardiac trauma.
Primary MVP
Primary MVP is the only variant of mitral prolapse that claims to be nosologically independent. The origin of primary MVP is associated with the pathology of the mitral valve leaflets, caused by a specific cause - mesenchymal inferiority as part of hereditary disorders (dysplasias) of connective tissue (HCT). NNCT is a group of genetically heterogeneous and clinically polymorphic pathological conditions, united by a violation of the formation of connective tissue in the embryonic and postnatal periods. The genetic heterogeneity of NNCT implies both a monogenic and multifactorial nature of the disease, and clinical polymorphism is associated with the ubiquity of connective tissue in the body and the variety of manifestations of congenital “weakness” of its individual components.
In accordance with modern concepts, two categories of NNCT are distinguished: classified (having agreed upon diagnostic recommendations) and unclassified (also known as dysplastic phenotypes). Consensus diagnostic recommendations include monogenic syndromes caused by mutations in the genes of extracellular matrix proteins, growth factor receptors, and matrix metalloproteinases. The most famous and clinically significant of them are Marfan and Ehlers-Danlos syndromes, MASS phenotype, joint hypermobility syndrome and others. The classified syndromes also include primary MVP. To date, three gene loci responsible for its occurrence have been found, located on the 11th, 13th and 16th chromosomes. The search for genes involved in the development of mitral prolapse continues; it is assumed that their identification will create the prerequisites for screening asymptomatic patients at risk of developing mitral regurgitation. However, despite all the attractiveness of molecular genetic research methods, one cannot help but note their inaccessibility in everyday practice. It is no coincidence that existing recommendations for NNCT give priority in the diagnosis of primary MVP to a combination of clinical and ultrasound data.
The morphological reflection of congenital “weakness” of connective tissue and a marker of primary MVP is the so-called myxomatous degeneration of the mitral valve leaflets. It is characterized by proliferation of the middle - spongy layer of the valve with excessive accumulation of glycosaminoglycans and disorganization of collagen fibrils (Fig. 1). In this case, the leaflet thickens, changes its mechanical properties, loses its ability to withstand pressure in the cavity of the left ventricle during systole and prolapses.
The clinical picture of primary MVP is varied; it can be either asymptomatic or clinically manifest. Symptoms of primary MVP are represented by a combination of the following syndromes:
- disturbances of intracardiac and general hemodynamics;
- manifestations of vegetative-vascular dystonia;
- other (extravalvular) manifestations of “weakness” of connective tissue.
It is this triad that determines the clinical uniqueness of primary MVP, distinguishing it from other variants of mitral prolapse. The degree of expression of each of these components may be different, which determines the significant clinical polymorphism inherent in primary MVP.
The main method and “gold standard” for diagnosing MVP is two-dimensional echocardiography. It allows you to establish the fact of mitral prolapse, assess the thickness of the mitral leaflets, and determine the degree of mitral regurgitation. These characteristics are extremely important in understanding the severity of the patient’s condition, prognosis and management tactics. Thus, an increase in the thickness of the valve by more than 5 mm (with a norm of 2–4 mm) is reliable evidence of its myxomatous degeneration, which is a morphological substrate and the main marker of primary MVP. Depending on the thickness of the sash, classic (with a sash thickness of 5 mm or more) and non-classical (less than 5 mm) PMC are distinguished. Since the outcomes of MVP are determined by the degree of disturbance of intracardiac hemodynamics, an equally important parameter is the severity of mitral regurgitation, assessed during a Doppler ultrasound study.
Autonomic dysfunction is recognized as the leading mechanism explaining the diverse symptoms of primary MVP, but the presence of asymptomatic patients does not allow us to unambiguously determine its pathogenetic role: whether it is the cause of MVP or a random combination. Nevertheless, most researchers consider changes in autonomic homeostasis to be an obligatory attribute of MVP. It is autonomic dysfunction that explains such common manifestations of “MVP syndrome” as cardialgia, most cardiac arrhythmias, instability of blood pressure, lipothymia, hyperventilation and asthenic syndromes. To explain the prevalence of autonomic dysfunction in MVP, many hypotheses have been proposed, including congenital changes in the perinervia, a systemic defect in biological membranes, perinatal damage to the hypothalamic structures, and finally, the recently actively discussed version of the pathogenetic role of magnesium deficiency.
But no matter how vivid the manifestations of autonomic dysfunction are, the basis of hemodynamic disturbances in MVP is still mitral regurgitation. Its inevitable consequence is volume overload and dilatation of the left heart, which leads to atrial fibrillation and progression of heart failure. Complications also include thromboembolism from myxomatically altered valves and the possibility of developing secondary infective endocarditis.
The cause of adverse consequences and complications of primary MVP is not only the valve mechanism. As has recently been shown, disturbances in general hemodynamics in this pathology occur not only through mitral regurgitation, but also through defects in the structure and function of the extracellular matrix of the myocardium, which are a consequence of the congenital “weakness” of connective tissue. These defects can cause diastolic dysfunction, decreased myocardial contractility and the development of secondary cardiomyopathy.
Recently, ideas about the severity of the consequences of MVP have been reassessed. Previously, MVP was considered a pathology with frequent and serious complications (including stroke, atrial fibrillation, heart failure) and a high need for surgical correction of mitral regurgitation. Contrary to previous knowledge, the results of the Framingham study gave reason to consider MVP as a benign condition with a low probability of adverse outcomes. In particular, the incidence of atrial fibrillation, cerebral stroke, and syncope in patients with MVP turned out to be comparable to similar outcomes in individuals with intact valves [1]. The question of risk stratification in MVP has become urgent. To date, pathological variants associated with high risk and poor prognosis have been identified. The likelihood of hemodynamic disorders increases with a high degree of mitral regurgitation and a leaflet thickness of more than 5 mm, indicating its myxomatous degeneration.
When discussing possible complications and outcomes of mitral prolapse, it is necessary to emphasize that primary MVP has a progressive course. Although it is a congenital pathology, it does not occur in newborns, is characterized by low incidence rates among children and young people, and affects mainly mature patients. The time interval preceding the clinical manifestation of MVP is the period during which an increase in myxomatous changes in the valve leaflet occurs up to its deformation to a degree that impairs the closure function and leads to mitral regurgitation. The appearance of the latter radically changes the well-being and fate of patients, which is clearly reflected in Fig. 2, which correlates the staged course of MVP with the likelihood of complications. Moreover, if thromboembolism, infective endocarditis, and “functional” arrhythmias are recorded with approximately the same frequency in different age periods, then the frequency of congestive heart failure and atrial fibrillation soars after 50 years.
The role of primary MVP in circulatory decompensation and the occurrence of atrial fibrillation in adulthood and old age is often underestimated. The manifestation of valvular pathology in elderly patients often becomes the cause of diagnostic errors. Practitioners are poorly aware of NNCT and, according to the prevailing stereotype, attribute circulatory failure and heart rhythm disturbances in people in the second half of life almost exclusively to coronary pathology. Often they are not embarrassed by either the absence of angina or scarring ECG changes, or the presence of a rough systolic murmur, reflecting the presence of mitral regurgitation.
A separate component of the clinical polymorphism of primary MVP are external and visceral markers of “weakness” of connective tissue. Since the connective tissue defect underlying the increased compliance of the valve leaflets is generalized, signs of mesenchymal inferiority are also determined from the skin, musculoskeletal system, and internal organs. The currently known visceral markers, as well as their most important clinical consequences and outcomes, are given in Table.
Since signs of systemic involvement of connective tissue are indirect confirmation that MVP belongs to NNCT (i.e., evidence of its “primary”), their identification is important from the point of view of diagnosis and assessment of prognosis. Thus, the likelihood of clinically and hemodynamically significant MVP is extremely low in individuals who do not have these signs. In order to unify approaches to assessing the systemic involvement of connective tissue, in particular, determining the threshold of stigmatization and structuring signs with highlighting the most informative, it is proposed to use the Ghent criteria (Ghent nosology, 2010) accepted by the world community for Marfan syndrome.
Recent times have been characterized by the emergence of new ideas about the cellular and molecular mechanisms of primary MVP. The leading role in the mechanisms of primary MVP is played by transforming growth factor β (TGF-β), a protein that activates the growth of fibroblasts and regulates the formation and degradation of the extracellular matrix. It was found that a number of manifestations of NNCT are caused by changes in the activity of TGF-β. In particular, increased expression of TGF-β was found in myxomatous valves. There is a natural desire to inhibit the increased activity of TGF-β in order to prevent the progression of myxomatosis, and such tools are already known - these are neutralizing antibodies (in experiment), angiotensin II receptor blockers and β-blockers (in experiment and clinic). The use of these agents opens up new ways to correct the consequences of impaired mesenchymal insufficiency and, perhaps, will serve as an alternative to surgical treatment.
However, the real possibilities of pathogenetic therapy for primary MVP still remain modest. Among the treatment methods relatively widely introduced into clinical practice, the use of magnesium preparations should be highlighted. The pathogenetic rationale for their use is the idea of primary MVP as a clinical form of genetically determined magnesium deficiency. It is known that under conditions of a lack of magnesium ions, the synthesis of connective tissue proteins slows down, and the activity of enzymes involved in the destruction of collagen and elastin, on the contrary, increases. In other words, under conditions of magnesium deficiency, connective tissue is destroyed faster than it is synthesized.
A number of studies have shown the fundamental possibility of eliminating the characteristic cardiac symptoms and ultrasound manifestations of MVP under the influence of magnesium preparations. One of the most famous of them belongs to a team of domestic authors under the leadership of Academician A.I. Martynov. A 6-month course of therapy with Magnerot at a dose of 3 g per day led to a decrease in the depth of prolapse and the degree of myxomatous degeneration of the valve leaflets. Along with this, a reduction in the clinical symptoms inherent in this category of patients was achieved [5]. Over the past time, many studies of similar design have been carried out, in which similar results were obtained.
Along with magnesium preparations, vitamins, other micro- and macroelements, and anabolics—drugs related to the metabolism of connective tissue and capable of influencing the biochemical mechanisms underlying myxomatous degeneration of the valve and its prolapse—may also be candidates for the role of means of pathogenetic treatment of primary MVP. All of them, in one or another combination, find use in the complex therapy of primary MVP.
When discussing the treatment of MVP, it is necessary to clearly understand that hemodynamically significant (accompanied by signs of heart failure) prolapse is a heart defect that requires surgical correction. Such patients should be promptly referred to a cardiac surgeon to resolve the issue of mitral valve replacement or plastic surgery. Commenting on the indications for surgical treatment of mitral regurgitation, we note that the current AHA/ACC2014 recommendations do not confirm the advisability of surgical treatment only for patients with preserved ejection fraction and left ventricular size.
Secondary MVP in syndromic NNCT
MVP can occur with monogenic connective tissue defects, such as Marfan, Loeys–Dietz, Ehlers–Danlos syndromes, osteogenesis imperfecta, and pseudoxanthoma elasticum. It accounts for 0.25–2% of cases of mitral prolapse. Among all monogenic syndromes, MVP is most often observed in Marfan syndrome - 75% of cases (and more severe variants with valvular myxomatosis - 28%) [6]. The prevalence of MVP in patients with Ehlers–Danlos syndrome is significantly lower—6% [7]. PMC in monogenic NNCT is classified as secondary, since it is part of the structure of the clinical and morphological manifestations of the corresponding pathology. However, it is characterized by the same myxomatous changes in the valves as primary MVP, and in this way it is similar to it.
MVP as a variant of the norm or a manifestation of a minor anomaly of cardiac development
The borderline degree of prolapse, the absence of myxomatous thickening of the leaflets, significant mitral regurgitation and family history allow us to consider MVP as a normal variant, a transient age-dependent phenomenon or a minor cardiac anomaly. It is these cases of mitral prolapse that most often occur in clinical practice, especially in adolescents, slender boys, girls and young women, creating the impression that MVP is extremely common in the population.
Among the causes of such “innocent” cases of MVP are congenital microanomalies of the architecture of the mitral complex, asynergy of contraction and relaxation of the myocardium, disruption of valvular innervation, etc. Separately, it should be noted cases of shallow, asymptomatic prolapse that occurs with age, arising in the puberty period due to the uneven development of individual elements of the valve mitral complex and their incomplete functional correspondence to each other. In this case, the area of the chordal-valve apparatus turns out to be excessive, as if stored for future use. At the end of puberty, as the volume and mass of the left ventricular myocardium increases, this discrepancy is often leveled out (and in women to a lesser extent than in men, which obviously explains the predominance of women among patients with MVP).
It is necessary to be aware that in the absence of data from a molecular genetic study, it is not possible to reliably exclude the preclinical stage of primary MVP in all these cases, which justifies recommendations for dynamic monitoring of such patients.
Half a century has passed since the first description of MVP by J. V. Barlow, who established the connection between late systolic murmur and mitral regurgitation. What results have we reached during this time, what conclusions can we draw?
- MVP is a syndrome inherent in different nosological forms and in each case requires the establishment of a nosological diagnosis.
- Clear diagnostic criteria for MVP have been established that should be followed.
- The ambiguity of the prognosis for MVP dictates the need for stratification of risk factors.
- Despite the capabilities of molecular genetic research methods, the diagnosis of PMC continues to be a synthesis of clinical and ultrasound manifestations with an assessment of signs of systemic involvement of connective tissue.
- Advances in understanding the mechanisms of development of myxomatosis open up new prospects for the treatment of primary MVP.
In conclusion, let us recall that both the All-Russian Scientific Society of Therapists and the Russian Society of Cardiology have issued a series of recommendations devoted to the diagnosis and treatment of NNST and MVP. By referring to these documents, a practitioner can clarify details that were not sufficiently covered in this article.
Literature
- Freed LA, Levy D, Levine RA et al. Prevalence and clinical outcome of mitral-valve prolapse // N. Engl. J. Med. 1999; 341 (1): p. 1–7.
- Sainger R., Grau J.B., Branchetti E. et al. Human myxomatous mitral valve prolapse: role of bone protein 4 in valvular interstitial cell activation // J. Cell. Physiol. 2012; 227 (6): p. 2595–2604.
- Boudoulas KD, Boudoulas H. Floppy mitral valve (FMV)/mitral valve prolapse (MVP) and the FMV/MVP syndrome: pathophysiologic mechanisms and pathogenesis of symptoms // Cardiology. 2013; 126 (2): p. 69–80.
- Delling FN, Vasan RS Epidemiology and pathophysiology of mitral valve prolapse: new insights into disease progression, genetics and molecular basis // Circulation. 2014; 129 (21): p. 2158–2170.
- Martynov A.I., Akatova E.V., Nikolin O.P. Results of long-term therapy with magnesium orotate in patients with mitral valve prolapse // Cardiovascular Therapy and Prevention. 2012; 11 (3): p. 30–35.
- Taub CC, Stoler JM, Perez-Sanz T. et al. Mitral valve prolapse in Marfan syndrome: an old topic revisited // Echocardiography. 2009; 26 (4): p. 357–364.
- Dolan AL, Mishra MB, Chambers JB, Grahame R. Clinical and echocardiographic survey of the Ehlers-Danlos syndrome // Br. J. Rheumatol. 1997; 36 (4): p. 459–462.
A. V. Klemenov, Doctor of Medical Sciences
GBUZ NO City Clinical Hospital No. 30, Nizhny Novgorod
Contact Information
DOI: 10.26295/OS.2019.64.93.014
Mitral valve prolapse: clinical options, modern concepts / A. V. Klemenov For citation: Attending physician No. 9/2019; Page numbers in the issue: 65-69 Tags: heart, mitral prolapse, congenital mesenchymal inferiority
Treatment and observation of a patient with fibrosis of the valve apparatus
Often on forums you can read the question of whether fibrosis can be treated using folk remedies. The answer is clear: there are no such recipes. This process is quite difficult in therapy even for modern medicine.
It is important to know that the prescription of medications is indicated only for the clinical picture of heart failure, in which the following are used:
- cardiac glycosides - Celanide, Digoxin, Strophanthin;
- diuretics - Trifas, Indap, Veroshpiron;
- if indicated, antihypertensive and antiarrhythmic drugs.
Medicines only minimize the symptoms caused by fibrosis without affecting the progression of the disease.
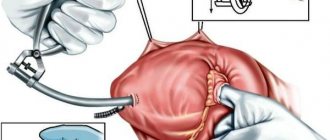
Radical treatment consists of the following methods:
valve prosthetics to replace the diseased structure with a mechanical or biological analogue. As a rule, a median sternotomy using a heart-lung machine is used;- mitral commissurotomy, closed or open, with the task of dissecting pathological connections between the valve leaflets;
- coronary artery bypass grafting;
- endovascular prosthetics. The essence of the method is to insert a catheter with an implant through the femoral vessels without general anesthesia. Indicated for patients with severe chronic diseases;
- valve transplantation (a relatively new technique).
Indications for surgical intervention in fibrosis:
- neglect of the process;
- wrinkling of valves, tendon threads;
- the presence of pronounced calcification.
After the operation, the patient should be under medical supervision of a cardiologist. The patient is indicated for annual examinations and treatment in a cardio- or cardio-rheumatological sanatorium.
Degrees of prolapse
In order to understand why mitral valve prolapse is dangerous, you need to know the severity of the disease. There are 3 of them in total.
The first is observed in 25% of patients and has no symptoms. It can be detected during an examination or a paid ultrasound of the heart.
In the second degree there are also no symptoms, the valve system remains unchanged throughout life.
Treatment and surgical intervention require the 3rd degree of prolapse.