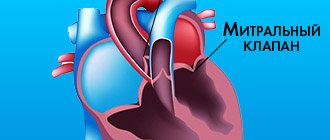
The heart valve system ensures the directed release of blood from one chamber to another, into the main vessels. The correct distribution of flow and the strength of myocardial contractions depend on the synchronous opening and closing of the valves. Through the aorta, blood enriched with oxygen and nutrients enters the general circulation.
Failure of the aortic valve leads to heart failure and is accompanied by impaired organ function.
Congenital bicuspid aortic valve (synonym - bicuspid aortic valve), according to its clinical manifestations, is not a harmless condition, but poses a risk of complications.
Inflammatory and atherosclerotic changes in the valves cause narrowing (stenosis) of the opening and varying degrees of insufficiency. The development of echocardiography has made it possible to identify pathology in childhood and determine early indications for aortic valve replacement.
Anatomical structure
The valve is located at the border of the aorta and left ventricle. Its main function is to prevent the return of blood flow to the ventricle, which during systole has already passed into the aorta.
The valve structure consists of:
- fibrous ring - a strong connective tissue formation that clearly separates the left ventricle and the initial part of the aorta;
- three semilunar valves - represent a continuation of the endocardial layer of the heart, consist of connective tissue and muscle bundles of fibers, the distribution of collagen and elastin allows them to close tightly, block the lumen of the aorta and redistribute the load on the vessel walls;
- sinuses of Valsalva - located behind the aortic sinuses, immediately behind the semilunar valves, from which the bed of the right and left coronary arteries begins.
The parts of the valve are connected to each other by adhesions (commissures)
Violation of the structure leads to a picture of a congenital defect (CHD) or acquired nature. Congenital heart disease is detected in a child during the neonatal period based on symptoms and auscultation.
The prevalence of bicuspid aortic valve (AAV) ranges from 0.5 to 2% among all congenital heart defects. Among patients with bicuspid AK, more than 80% are male [41, 68, 69]. It is generally accepted that bicuspid AV is not a variant of the development of AV, but a congenital heart defect [5, 6, 12, 13, 20, 30, 36, 40, 45, 56, 59, 62, 68, 70]. In patients with bicuspid aortic valves, 66% of cases have aortic insufficiency of varying degrees [45]. Indications or contraindications for surgical treatment of patients with bicuspid aortic valves are not clearly defined.
According to C. Ward, in terms of incidence rate, bicuspid aortic valve ranks first among all congenital heart defects [70]. According to Yu.V. Belousov [1], in 80% of cases, pathological changes in the aortic root are caused by congenital pathology of the aortic valve (bicuspid and extremely rarely monocuspid aortic valve).
In 1926, M. Abbott and W. Hamilton [3] described the relationship between bicuspid aortic aneurysm and rupture of aortic aneurysm in an adult patient. Most often, bicuspid aortic valve is combined with acquired diseases such as aneurysm of the ascending aorta, stenosis and insufficiency of the aortic valve, aortic dissection and rupture, and bacterial endocarditis [4, 12, 37, 51, 56-58]. The association between bicuspid aortic valves and aortic stenosis, regurgitation, and infective endocarditis has been known for more than 150 years, and the relationship between bicuspid aortic valves and aortic dissection and rupture has been known for more than 75 years [3, 54]. V. McKusick et al. [42] in their work supported the theory of the histopathology of the aortic wall in patients with bicuspid aortic valve and dissection of the ascending aorta, in particular linking it with medianecrosis. The term “medionecrosis” was introduced by O. Gsell [27, 31] in 1928, taking a strong position in the pathogenesis of aneurysms and dissections of the ascending aorta. Clinical studies [39] performed in recent years clearly demonstrate the relationship between bicuspid aortic valves and degenerative changes in the wall of the ascending aorta, which consist of elastin fragmentation and disruption of the orientation of smooth muscle cells. These results are consistent with reports by other authors indicating similar changes in the aortic wall during its dissection in patients with bicuspid aortic valves [26], with aneurysms of the ascending aorta [35] and with anuloaortic ectasia [21, 61]. Such histological changes are detected in bicuspid aortic valves (in 45% of patients gross changes in the aortic wall were found) much more clearly than in tricuspid aortic valves (only in 9% of patients) [39]. In the work of M. Bauer et al. [8] conducted morphometric studies of the aortic wall obtained during surgery in 107 patients with bicuspid aortic valves. It was shown that in patients with bicuspid aortic valves, the elastic lamina of the aortic media is thinner than in patients with tricuspid aortic valves, while the total thickness of the aortic media did not differ significantly in both groups. Similar data were obtained by Mauro de Sa [39], M. Nataatmadja [44].
Although the bicuspid aortic valve can function normally, it is a substrate for the occurrence of aneurysm and dissection of the ascending aorta [25, 48, 70]. According to a study conducted by R. Beroukhim et al. [9], during an echocardiographic examination of children (n=101) with bicuspid AV and 98 children with tricuspid AV, the sizes of the ascending aorta in the first group of children were larger than in the second (2.3±0.6 and 1.8±0. 5 cm, respectively; p<0.0001).
In bicuspid aortic aneurysms, aneurysms of the ascending aorta are localized along its anterior surface. In addition, diffuse dilatation of the ascending aorta is common. Dissection or rupture of the aortic wall in the presence of a bicuspid aortic valve occurs 8–10 times more often than in a tricuspid aortic valve [7, 13, 18, 22, 36, 67, 70]. According to the International Registry of Acute Aortic Dissections, the incidence of dissections with a diameter of the ascending aorta of more than 5.5 cm is statistically significantly higher in patients with bicuspid aortic valves [65]. When comparing different age groups of patients with bicuspid and tricuspid aortic valves, the group with bicuspid aortic valves showed a statistically significantly higher incidence of ascending aortic aneurysms [6, 15, 29, 67, 71]. The age group was dominated by patients under 40 years of age [15, 38]. Even with a normally functioning bicuspid aortic valve, aneurysms and aortic dissection occur in 50–69% of cases [45, 52]. According to foreign authors, the rate of increase in aortic diameter in patients with bicuspid aortic valves ranges from 0.2 to 1.9 mm per year [15, 16, 23, 38, 47]. Some studies [63] demonstrated that the expansion of the ascending aorta in patients with bicuspid aortic valves was 2.1 mm per year with an initial aortic diameter of 35-40 mm and even 5.6 mm per year with a diameter of 60 mm.
Aortic diameter is an important prognostic criterion for aortic dissection and rupture [14, 15, 19]. A study of 1,600 cases of aortic aneurysm with dissection found that for aortic diameters greater than 60 mm, the annual rupture rate was 3.6% and the annual dissection rate was 3.7%. The overall incidence of all these complications, including deaths, was 14.1%, i.e. 2 times higher than the incidence of similar complications in patients with aneurysms with a diameter of 50 mm [19]. The average diameter of the ascending aorta at dissection or rupture in patients with bicuspid aortic valves in this study was 59–60 mm (range, 3.0 to 10 cm) [14, 65]. In separate studies examining dissections of aortic aneurysms in patients under 40 years of age, in 24% of cases dissections were recorded in patients with bicuspid aortic aneurysms [26].
Despite the above, the authors [55] argue that the presence of a bicuspid aortic valve does not affect the occurrence of an aneurysm of the ascending aorta. O. Yoshihiro [72], based on a similar opinion, performs plasty of bicuspid aortic valve, demonstrating good immediate results. However, it is unknown how a bicuspid aortic valve will behave in the long-term postoperative period.
Back in 1886, W. Osler [49] put forward a hypothesis about the relationship between infective endocarditis and bicuspid aortic valve, in which he was not mistaken. The results of further studies conducted by a number of authors [10, 24, 48, 50, 64] show that in patients with bicuspid aortic valves, the incidence of infective endocarditis varies from 10 to 30%. According to S. Higgins [33] and A. Stewart [66], of the total number of observations, the substrate of infective endocarditis was in 25% of cases the leaflets of the bicuspid aortic valve, with the highest frequency of lesions in children and adolescents. In observations conducted by R. Grant et al. [28], in patients with bicuspid aortic valves younger than 40 years of age, the cause of death in 30% of cases was infective endocarditis. According to the authors [4, 58], in patients with bicuspid aortic valves, infective endocarditis is the cause of severe valvular insufficiency and leaflet perforation with a frequency of 43 to 60%.
It has been proven that bicuspid aortic valve serves as a trigger in triggering the development of aneurysms of the ascending aorta, stenosis and insufficiency of the aortic valve, dissection and rupture of the aorta, and endocarditis. However, in none of the studies studied, the authors [9, 15, 32, 46, 52, 71, 72] do not answer the question of why aneurysms of the ascending aorta are more common in patients with bicuspid aortic valves than in patients with tricuspid aortic valves.
The lack of understanding of the mechanism leading to the development of ascending aortic aneurysms in bicuspid aortic valves is that the aortic root is not considered in terms of physical processes during the cardiac cycle. In structural and functional terms, the valves of the aortic valve and the aortic root should be considered as a single whole. The components of the aortic root are the aortoventricular crest, commissural rods and arched ring, connected into a complete frame that maintains the stereometry of the aortic bulb and controls the operation of the leaflets. The fibrous frame of the AC serves as a stress concentrator; this structural physiological structure reduces the load on the valve leaflets due to the redistribution of forces in systole and diastole [2].
The work cycle of the heart consists of rhythmic processes of systole and diastole, indirectly replacing each other. In the diastole phase, the left ventricle is filled with a volume of blood, which in the systole phase is ejected through the aortic valve into the ascending aorta. The valve leaflets open under the pressure of the blood volume created in the left ventricle of the heart according to the Frank-Starling law. The closure of the AV valves occurs under the influence of the pressure force of the blood volume, which interacts with the AV valves according to the principles described by B. Bellhouse [11]. If the valves of the aortic valve are not changed, the volume of blood is retained in the ascending aorta until the next volume arrives. Anatomically, the unit area of the aorta is designed for a uniform pressure force of a certain volume of blood arising during the diastole of the left ventricle and the reverse flow of blood under the influence of gravity. This volume and this force of fluid pressure on the aortic wall are created due to the distribution of the fluid volume between the three valves of the aortic valve. Thus, the pressure force of the shock wave created by the reverse flow of blood volume in the ascending aorta is dampened. To better understand these processes, it is necessary to know the difference between “fluid pressure” and “fluid pressure force,” since these concepts are not equivalent. The pressure of a liquid is a constant value equal to the product of the density of the liquid, the height of the liquid column and the acceleration of gravity. Pressure force is the product of fluid pressure and the surface area on which this pressure is applied. Now, considering the aorta as a vessel containing fluid, we can understand that the pressure force exerted on its bottom, and in particular on the AC, falls evenly on all 3 leaflets. The shock wave of the reverse flow of fluid is “quenched” evenly and the pressure force is evenly distributed on the walls of the aorta; overload of the fibrous ring of the aortic valve, commissural rods and sinotubular crest does not occur. Thus, conformational changes in the aortic root, which are a predisposing factor in the development of aneurysm of the ascending aorta and aortic insufficiency, are prevented.
In bicuspid aortic valves, the spatial arrangement of the aortic root structures is disrupted. The AC valves are formed as right and left or as anterior and posterior [2]. The latter have different areas [70], in contrast to the tricuspid AC, in which the area of the valves is approximately equal or differs slightly. These anatomical changes, due to the uneven distribution of pressure on the valves of the aortic valve, lead to overload of the previously identified structures and lead to the formation of aneurysms of the ascending aorta and aortic insufficiency. The pressure force created by the reverse flow of fluid in the ascending aorta during diastole “falls” perpendicularly onto both leaflets of the bicuspid aortic valve. The greater the surface area on which this force is exerted, the greater the pressure force. Considering the size of the unchanged ascending aorta, its shape resembles a cylinder tapering upward, with this shape of the vessel, the force of not only the poured liquid acts on the bottom, but also the pressure force of the walls of the vessel itself (in our case, the aorta). The pressure force of the reverse blood flow in a bicuspid aortic valve on the leaflet with a larger area is greater than on the leaflet with a smaller area. This force is increased by the force of pressure on the walls of the vessel, which inevitably entails overstraining the structures of the AC that are under maximum load - the commissures and the attachment points of the pathologically altered valves of the AC - the walls of the sinuses of Valsalva. In these zones, the greatest stretching and bending of the valves occurs, which leads to trophic tissue disorders, expansion of the ascending aorta, calcium deposition on the valves of the aortic valve, as well as aortic dissection [2]. With bicuspid aortic valves, not only conformational changes occur, but also changes in the flow of the blood stream passing through the aortic ring. In patients with bicuspid aortic valves, regardless of the presence of an ascending aortic aneurysm, so-called nested “helical flows” were found at peak systole in the ascending aorta. These flows play a trigger role in the occurrence of an aneurysm of the ascending aorta. No similar flows were found in patients with tricuspid aortic valve [43].
Taking into account the presented data, it can be stated that bicuspid aortic valve is one of the reasons for the formation of an aneurysm of the ascending aorta. The question of the surgeon's tactics when detecting a bicuspid aortic valve during surgery, during intervention for an aneurysm of the ascending aorta, remains unresolved. If the bicuspid aortic valve is wealthy and the patient’s age at the time of surgery is over 60 years, it is logical to preserve the aortic valve, since its service life will be longer than the patient’s life expectancy. Another question is if we are dealing with a young patient, younger than 50-55 years old. The question of the advisability of re-implantation of a bicuspid aortic valve into an aortic root prosthesis is currently open.
How does the aortic valve work?
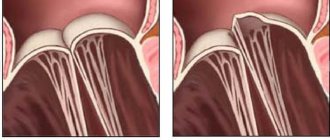
The tricuspid structure of the aortic valve is distinguished from the bicuspid mitral valve by the absence of papillary muscles and tendon chords. Therefore, it opens and closes only under the influence of the difference in pressure in the cavity of the left ventricle and the aorta.
During opening, elastin fibers from the ventricle press the valves against the walls of the aorta, opening the hole for blood flow. At the same time, the aortic root (the initial part) contracts and pulls them towards itself. If the pressure in the ventricular cavity exceeds the pressure in the aorta, then blood flows into the vessel.
The valves close with swirling currents in the area of the sinuses. They move the valve away from the walls of the aorta towards the center. Elastic flaps close tightly. The closing sound is heard with a stethoscope.
Anatomy of the heart
A healthy heart is a strong, continuously working organ, about the size of a fist and weighing about half a kilogram.
In addition to maintaining steady, normal blood flow, it quickly adapts and adapts to the body's ever-changing needs.
For example, the heart pumps more blood when it is active and less when it is at rest. During the day, the heart produces an average of 60 to 90 beats per minute - 42 million beats per year!
The heart is a two-way pump that circulates blood throughout the body. It consists of 4 chambers.
A muscular wall called the septum divides the heart into left and right halves. Each half has 2 chambers.
The upper chambers are called atria, the lower chambers are called ventricles. The right atrium receives all the blood returning from the upper and lower parts of the body.
Then, through the tricuspid valve, it sends it to the right ventricle, which in turn pumps blood through the pulmonary valve to the lungs.
In the lungs, the blood is enriched with oxygen and returns to the left atrium, which sends it through the mitral valve to the left ventricle.
The left ventricle pumps blood through the arteries through the aortic valve throughout the body, where it supplies the tissues with oxygen. Oxygen-depleted blood returns through the veins to the right atrium.
Four valves (tricuspid, pulmonary valve, mitral, aortic) act as a door between the chambers, opening in one direction.
These valves help blood move forward and prevent it from flowing in the opposite direction.
The petals of a healthy valve are thin, flexible tissue of perfect shape. They open and close when the heart contracts or relaxes.
Heart valves can become abnormal due to birth defects. They can become damaged or scarred due to rheumatic fever, infection, hereditary factors, age or heart attacks.
The mitral valves are most susceptible to such changes.
Regardless of the case, the heart valve can become stenotic (narrowed opening) or insufficient (does not close completely).
With valve stenosis, the heart must work harder to pump the required amount of blood through the narrowed opening.
Valve insufficiency causes blood to flow backwards through the valve after it closes. Once again, the heart has to work harder to pump enough blood for the body's needs to make up for the deficiency caused by the backflow of blood.
Both cases - stenosis and failure - force the heart to work harder to pump the required amount of blood. This extra work can weaken the heart, cause it to enlarge, and cause various diseases.
to the top of the page
Congenital changes in the aortic valve
The exact causes of congenital disorders are still unknown. More often it occurs simultaneously with another congenital heart disease - the mitral valve.
The most common developmental defects:
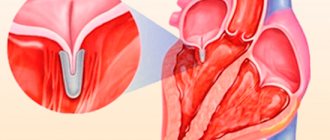
- formation of two leaflets rather than three (bicuspid aortic valve);
- one of the sashes is larger than the others, stretches and sags;
- one valve is smaller than the others, underdeveloped;
- holes inside the doors.
Aortic valve insufficiency ranks second in frequency after mitral valve defects. Usually combined with stenosis of the aortic lumen. More often found in boys.
Acquired vices
The causes of acquired defects are severe chronic diseases, so they often develop in adulthood. The greatest connection has been established with:
- rheumatism;
- septic conditions (endocarditis);
- previous pneumonia;
- syphilis;
- atherosclerosis.
The nature of pathological changes is different:
- With rheumatic lesions, the valves are soldered at the base and wrinkled.
- Endocarditis deforms the valves, starting from the free edge. Here, warty growths are formed due to the proliferation of colonies of streptococci, staphylococci, and chlamydia. Fibrin is deposited on them and the leaflets fuse together, losing the ability to completely close.
- With atherosclerosis, the lesion passes from the aortic wall, the valves thicken, fibrosis develops, and calcium salts are deposited.
- Syphilitic changes also spread to the valves of the aorta, but are accompanied by the death of elastic fibers and expansion of the fibrous ring. The valves become dense and inactive.
The causes of the inflammatory process can be autoimmune diseases (lupus erythematosus), chest injuries.
In older people, atherosclerosis of the aortic arch leads to expansion of the root, stretching and sclerosis of the valves.
Pathological changes in damage to the aortic valves
The result of congenital and acquired changes is the formation of insufficient closure of the valves, this is expressed in the return of part of the blood to the cavity of the left ventricle when it relaxes. The cavity expands and lengthens in size.
Forced intensification of contractions causes, over time, a breakdown of compensatory mechanisms and hypertrophy of the muscular layer of the left ventricle. This is followed by dilation of the left venous opening connecting the ventricle to the atrium. Overload from the left sections is transmitted through the pulmonary vessels to the right heart.
The impaired ability of the valve leaflets to close tightly leads to the formation of insufficiency and prolapse under the influence of reverse blood flow. Usually, aortic stenosis occurs simultaneously. In the clinical picture we can talk about the predominance of one type of defect. Both increase the load on the left ventricle of the heart. Features of the course must be taken into account when choosing a treatment method.
Postoperative period and discharge
Upon admission to the intensive care unit, the condition is severe and corresponds to the volume of surgical intervention.
In the postoperative period, sinus rhythm was restored, followed by a breakdown into atrial fibrillation. An attempt at recovery with a short-term effect. It is recommended to perform electrical pulse therapy three months after surgery.
On the fourth day after surgery, the patient was transferred from intensive care to the department in satisfactory condition.
Place of the bicuspid valve among aortic defects
The incidence of bicuspid aortic valve detection among children reaches 20 cases per thousand newborns. In adults it is 2%. For most people, two valves are enough to ensure normal blood circulation throughout a person’s life and do not require treatment.
On the other hand, when examining children with congenital heart disease in the form of aortic stenosis, up to 85% of cases reveal a variant of the bicuspid aortic valve. In adults, similar changes are found in half of the cases.
The “throughput” area of the aortic opening depends on the options for valve fusion.
Usually one of the two valves is larger than the other, the opening has an asymmetrical appearance like a “fish mouth”
If the congenital pathology of heart disease is “layered” with causes of an infectious nature, atherosclerosis of the aorta, then the valves fail faster than usual and undergo fibrosis and calcification.
How does valvular insufficiency manifest?
Symptoms of incomplete closure of the aortic valve begin to appear if the reverse flow of ejected blood reaches 15–30% of the volume of the ventricular cavity. Before this, people feel good, even play sports. Patients complain about:
- heartbeat;
- headaches with dizziness;
- moderate shortness of breath;
- feeling of pulsation of blood vessels in the body;
- angina pain in the heart area;
- tendency to faint.
With decompensation of the cardiac adaptation mechanisms, the following appears:
- dyspnea;
- swelling in the limbs;
- heaviness in the hypochondrium on the right (due to stagnation of blood in the liver).
During the examination, the doctor notes:
- pale skin (reflex spasm of peripheral small capillaries);
- pronounced pulsation of the cervical arteries and tongue;
- change in pupil diameter in accordance with the pulse;
- In children and adolescents, the chest protrudes due to strong heartbeats into the non-ossified sternum and ribs.
Intensified beats are felt by the doctor when palpating the heart area. Auscultation reveals a typical systolic murmur.
Blood pressure measurement shows an increase in the upper number and a decrease in the lower one, for example, 160/50 mmHg. Art.
Causes and symptoms
Heart valve defects can be congenital or acquired. The main causes of the development of heart valve defects are rheumatism, infections, myocardial and cardiovascular diseases.
Congenital heart valve defects develop before birth and depend on how the pregnancy progressed. Congenital heart valve defects are an extremely rare diagnosis, diagnosed only in 1% of cases. Congenital defects include defects of the aortic and pulmonary valves, which are treated by surgical intervention in the first years of the patient’s life.
Purchased . Acquired heart valve defects include transformations of the valve structure due to infections, inflammation, previous heart attacks, etc. Most of them arise as a result of a gradual change in the structure of the heart; in some cases, rheumatism leads to the defect. All congenital and acquired defects have related symptoms that can appear at any age:
- increased heart rate,
- dyspnea,
- swelling,
- other manifestations of heart failure.
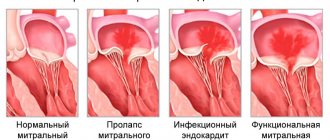
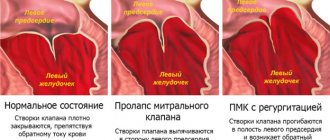
Initially, they appear during physical activity, but as pathologies develop, they will begin to appear in a calm state. Among the types of heart valve defects, mitral valve prolapse is the most common. It occurs during heart contractions when the valve leaflets in the left atrium sag. The walls of the valve lose elasticity and it “leaks”. Prolapse can be primary or secondary:
- Primary prolapse refers to congenital valve defects. Connective tissue pathologies in this case are a genetic predisposition.
- Secondary prolapse is an acquired defect. It occurs due to chest injury, rheumatism or myocardial infarction.
Prolapse does not have serious health consequences, and its symptoms do not interfere with life. However, they may not appear for a long time and most often bother people in old age, which is why they are written off as “age-related.” If you do not pay attention to the symptoms in time, complications may arise, such as arrhythmia and heart failure.
Symptoms also include complaints of pain in the heart area. They arise against the background of anxiety, are not associated with physical activity and are not relieved with medication. The pain is not intense, but long-lasting, accompanied by anxiety and rapid heartbeat.
The role of valves in the formation of aortic stenosis
With repeated rheumatic attacks, the aortic valves shrink, and the free edges become so welded together that they narrow the outlet. The fibrous ring becomes sclerotic, further increasing the stenosis.
Symptoms depend on the degree of narrowing of the opening. A critical stenosis is considered to be a diameter of 10 mm2 or less. Depending on the area of the free aortic opening, it is customary to distinguish between the following forms:
- light - more than 1.5 cm2;
- moderate - from 1 to 1.5 cm2;
- severe - less than 1 cm2.
Patients complain about:
- pain similar to angina attacks is caused by insufficient blood flow into the coronary arteries;
- dizziness and fainting due to brain hypoxia.
Signs of heart failure appear in the event of decompensation.
During the examination, the doctor notes:
- pale skin;
- palpation is determined by a shift to the left and down the apex impulse, “trembling” at the base of the heart on exhalation, like a “cat’s purr”;
- hypotension;
- tendency to bradycardia;
- typical sounds on auscultation.
What is the treatment for patients with bicuspid aortic valve?
If the patient is not bothered by anything, he is able to perform the physical activities he needs, and according to ultrasound there are no signs of valve dysfunction and heart overload, then there is no need for treatment. The patient should be regularly monitored by a cardiologist.
If the patient has characteristic complaints and cardiac ultrasound reveals a significant dysfunction of the valve (for example, high-grade insufficiency or critical stenosis) and signs of heart overload, then the doctor may decide to replace the aortic valve.
Make an appointment through the application or by calling +7 +7 We work every day:
- Monday—Friday: 8.00—20.00
- Saturday: 8.00–18.00
- Sunday is a day off
The nearest metro and MCC stations to the clinic:
- Highway of Enthusiasts or Perovo
- Partisan
- Enthusiast Highway
Driving directions
Survey data
On an x-ray (including fluorography), the expansion of the aortic arch and enlarged left and right ventricles are clearly visible.
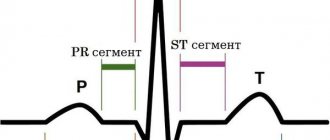
ECG - shows a shift to the left of the electrical axis, signs of myocardial hypertrophy, extrasystoles are possible.
Phonocardiographic signs allow you to objectively examine heart murmurs.
Ultrasound or echocardiography - indicates an enlargement of the left ventricle, most accurately characterizes the pathology of the valves (changes in structure, flutter of the valves, width of the residual opening).
Dopplerography is the most informative
The Doppler ultrasound method allows:
- see the return flow of blood;
- diagnose the degree of valve prolapse (internal deflection);
- establish the compensatory capabilities of the heart;
- determine indications for surgical treatment;
- assess the severity of stenosis by violation of the normal pressure gradient (from 3 to 8 mm Hg).
In the functional diagnosis of aortic stenosis using Dopplerography, it is customary to take into account the following gradient deviations (differences between pressure in the aorta and left ventricle):
- mild stenosis - less than 20 mm Hg. Art.;
- moderate - from 20 to 40;
- severe - over 40, usually 50 mmHg. Art.
The development of heart failure is accompanied by a decrease in the gradient to 20.
A type of echocardiography, the transesophageal version, is carried out by inserting a special sensor with an esophageal probe closer to the heart. It makes it possible to measure the area of the aortic annulus.
By catheterizing the chambers of the heart and blood vessels, the pressure in the cavities is measured (along the gradient) and the characteristics of the blood flow are studied. This method is used in specialized centers for diagnosis in people over 50 years of age, if it is impossible to decide otherwise on the method of surgical intervention.
Congenital defects are suggested to be operated on after 30 years of age, earlier - only with rapid decompensation
Diagnosis of heart valve diseases
After listening to the symptoms you describe and examining your medical record, the doctor will measure your pulse and blood pressure and listen to your heart using a stethoscope.
If your doctor suspects that you have a heart disease, he may ask you to undergo a series of special diagnostic tests that will help make an accurate diagnosis and prescribe the necessary treatment.
One of these research methods is a non-invasive method, i.e. which does not require any internal intervention.
Another type of research is invasive: with the help of instruments inserted into the body, which, as a rule, causes only minor inconvenience to the patient.
Chest X-ray
This test allows the doctor to obtain valuable information about the size of the heart, heart chambers and the condition of the lungs.
Electrocardiogram (ECG)
An electrocardiogram monitors the electrical current passing through the heart and stimulates the chambers to contract. An ECG is especially useful in diagnosing abnormal heart rhythms and rates.
These studies also show muscle enlargement or damage, and the presence of congestion on one side or another of the heart.
Echocardiogram (EchoCG)
This test is carried out using a “small” microphone placed on the surface of the chest, which emits high-frequency sound waves.
Sound waves are reflected back (hence the term "echo") from each layer of the heart wall and valves and then displayed on a monitor screen. The “echo” image from different points allows you to see a cross-section of the heart at the moment of its operation.
During the echo, the speed of blood flow is also recorded, the direction of blood movement is monitored: is the blood moving in the normal forward direction or is there a reverse movement (as in the case of valve insufficiency).
A narrowed (or stenotic) valve causes increased blood flow. The degree of valve stenosis is in many cases accurately determined by the increased blood flow velocity.
This test will not only show how the heart valves work, it will also provide useful and comprehensive information about the size of the heart chambers, as well as the thickness and function of the heart muscle.
Cardiac catheterization and angiogram
These tests are done by inserting a thin, hollow tube (catheter) through a vein or artery in the arm or groin area and into the chambers of the heart, using X-rays.
During catheterization, the pressure in the chambers of the heart is measured and the volume of blood in the bloodstream is determined.
Angiography consists of the injection of a radiopaque contrast agent, which is visible using X-rays and allows you to evaluate the work of the heart in pumping blood, the function of the valve and the patency of the arteries (coronary) that supply blood to the heart muscle.
Despite the fact that similar studies have been routinely carried out before, it is not at all necessary that they are needed in your case if the information obtained by echocardiography is complete and accurate.
In many cases, the only invasive test required before surgery is a coronary angiogram if it is determined that the patency of one or more arteries is impaired.
If there are blockages in the coronary arteries, the doctor will usually perform bypass surgery at the same time as heart valve surgery.
to the top of the page
Treatment without surgery
Treatment of narrowing of the orifice and aortic valve insufficiency is required only if the onset of decompensation is suspected, arrhythmia is detected, or severe damage is detected. Correct and timely use of medications allows you to avoid surgery.
Groups of pharmacological drugs are used that enhance myocardial contractility, making it possible to prevent arrhythmias and the manifestation of failure. These include:
- calcium antagonists;
- diuretics;
- β-blockers;
- drugs that dilate coronary vessels.