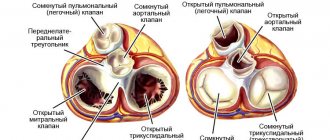
In the group of congenital heart defects, tetralogy of Fallot ranks firmly in tenth place. The prevalence among “blue” defects is half. In medical reports and reference literature, the abbreviation CHD is often used, which is synonymous with the term “congenital heart disease.”
In the International Classification of Diseases ICD-10, it is included in the group of congenital anomalies under code Q21.3. An unusual combination of disturbances in the formation of the heart and main vessels was described in 1888 by A. Fallot as a separate syndrome. His name remains in the history of medicine.
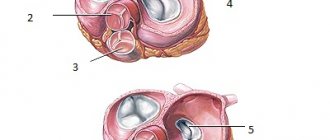
What anomalies does the syndrome consist of, anatomical features
Tetralogy of Fallot includes a combination of four anomalies:
- defect in the interventricular septum;
- right-sided position of the aorta (as if “sitting astride” on both ventricles);
- stenosis or complete fusion of the pulmonary artery, it lengthens and narrows due to rotation of the aortic arch;
- pronounced right ventricular hypertrophy of the myocardium.
Among the combinations of defects with pulmonary artery stenosis and septal defects, there are 2 more forms, also described by Fallot.
The triad consists of:
- holes in the interatrial septum;
- pulmonary stenosis;
- right ventricular hypertrophy.
Pentad - adds to the first option the damaged integrity of the interatrial septum.
In most cases, the aorta receives a large volume of blood from the right side of the heart without sufficient oxygen concentration. Hypoxia is formed according to the circulatory type. Cyanosis is detected in a newborn child or in the first years of a baby’s life.
As a result, the infundibulum of the right ventricle narrows, and a cavity is formed above it, similar to an additional third ventricle. The increased load on the right ventricle contributes to its hypertrophy to the thickness of the left.
The only compensatory mechanism in this situation can be considered the appearance of a significant collateral (auxiliary) network of veins and arteries supplying blood to the lungs. The open botal duct temporarily maintains and improves hemodynamics.
Tetralogy of Fallot is typically associated with other developmental anomalies:
- non-closure of the ductus botallus;
- accessory superior vena cava;
- additional coronary arteries;
- Dandy Walker syndrome (hydrocephalus and underdevelopment of the cerebellum);
- in ¼ of patients the embryonic right aortic arch remains (Corvisart's disease);
- congenital dwarfism and mental retardation in children (Cornelia de Lange syndrome);
- defects of internal organs.
Recommendations for pulmonary valve replacement
American Heart Association Guidelines (2008)
Pulmonary valve replacement should be considered for severe pulmonary regurgitation and when clinical symptoms or decreased exercise capacity occur (Class I, Level of Evidence B).
Pulmonary valve replacement should be performed in the absence of symptoms in one of the following cases (Class IIa, Level of Evidence B/C):
- moderate to severe right ventricular dysfunction.
- moderate to severe dilatation of the right ventricle.
- development of atrial or ventricular arrhythmia.
- moderate to severe tricuspid regurgitation.
Pulmonary valve replacement for right ventricular outflow tract obstruction should be performed in the following cases (Class IIa, Level of Evidence C):
— peak pressure gradient according to echocardiography >50 mm Hg.
- pressure in the right ventricle is more than 0.7 pressure in the left ventricle.
- progressive or severe dilatation of the right ventricle.
Canadian Heart Association Guidelines (2009)
Pulmonary regurgitation causing severe right ventricular dilatation (right ventricular ejection volume >170 ml/m2) (Class IIa, Level of Evidence C).
Moderate or severe right ventricular dysfunction (Class IIa, Level of Evidence C).
Tricuspid regurgitation, arrhythmias, or symptoms associated with decreased exercise intolerance (Class IIa, Level of Evidence C).
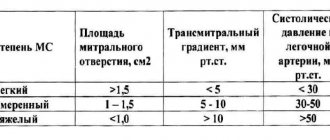
Residual pulmonary stenosis with a pressure of at least 2/3 of the systemic pressure (class IIa, level of evidence C).
European recommendations (2010)
Pulmonary valve replacement should be performed in symptomatic patients with severe pulmonary regurgitation or pulmonary stenosis (right ventricular systolic pressure >60 mmHg and tricuspid regurgitation velocity >3.5 m/s) (Class I, Level of Evidence C).
Pulmonary valve replacement should be considered in asymptomatic patients in one of the following cases (Class IIa, Level of Evidence C):
— objective decrease in tolerance to physical activity.
- progressive dilatation of the right ventricle.
- progressive systolic dysfunction of the right ventricle.
- progressive tricuspid regurgitation (moderate or severe).
— obstruction of the outflow tract of the right ventricle, systolic pressure in the right ventricle >80 mm Hg. and tricuspid regurgitation velocity >4.3 m/s).
- sustained atrial or ventricular arrhythmias
Surgery
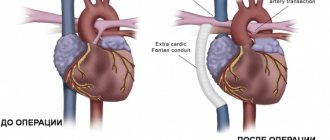
The question of choosing the optimal conduit for reconstruction of the right ventricular outflow tract still remains open. Among the variety of conduits available, there is not a single conduit that meets the parameters of an ideal prosthesis. Being exposed to the aggressive influence of surrounding tissues, as a result of degenerative processes, the conduit eventually loses its original properties and its function is impaired [35].
Pulmonary homograft is the “gold standard” in reconstruction of the right ventricular outflow tract, but early calcification of the graft, high cost and limitations in the size of the conduit forced us to look for an alternative [36]. To solve this problem, a xenoconduit from the jugular vein of a bull, Contegra, was developed in 1999 and purchased in 2001. Over the past decade, the Contegra xenoconduit has been widely used by many surgeons around the world due to the availability of a range of sizes from 12 to 22 mm, relatively low cost, and low incidence of calcification [37]. T. Breymann et al. [38] reported a low reoperation rate in patients with Contegra compared with patients with implanted homografts after 4 years of follow-up. J. Brown et al. [40] described excellent early and mid-term results of right ventricular outflow tract reconstruction with a Contegra xenoconduit. The authors recommend using Contegra as the conduit of choice during operations to form the outflow tract from the right ventricle [37—39].
Despite encouraging initial results, many centers reported frequent distal xenoconduit stenoses. B. Meyns et al. [41] observed severe distal stenoses in 51% of patients with Contegra xenoconduit 2 years after surgery. V. Gober et al. [42] reported supravalvular stenoses requiring reintervention in 15.8% of Contegra patients with a mean follow-up of 18 months. In a meta-analysis [42] and a number of other works [43, 44], it was noted that the most common cause of conduit dysfunction developing during the first year after surgery is stenosis of the distal anastomosis caused by pseudointimal hyperplasia. This complication develops more often the younger the patient’s age and the smaller the diameter of the conduit [45]. In addition, in addition to conduit stenosis, some centers describe xenoconduit aneurysms, thrombosis, and endocarditis [26, 45].
Another type of conduit that is used in right ventricular outflow tract surgery is Dacron grafts with built-in biological valves. The main advantage of such conduits is a complete set of all sizes and a relatively low price. A. Corno et al. [46] believe that the disadvantages of such grafts are frequent stenosis due to the growth of pseudointima and calcification of the biological valve leaflets. In the early postoperative period, thrombosis may be observed in these conduits more often than in a homograft or Contegra, due to the structural features of the wall, so anticoagulant therapy should be selected more carefully in such patients. Despite these data, E. Belli et al. [47] note the absence of the need for reoperations when using Hancock up to 98% within 1 year and 81% within 5 years. The authors call the growth of pseudointima the main reason for the development of conduit stenosis. K. Vitanova et al. [36], comparing Hancock, Contegra and homografts, found that after 5 years there was no stenosis in 69.1±7.9% of patients for Hancock, 75.1±9.1% for Contegra and 85.4±5.6 % for homografts. Conduit valve failure was 91.7±4% for homografts, 74.6±9.1% for Contegra, and 86.9±7.4% for Hancock at 5 years. The authors cite the heterotopic position of the graft as the only risk factor for conduit dysfunction. In conclusion, the authors note that the terms of reprosthetics of Contegra, Hancock conduits and homografts are comparable.
Transcatheter interventions
Since its first procedure in 2000, percutaneous transcatheter pulmonary valve replacement has become widely accepted as a minimally invasive alternative for patients undergoing radical repair of tetralogy of Fallot [48]. Currently, the most used transcatheter valves have a number of disadvantages, such as stent fracture, limited size and the inability to install the system in the native outflow tract; this has served as an incentive for the production of new devices with a larger range of sizes and a more durable stent design. In table 2 shows the characteristics of valves that are being tested or are already available on the market.
Table 2. Characteristics of transcatheter pulmonary valves
Studies conducted after placement of transcatheter valves show good short- and medium-term results [7]. S. Virk et al. [49] showed in their study that after implantation of transcatheter valves, exercise tolerance increases and right ventricular function is restored, and the procedure is accompanied by a low rate of complications. There are currently no studies comparing transcatheter technologies and conduits for right ventricular outflow tract replacement; however, the functional state of the right ventricle, according to MRI, seems comparable [50, 51]. Despite the many positive qualities of transcatheter technologies, there is one important unresolved issue - infective endocarditis after valve placement. This was first noted in the review by J. Buber et al. [52], in which out of 155 patients, 5 were diagnosed with infective endocarditis. More recent studies have shown that the development of endocarditis with transcatheter intervention is 10-15% compared with open surgery, after which the incidence of infective endocarditis does not exceed 2% [53, 54]. The authors cite stent disposition, obstruction of the right ventricular outflow tract, and abrupt cessation of aspirin intake as the main risk factors [54].
Another unresolved issue in transcatheter surgery is the timing of valve implantation. Historically, the main goal of reintervention after radical repair of tetralogy of Fallot has been to avoid right ventricular dysfunction or dilatation (for late intervention) and the need for reoperation (for early intervention). Currently, the indications for endovascular pulmonary valve replacement are the same as for open surgery. However, some authors recommend performing the operation at an earlier stage, without waiting for the development of clinical symptoms. They install a valve of the maximum size acceptable for the patient, understanding that if it is dysfunctional, it is possible to avoid open surgery and implant a valve prosthesis using the transcatheter “valve-to-valve” technique [7, 54, 55].
Thus, modern methods and technologies of cardiac surgery provide a wide range of parameters for the prevention and correction of developed right ventricular dysfunction in patients after radical correction of tetralogy of Fallot. The rapid expansion and development of transcatheter surgery allows achieving satisfactory immediate and mid-term results comparable to those after repeated open interventions, exposing patients to less risk. At the same time, questions about clear criteria and timing for re-intervention remain controversial. Perhaps the wider introduction of MRI methods for cardiac diagnostics into everyday practice and careful monitoring of patients in the long-term postoperative period will make it possible to timely identify dysfunction of the right ventricle at an early stage and determine the exact scope of the necessary intervention.
Causes
The causes of the anomaly are considered to be effects on the fetus in the early stages of pregnancy (from the second to the eighth week):
- infectious diseases of the expectant mother (rubella, measles, influenza, scarlet fever);
- taking alcohol or drugs;
- treatment with hormonal drugs, sedatives and sleeping pills;
- toxic effect of nicotine;
- intoxication with industrial toxic substances in hazardous industries;
- a hereditary predisposition is possible.
The use of pesticides in the garden without respiratory protection affects not only the health of the woman, but also her offspring
The important thing is that at a short period of time a woman may not notice pregnancy and independently provoke fetal pathology.
Types of tetralogy of Fallot
It is customary to distinguish 4 types of tetralogy of Fallot according to the characteristics of anatomical changes.
- Embryological - narrowing is caused by anterior displacement of the septum to the left and low localization. Maximum stenosis coincides with the level of the anatomical demarcation muscle ring. In this case, the structures of the pulmonary valve are practically unchanged, moderate hypoplasia is possible.
- Hypertrophic - pronounced hypertrophy of the exit zone from the right ventricle and the dividing muscle ring is added to the mechanism of the previous type.
- Tubular - obstruction is caused by incorrect division in the embryonic period of the common arterial trunk, due to which the pulmonary cone (the future of the pulmonary artery) turns out to be underdeveloped, narrowed and short. At the same time, it is possible to change the valve apparatus.
- Multicomponent - all of the listed factors are partially involved in the formation.
Introduction
Tetralogy of Fallot belongs to a group of common congenital heart defects; the frequency of this defect in newborns and infants is 5.6–14% of cases [1–3]. The natural course is unfavorable, so patients undergo surgical treatment in infancy. Despite the significant improvement in the immediate results of surgical treatment of tetralogy of Fallot, long-term results still cannot be called satisfactory [4, 5]. The main reason for the complicated course of the long-term period is dysfunction of the right ventricle due to long-term severe pulmonary regurgitation [6]. Currently, an adequate strategy for surgical treatment of right ventricular dysfunction is a current topic of discussion regarding congenital heart defects [7]. For many years, the only treatment for right ventricular dysfunction was to construct the right ventricular outflow tract with a xenograft or homograft, but with the development of endovascular surgery, transcatheter technologies have emerged as an alternative treatment option. This article reviews current problems and approaches to the treatment of right ventricular outflow tract dysfunction after radical correction of tetralogy of Fallot.
Data from EACTS, ECHSA and STS Congenital Database
In 2012, data from the European Congenital Database were published for the first time in EJCTS. The study included 6654 patients operated on for tetralogy of Fallot between 1999 and 2011. The study included 119 centers, 27 of which were outside Europe. In 57.5% of cases, transannular plastic surgery was performed, in 19.7% - radical correction without transannular plastic surgery, but with the use of ventriculotomy, in 18.2% - transatrial - transpulmonary correction without ventriculotomy. Thus, the rate of ventriculotomy for radical correction of tetralogy of Fallot was 77%, and overall hospital mortality was 2.58%. The study showed that after transannular plastic surgery the risk of complications in the early and late postoperative period is significantly higher than after using other types of reconstruction [8]. Similar data were obtained when analyzing the STS Database. The rate of ventriculotomy was 52% with an in-hospital mortality rate of 1.3% and a high rate of complications in the long-term period [9].
In conclusion of this analysis, the authors note the obvious uncertainty of the optimal and generally accepted strategy for surgical treatment of tetralogy of Fallot and the need for further modern research [9].
Modern strategy for surgical treatment of tetralogy of Fallot
The modern concept of surgical treatment of tetralogy of Fallot aims to minimize the neurological problems of the frequency of ventriculotomy and reoperations, the rate of perioperative mortality and the severity of the postoperative period [10]. In addition, in the long term, this concept should help to minimize the involvement primarily of pulmonary valve insufficiency and right ventricular dysfunction and arrhythmia.
Currently, this approach is becoming increasingly popular, and many large centers have adopted and are developing this strategy [11–13]. The advantages of this approach are the preservation of the intrinsic structures of the right ventricle, reduction of pulmonary regurgitation and right ventricular dysfunction [11]. As a rule, a Z score of the pulmonary ring of more than –3 allows almost all patients to undergo radical correction of this type without any significant problems [11, 12]. A fairly narrow retained ring may contribute to an increase in the residual gradient [11], but in some studies up to 30% of patients had an RV/LV ratio greater than 0.7 (0.7–0.9) without increasing mortality, and this had little effect on severity postoperative period [14]. Many studies have noted the fact of a decrease in dynamic obstruction of the outflow tract from the right ventricle after surgery, including due to the growth and development of the initially narrow valve ring of the pulmonary artery. There is also evidence that this approach does not affect the reoperation rate, which does not exceed 5% [11].
Modern studies assessing the strategy of preserving the native outflow tract from the right ventricle are rare, the immediate results are very optimistic, the long-term results are practically unstudied, therefore this area is the most relevant and promising for study in the surgical treatment of tetralogy of Fallot.
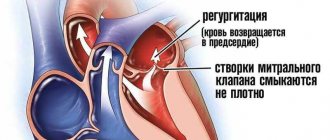
Pulmonary valve dysfunction
Surgical treatment of tetralogy of Fallot is aimed at closing the ventricular septal defect and eliminating obstruction of the right ventricular outflow tract. In the presence of severe hypoplasia of the pulmonary valve ring, massive infundibulectomy and transannular repair are required, which leads to the development of severe pulmonary regurgitation [15]. The latter leads to chronic volume overload of the right ventricle, leading to progressive dilatation and dysfunction of the right ventricle [6, 7, 15, 16]. Over time, patients have reduced exercise tolerance, develop atrial and ventricular arrhythmias, and also have a high risk of sudden cardiac death within 3–4 decades of life [17].
In the case of correction of tetralogy of Fallot with anomalies of the coronary arteries, the branches of which cross the outflow tract of the right ventricle, the only available option is conduit implantation. Over time, deformation and stenosis of the conduit occurs, which, due to pressure overload, leads to concentric hypertrophy of the right ventricle, increased diastolic pressure in its cavity and increased pressure in the right atrium [7]. Pressure overload and right ventricular hypertrophy are risk factors for poor outcome, including sustained ventricular tachycardia and sudden cardiac death in the long term after radical correction of tetralogy of Fallot [18].
Timing of pulmonary valve implantation
Currently, there are a large number of publications that note the connection between dilatation and dysfunction of the right ventricle in the long-term period with severe pulmonary regurgitation [6, 7, 15, 16, 19] (see figure). Particular attention is paid to the optimal timing of treatment of pulmonary regurgitation. Surgical treatment is indicated for patients with clinical symptoms, while relatively asymptomatic processes are not observed in generally accepted tactics. Proponents of early correction rely on the pathogenetic influence of residual regurgitation of systemic valves on the ventricles of the heart. Experience in studying the mechanism of action of chronic aortic insufficiency on the left ventricle shows that after a compensatory phase, which lasts for many years, a decompensation phase begins - irreversible changes in the myocardium develop, characterized by its dysfunction and even the formation of scars. Taking this into account, treatment of chronic pulmonary valve insufficiency should be carried out in the early stages, before the development of irreversible changes in the myocardium at the cellular level.
Outcomes of massive pulmonary regurgitation.
Right ventricular dysfunction
J. Therrien et al. [20] introduced the concept of “too late surgery” to restore or maintain normal contractility of the right ventricle, the function of which has deteriorated due to massive pulmonary regurgitation. These data were confirmed in a recent meta-analysis by Ferraz Cavalcanti et al. [21], who showed no increase in right ventricular ejection fraction after correction of pulmonary regurgitation. T. Geva et al. [22] also believe that a right ventricular ejection fraction of less than 45% is the main predictor of the development of permanent right ventricular dysfunction in the long-term postoperative period. However, there are studies that indicate an increase in ejection fraction after elimination of pulmonary regurgitation [23]. It has now been proven that right ventricular failure indirectly affects left ventricular function and the risk of sudden cardiac death. A large prospective multicenter study in which 873 patients underwent cardiac MRI to assess right ventricular function found that right ventricular dysfunction was a predictor of sudden cardiac death, sustained ventricular tachycardia combined with left ventricular dysfunction, atrial fibrillation, and right ventricular hypertrophy [ 18]. In this regard, surgical treatment should be carried out before the development of right ventricular dysfunction.
Right ventricular remodeling
One of the important topics in the last decade is the long-term preservation of right ventricular function and the identification of early predictors of right ventricular remodeling. With the development of MRI technology, it has become possible to determine the exact anatomy of the right ventricle and its function. MRI technologies have helped identify threshold values of right ventricular size at which correction of pulmonary regurgitation will lead to reverse remodeling of the right ventricle and restoration of its function. J. Therrien et al. proposed as threshold values the right ventricular end-diastolic volume index (EDV) of 170 ml/m2 or the end-systolic volume index (ESV) of 85 ml/m2, but later researchers proposed lower values of these indicators, leaning towards earlier surgical intervention (Table 1) [23-25]. Recently, greater attention has been paid to end-systolic volume as a more sensitive marker of preservation of right ventricular function and remodeling [26].
Table 1. Evolution of right ventricular size thresholds for correction of residual pulmonary regurgitation in tetralogy of Fallot
Indications for pulmonary valve replacement
In addition to the previously given parameters of the right ventricle - EDV, ESV and ejection fraction - you need to focus on the following factors: the ratio of EDV of the right ventricle should be 2 times higher than the EDV of the left ventricle, the ejection fraction of the left ventricle is less than 55%, aneurysm of the outflow tract from the right ventricle, QRS more than 160 m/s, stable tachyarrhythmia [30].
In case of pulmonary valve stenosis, the indication for surgical treatment is an increase in pressure in the right ventricle exceeding 2/3 of the systemic pressure [31]. Surgical treatment should begin with balloon dilatation of the valve and only if valvuloplasty fails, open surgery should be performed [31].
In case of combined pulmonary valve disease, indications for surgery are still a controversial topic. Most authors recommend performing surgery when symptoms of a dominant pulmonary valve lesion appear [31, 32]. However, Y. Kim and E. Ruckdesche [7[ advise using an individual approach to each patient, explaining this by earlier dysfunction of the right ventricle with combined lesions.
Features of hemodynamics
The severity of the defect is determined by the degree of narrowing of the diameter of the pulmonary artery. To diagnose and determine treatment tactics, it is important to distinguish three types of anomalies:
- with complete fusion (atresia) of the arterial lumen: the most severe disorder, with a large interventricular foramen, the mixed blood of both ventricles is directed predominantly to the aorta, oxygen deficiency is pronounced, in the case of complete atresia, blood enters the lungs through the open ductus arteriosus or through collateral vessels;
- acyanotic form: with moderate stenosis, the obstacle to the blood flow from the right ventricle can be overcome by a lower pressure than in the aorta, then the blood discharge will take a favorable path from the artery to the vein, the defect variant is called “white”, since cyanosis of the skin does not form;
- cyanotic form with stenosis of varying degrees: caused by the progression of obstruction, discharge of blood from right to left; this causes a transition from the "white" form to the "blue" form.
Morphology and hemodynamic disorders in tetralogy of Fallot
It includes 4 anatomical components:
- Dextraposition of the aorta (right position) - the aorta partially departs from the right ventricle, but with the maintenance of blood flow in it dominantly from the left ventricle.
- Right ventricular hypertrophy – it develops with age.
- Narrowing of the pulmonary trunk.
- A ventricular septal defect is a large and non-restrictive defect through which the right and left ventricles communicate with hemodynamic impairment.
Hemodynamic disturbances with Fallot's disease are caused by the fact that blood from the right ventricle (venous) during its systole is in insufficient quantity and hardly enters the pulmonary trunk, after which there is a lack of circulating blood volume in the pulmonary circulation. At the same time, most of that same venous blood, through a defect in the interventricular septum, enters the left ventricle, and then the aorta, and mixes with arterial blood, causing the appearance of skin cyanosis.
Symptoms
The clinical picture appears:
- significant cyanosis - located around the lips, in the upper half of the body, intensifies when the child cries, feeds, strains;
- shortness of breath - has a paroxysmal nature associated with physical activity, the child takes the most comfortable “squatting” position, due to a temporary reflex additional spasm of the pulmonary artery and the cessation of blood oxygen saturation by 2 times;
- fingers in the shape of “drum sticks”;
- physical underdevelopment and weakness of children; running, outdoor games cause increased fatigue, dizziness;
- convulsions - associated with hypoxia of brain structures, blood thickening, and a tendency to thrombosis of cerebral vessels.
The form of the disease depends on the age of the child, and it determines the adequacy of compensation; in a newborn, cyanosis is visible on the face, hands and feet
There are:
- early manifestations in the form of cyanosis immediately after birth or in the first 12 months of life;
- The classic course is considered to be the manifestation of cyanosis at two to three years of age;
- severe form - paroxysmal clinical picture with shortness of breath and cyanosis;
- late - cyanosis appears only by 6 or 10 years;
- acyanotic form.
An attack of shortness of breath can occur at rest: the child becomes restless, cyanosis and shortness of breath intensify, the heart rate increases, loss of consciousness with convulsions and subsequent focal manifestations in the form of incomplete paralysis of the limbs is possible.
Degrees of acute heart failure:
I degree - cyanosis of the mucous membranes is noted (disappears after oxygen therapy), dullness of heart sounds, expansion of the boundaries of cardiac dullness. Heart rate is 20-30% higher than normal, shortness of breath is 30-50% higher than normal,
II degree - characterized by pronounced cyanosis of the mucous membranes, acrocyanosis, periorbital edema, dullness of heart sounds, expansion of the borders of the heart, enlargement of the liver by 2-3 cm. Heart rate increases by 30-50%, respiratory rate - by 50-70%. There are manifestations of congestion in the pulmonary circulation, and the development of peripheral edema against the background of oliguria.
III degree - symptoms continue to increase, accompanied by tachycardia (increase in heart rate by 50-60%), tachypnea (RR - by 70-100% and higher), the clinical picture of pre-edema of the lungs develops. In the terminal period, bradycardia, bradypnea, muscle hypotension, areflexia develop, and blood pressure decreases.
Emergency care for acute left ventricular failure:
- give the patient an elevated position, half-sitting with legs down; You can apply venous tourniquets to the lower extremities for 15-20 minutes;
- ensure patency of the upper respiratory tract. At the “first aid” stage – inhalation of humidified oxygen;
- administer 1% solution of furosemide (Lasix) 0.1-0.2 ml/kg (1-2 mg/kg) intramuscularly or intravenously. If there is no effect after 15-20 minutes. – repeat the administration of the drug;
- for high or normal blood pressure - glyceryl trinitrate (nitroglycerin) ½ (0.25 mg) - 1 tablet. (0.5 mg) under the tongue for children over 12 years of age.
- with high blood pressure and hyperkinetic variant of myocardial failure upon arrival of the ambulance team:
- administer glyceryl trinitrate 2-5 mcg/kg intravenously;
- administer 0.25% droperidol solution 0.1 ml/kg intravenously or intramuscularly;
- according to indications, a single administration of 2.5% benzogexonium solution to children 1-3 years old at a dose of 1-3 mg/kg, over 3 years old - 0.5-1 mg/kg;
- if blood pressure decreases, administer a 3% solution of prednisolone 2-3 mg/kg (or hydrocortisone 5-15 mg/kg) intravenously. With increasing clinical signs of pulmonary edema - 4% dopamine solution intravenously titrated to 3-6 mcg/kg/min (dosage is selected individually);
- if the child is excited, administer 0.5% diazepam (seduxen) solution 0.02-0.05 ml/kg (0.1-0.3 mg/kg) intramuscularly;
- in case of severe bradycardia or severe bronchospasm, administer 2.4% aminophylline solution 0.15 ml/kg (4 mg/kg) intravenously.
Urgent hospitalization in the intensive care unit. The patient is transported in a semi-sitting position, while receiving oxygen therapy.
Diagnostics
The diagnosis is made by observing the child and the presence of objective signs. Information from relatives about development and activity, attacks with loss of consciousness and cyanosis are taken into account.
When examined in children, attention is drawn to the cyanotic nature of the lips and the altered shape of the terminal phalanges of the fingers. Rarely does a “heart hump” form.
On percussion, the borders of the heart are not changed or expanded in both directions. During auscultation, a rough systolic murmur is heard to the left of the sternum in the fourth intercostal space due to the passage of blood flow through the hole in the interventricular septum. It is better to listen to the patient in the supine position.
On the radiograph, the contours of the cardiac shadow resemble a “shoe” directed to the left
Due to the absence of the pulmonary artery arch, retraction occurs in the place where the vessels are usually located. A depleted lung appears more transparent. Enlargement of the heart to a large size does not occur.
A general blood test determines the adaptive response to hypoxia in the form of an increase in the number of red blood cells and an increase in hemoglobin.
Ultrasound diagnostics using a conventional ultrasound machine or Doppler ultrasound allows you to accurately determine changes in the chambers of the heart, abnormal development of blood vessels, the direction and magnitude of blood flow.
The ECG shows signs of right-sided cardiac hypertrophy, possible right bundle branch block, and the electrical axis is significantly deviated to the right.
Probing of the cavities of the heart with measurement of pressure in the chambers and vessels is carried out in specialized clinics when deciding on surgical treatment.
Less commonly, coronary angiography and magnetic resonance imaging may be required.
In differential diagnosis, it is necessary to exclude a number of diseases:
- transposition of the pulmonary artery causes a significant enlargement of the heart as the child grows;
- fusion at the level of the tricuspid valve promotes hypertrophy not of the right, but of the left ventricle;
- Eisenmenger's tetralogy - a defect accompanied not by fusion, but by dilation of the pulmonary artery; its pulsation and characteristic pattern of pulmonary fields are determined on an x-ray;
- stenosis of the pulmonary artery lumen is not accompanied by a “shoe” picture.
Doppler ultrasound helps to distinguish atypical forms.
Treatment
Drug therapy for a patient with tetralogy of Fallot is carried out only for the purpose of preparing for surgery or in the postoperative period. The only goal is to support the myocardium, preventing possible thrombosis after attacks and impaired coronary and cerebral circulation.
The patient is shown:
- inhalation of an oxygen-air mixture through nasal catheters or in an oxygen tent; newborns are kept in special intensive care units to reduce hypoxia;
- A solution of Reopoliglyukin, Eufillin is administered intravenously (in the absence of tachycardia);
- due to tissue acidosis, sodium bicarbonate solution is required.
It is impossible to treat a patient without surgical assistance
Operations can be:
- emergency measure of temporary assistance;
- shunt type for relieving blood flow along a new channel;
- a radical choice with correction of the ventricular septal defect and the location of the aorta.
As an emergency, the creation of an artificial connection (anastomosis) between the aorta and pulmonary artery using a prosthesis is used.
It is used as the first stage of surgical intervention for newborns and young children. It is believed that such actions allow you to prepare the child and avoid complications during further treatment, reducing the risk to 5–7%.
It is necessary to decide on the final planned correction of the defect before the age of three years. Temporary anastomoses can be performed between the subclavian and pulmonary arteries.
Radical surgery includes plastic surgery of the right ventricular exit cone, elimination of a hole in the interventricular septum, and valvotomy (dissection of an overgrown pulmonary valve). It is performed on an open heart and requires the use of a heart-lung machine.
The first days after surgery already show improvement in hemodynamics
Surgical treatment of tetralogy of Fallot in adults.
K.B. Babajanov, F.F. Turaev, A.A. Dzhumaniyazov, N.Sh. Bakhritdinov.
Republican Specialized Center for Surgery named after. acad. V. Vakhidova, Tashkent, Uzbekistan.
Tetralogy of Fallot (TF) is a congenital heart defect with poor pulmonary blood flow. It accounts for 25–40% of all pathology of the cardiovascular system and represents one of the most pressing problems of modern cardiac surgery [1, 18, 20]. Patients with TF are mainly operated on in childhood, but approximately 17–20% of them seek surgical help late for various reasons [2, 16, 19]. According to the European Journal of Epidemiology (1993), the incidence of TF is 2.2 cases per 10,000 newborns. M. Norgaard in 1999 gives a figure of 0.1 cases per 1000 live births. TF occurs with greater frequency in children born at low birth weight and lower gestational age with a sex ratio of 2.5 with a male bias [18, 19]. To this day, views on age restrictions on indications for surgery, stages and methods of surgery remain controversial.
In 1888, the Marseille pathologist Etinne-Louis Arthur Fallot described the clinical picture and pathological anatomy of congenital heart disease, which is characterized by a combination of four anatomical signs: a) narrowing of the pulmonary artery (PA), b) defect of the membranous part of the interventricular septum (VSD), c ) displacement of the aortic mouth towards the right ventricle (RV), d) hypertrophy of the RV myocardium [5, 15, 21].
The results of a morphological study of TF at the Institute of Cardiovascular Surgery named after. A.N. Bakulev showed that typical anatomical signs of the defect are: 1) displacement of the conical septum anteriorly and to the left; 2) disturbance in the development of pancreatic structures; 3) the presence of stenosis of the outflow tract of the pancreas, as well as stenosis of the annular and trunk of the pulmonary artery; 4) non-restrictive VSD; 5) dextroposition of the aorta, which should be understood as displacement of the orifice in relation to the interventricular septum and counterclockwise rotation of the arterial cone; 6) the presence of mitral-aortic fibrous contact; 7) hypertrophy of the pancreas myocardium [4].
A feature of TF in adult patients (over 16 years old according to the classification of A.F. Tour, 1988) from the above anatomical signs is a large subaortic non-restrictive VSD and severe hypertrophy of the RV myocardium [4, 19]. According to JK Kirklin (1983), the location of the atrioventricular bundle in TF in adult patients also has its own characteristics, namely: it is located along the base of the partially formed membranous part of the interventricular septum [18, 19]. The projection of the branching of the atrioventricular bundle onto the legs falls, respectively, on the most inferior part anterior to the conus papillary muscle, or at the place of attachment of the commissure between the anterior and septal leaflets of the tricuspid valve to the crest of the interventricular septum. Knowledge of these landmarks allows the surgeon to avoid damage to the conduction system during VSD repair [18, 25]. In patients with TF, collateral circulation is developed between the systemic and pulmonary circulation, which is especially pronounced in adult patients, which must be taken into account during surgery [15, 18, 19].
It has been established that in patients with TF, pancreatic myocardial hypertrophy appears already from the first year of life and subsequently increases, while in patients of preschool age it is associated with accelerated growth of cardiomyocytes against the background of continued differentiation, and in patients over 16 years of age - with hypertrophic growth of already mature differentiated cardiomyocytes [2, 3, 5, 7].
The nomenclature of the defect consists of the topic of PA stenosis, which can be valvular, subvalvular (infundibular), supravalvular, mixed, and its degree [4, 7, 22, 26]. At the same time, in adults, the infundibular type predominates (86.6%), which determines surgical tactics in these patients [17, 23, 24]. The degree of stenosis varies within different limits. Thus, the ratio of the diameters of the aorta and pulmonary trunk is normally 0.98–1.00, and with TF it is 0.16–0.70. If the diameter of the pulmonary trunk is less than 60% of the diameter of the ascending aorta, this indicates its hypoplasia [7, 26].
Depending on the amount of discharge through the VSD, J. Kirklin divides patients with TF into three groups [18, 19]:
- Group 1 (severe form of the defect) – patients in whom the volume of the right-left shunt is 45% or more of the minute volume of the systemic circulation;
- Group 2 (moderate form of the defect) – patients with a shunt volume of 25–45%;
- Group 3 (mild form of the defect) – shunt volume – 10–25% of the large circle.
N.M. Amosov (1983) distinguishes 4 stages according to the severity of the defect [1]:
- moderate severity - no or almost no cyanosis at rest, moderately limited physical activity, children rarely squat, hemoglobin content in the blood is not higher than 10.5 mmol/l (17 g%);
- moderate severity – significant limitation of physical activity, frequent squatting position, cyanosis, presence of nail phalanges in the form of “drum sticks”, hemoglobin content in the blood within 10.5–12.4 mmol/l (17–20 g%);
- severe - limited mobility, severe cyanosis, frequent dyspnea-cyanotic attacks (OCB), hemoglobin content in the blood 12.4–14.3 mmol/l (20–23 g%).
- extreme severity - central nervous system occurs with minimal physical activity, patients are practically deprived of the ability to move, severe cyanosis, hemoglobin content in the blood is higher than 14.3 mmol/l (23 g%), often there is no systolic murmur.
The features of intracardiac hemodynamics during TF in adults are such that the ventricles function in fundamentally different hemodynamic states. The pancreas operates under constant pressure overload and hypertrophies over time. The left ventricle (LV), on the contrary, operates under conditions of volumetric underload, and its cavity is small [9, 11, 12]. ON THE. Belokon (1991) believes that functional underload of the left parts of the heart in TF is the cause of relative LV hypoplasia, which is absent in adult patients with pronounced collateral circulation. Through these collaterals, so much blood can flow into the pulmonary circulation that even with atresia of the PA orifice, patients have a fairly moderate degree of cyanosis [1, 19, 20, 24].
Clinical manifestations of TF in an adult patient depend on the severity of two anatomical defects: pulmonary stenosis and VSD [3, 18].
49.4% of children with TF have cyanosis from birth. As the child grows, his motor and emotional activity increases, and also due to obliteration of the ductus arteriosus, cyanosis becomes more pronounced over time in adult patients (91.2%) [18, 19, 23]. Severe cyanosis is observed in approximately 14% of cases. Often, cyanosis may not be diagnosed during clinical examination. This happens in patients with the so-called pale (acyanotic, pink) form of TF, when pulmonary blood flow is reduced to acceptable values. However, in such cases, auscultation reveals a systolic murmur on the PA [3, 15].
In 40–81.5% of patients with TF in early childhood, OCP periodically occur - episodes of paroxysmal dyspnea with severe cyanosis, which is a pathognomonic sign of the disease [3, 13]. They usually appear at the age of 2 months, and then may disappear (usually from two years) due to the development of collateral circulation. OCPs are especially common in adult patients [14, 16, 23]. The etiological cause of attacks in TF is unclear. Wario (1957) suggested that attacks originate in connection with spasm of muscle fibers at the site of narrowing of the outflow tract of the pancreas, which stops the flow of blood into the PA [5]. Recently, researchers have been inclined to believe that this is one of the types of mechanocardiac reflexes from the pancreas. Crying, defecation, feeding, hot weather, infection, cardiac catheterization, and supraventricular tachycardia may act as trigger mechanisms [16, 17, 21].
The main complaints of patients with TF are shortness of breath, weakness, headaches, and dizziness. Exercise tolerance is usually reduced. Sick children often squat, which leads to an increase in total peripheral resistance and a decrease in the right-to-left shunt. Characteristic changes in the terminal phalanges of the fingers of the extremities or symptoms of “watch glasses” and “drumsticks” appear depending on the degree of hypoxemia on average in the 1st–2nd year of life, and in the adult cohort of patients the above changes in the fingers are determined in almost all (99.8 %) [18, 19]. Typical auscultatory signs of the defect in adult patients are a low-amplitude first tone, an accentuated second tone, which is represented by the aortic component, and a high-amplitude systolic murmur with an epicenter in the 2nd-3rd intercostal space on the left at the sternum. The intensity and duration of systolic murmur is inversely related to the severity of the defect and the severity of PA stenosis [20, 21].
In addition to generally accepted clinical research methods, it is possible to establish an accurate diagnosis of TF only with the help of special research methods, the main of which are transthoracic echocardiography and catheterization of the cardiac cavities. Echocardiography as a non-invasive research method allows you to directly determine the magnitude of aortic displacement, VSD, the degree of pulmonary stenosis and RV hypertrophy. During sounding, normal or reduced pressure in the PA, a systolic pressure gradient between the PA and the RV, equal pressure in both ventricles, arterial blood discharge at the level of the RV, and the architecture of the pulmonary trunk and its branches are determined. The use of angiocardiography made it possible to develop a number of diagnostic and prognostic criteria that make it possible not only to make or confirm the diagnosis of TF, but also to determine the optimal treatment tactics in each specific anatomical situation. S. Nakata began to calculate the PA index to determine the degree of narrowing of the branches of the pulmonary trunk. The index is the ratio of the sum of the cross-sectional areas of the right and left branches of the aircraft to the surface area of the body. According to these studies, the Nakata index should normally be (330±30) mm2/m2.
Finally, the structures of the heart can be visualized using the magnetic resonance method, which has gained great popularity among cardiologists in the last 15 years [6, 13]. This non-invasive method allows you to obtain adequate, and sometimes unique information, in comparison with the now routine echoscopy and X-ray cardioangiography. Magnetic resonance imaging allows you to accurately measure the size of the pancreas and especially its outflow tract, calculate indicators of pancreatic diastolic function, determine myocardial mass, and also provides a quantitative assessment of the volume of regurgitation on the pulmonary valve, which is especially important for assessing the result of radical correction of the defect in the long term [6] .
All types of surgical interventions for TF can be divided into two large groups: palliative and radical. Palliative operations are surgical interventions that are aimed at alleviating the patient’s condition and eliminating life-threatening symptoms and conditions. Palliative operations include subclavian-pulmonary anastomosis according to Blalock–Taussing, supplemented by other original developments. So, in 1960 A.A. Vishnevsky performed anastomosis using an arterial lyophilized homograft. In 1962, J. Klinner described the technique of such anastomosis with a Teflon tube, and de Leval in 1980 - with a polytetrafluoroethylene prosthesis (Core-Tech). Another part of the development concerned the search for new places to create a connection between the two circles of blood circulation. The main ones are: Potts anastomosis between the descending thoracic aorta and the left branch of the pulmonary artery, Waterstone-Cooley anastomosis between the ascending aorta and the right branch of the pulmonary artery, and Schumaher anastomosis between the ascending aorta and the right pulmonary artery using a vascular graft.
Palliative surgery is indicated in children with severe, recurrent attacks of hypercyanosis; in the case where the coronary artery crosses the outflow tract of the pancreas (ROV), radical surgery can be postponed until the child reaches an age at which it is possible to create a communication between the pancreas and the pulmonary trunk; with hypoplasia of the LA; in cases where heart defects associated with TF prevent radical surgery. Palliative operations allow the patient to survive the critical period [1, 2, 4, 19].
In relation to TF, a two-stage treatment method can also be considered in this way [4]. Systemic-pulmonary anastomosis eliminates such important risk factors as hypoxemia, central nervous system, and the need to take beta-blockers. Due to the increase in blood flow to the left parts of the heart, they adapt to the increased load. Increasing arterial blood oxygen saturation activates metabolism. All this significantly increases the patient’s chances of successfully undergoing radical surgery.
But according to Pacifico (1990) and Ilbavi (1990), it is better to undertake primary radical correction of the defect in early childhood and infancy, since the one-stage correction method makes it possible to avoid the additional risk of two operations, the occurrence of early and late complications of bypass operations (anastomotic thrombosis, partial or complete occlusion of the PA branch, development of pulmonary hypertension with hyperfunction of the anastomosis) [2, 16, 19]. But at the same time, they note that in 17–20% of cases patients seek surgical help late for various reasons.
The technique of radical surgery in patients with TF was first proposed by Lillehei in 1955 and co-authors. It consists of closing the VSD with a synthetic patch and eliminating the obstruction of the outflow tract from the pancreas. Subsequently, the surgical technique was improved by J. Kirklin and began to be called classical; after eliminating the stenosis of the RVOT, its wall was not sutured, but underwent plastic surgery with a patch of suitable sizes. In adult patients, due to severe infundibular obstruction of the RVOT during surgery, it is most often necessary to make a longitudinal incision of the RVOT, which leads to additional trauma to the heart, lengthening the time of artificial circulation, and also due to the well-developed collateral circulatory network, technical difficulties arise with hemostasis [ 21, 22].
In order to improve the technique for eliminating infundibular stenosis, pericardial patches are used for RVOT repair [18, 19]. In 1963, a transatrial approach was proposed for radical correction of TF [2, 12], which significantly reduced the incidence of complications and mortality in adult patients. Its essence was to avoid ventriculotomy, and therefore injury to the RV myocardium. This technique involved closing the interventricular defect and resection of the hypertrophied myocardium using access through the right atrium and the right atrioventricular orifice. Then, to make infundibular resection more radical, it was proposed to combine the transatrial approach with the transpulmonary one [3]. The method has become widespread in the Institute of Agricultural Sciences of the Academy of Medical Sciences of Ukraine since 1995 (V.V. Lazorishinets et al.). However, its adequate use is possible only in 30–40% of patients with TF.
According to V.P. Podzolkova et al (2004), in operated adult patients with TF, a good result, in terms of the ratio of pressure in the RV to pressure in the LV less than 0.5, was obtained in 57.6% of cases, satisfactory (0.5–0.7) – in 32.6% of cases. And only 9.8% of patients had a poor hemodynamic result, that is, the pressure ratio in the RV and aorta was more than 0.7. At the hospital stage, mortality due to acute heart failure, sepsis, pulmonary embolism and severe brain damage was 8.3%. In the period from 1 to 18 years, 74.4% of patients who underwent radical correction of TF at the age of over 18 years were examined. Based on the examination data, 58.9% of patients were classified into functional class I, 34.4% into functional class II, and 6.7% into functional class III according to the NYHA classification. The main reasons for unsatisfactory results were: VSD recanalization, high residual RV-PA gradient (above 60 mmHg) and low ejection fraction.
Analysis of literature data indicates that views on age restrictions in indications for surgery, stages and methods of surgery, as well as the relevance of diagnosis and surgical treatment of tetralogy of Fallot over the age of 18 years remain controversial, and rehabilitation of adults who have undergone surgery , unlike children, develops into a complex medical and social problem.
Literature
- Amosov N.M., Bendet Ya.A. Therapeutic aspects of cardiac surgery. – K., 1983. – P. 68-80.
- Amosov N.N., Zinkovsky N.F. Surgical treatment of tetralogy of Fallot. – K.: Health, 1982. – P. 151-158.
- Burakovsky V.I., Bockeria L.A. Cardiovascular surgery. – 1996. – P. 167-188.
- Burakovsky V.I., Bukharin V.A., Podzolkov V.P., Plotnikova L.R. Radical surgical treatment of tetralogy of Fallot // Recent advances in open heart surgery. – M., 1988. – P. 10-29.
- Vishnevsky A.A., Galankin N.K., Krymsky L.D. Tetralogy of Fallot. – M., 1969.
- Dynnik I.B. Methods of radiological diagnosis for tetralogy of Fallot // Bulletin of radiology and radiology. – 1989. – No. 1. – P. 28-32.
- Egorova I.F. and others. Thoracic and cardiovascular surgery. – No. 4. – 2001. – P. 9.
- Anderson RH, Devine WA, Del-Nido R. The Surgical anatomy of tetralogy of Fallot with pulmonary atresia rather than pulmonary stenosis see comments // J. Card. Surg. – 1991. – Vol. 6. – No. l. – P. 41-58.
- Bastos P., Campos J., Cunha D. Left ventricular function after total correction of tetralogy of Fallot // Eur. Heart J. – 1991. – Vol. 12. – P. 1089-1097.
- Borow KM, Green LH, Castaneda AR et al. Left ventricular function after repair of tetralogy of Fallot and its relationship to age at surgery // Circulation. – 1990. – Vol. 61. – P. 1150-1158.
- Dubost Ch., Blondesu Ph., D Allaines S. et al. Results a long terme de la reparation complete de la tetralogie de Fallot Coeur. – 1977. – Vol. 8, No. 3. – P. 697-707.
- Ergin MA, Griepp BB Total correction of tetralogy of Fallot // J. Thorac. Cardiovasc. Surg. – 1979. – Vol. 77. – No. 3. – P. 469-473.
- Fogelman R., Nykanen D., Smallhorn J. et al. // Circulation. – 1995. – Vol. 92. – P. 881-885.
- Gustafson RA, Murray GF, Warden HE et al. Early primary repair of tetralogy of Fallot // Ann. Thorac. Surg. – 1988. – Vol. 45. – P. 235-241.
- Hammon JD, Henry CI, Merril WH et al. Tetralogy of Fallot; selective surgical management can minimize operative mortality // Ann. Thorac. Surg. – 1985. – Vol. 40. – P. 280.
- Hu DG, Seward JB, Puga FJ et al. Total correction of tetralogy of Fallot at age 40 years and older: long-term follow-up // J. Amer. Coll. Cardiology. – 1985. – Vol. 5. – P. 40-44.
- Hughes CF, Lim YC, Cartmill TB et al. Total intracardiac repair for tetralogy of Fallot in adults // J. Thorac. Surg. – 1987. – Vol. 43, No. 6. – P. 634-638.
- Kirklin JK Tetralogy of Fallot: Principles of surgical management // Mod. Probl. Paediat. – 1983. – Vol. 22. – P. 139-151.
- Kirklin JW, Barratt-Boyes GG Cardiac Surgery. – N.-Y.: Wiley, 1986.
- Malm JM, McMicholas KW Tetralogy of Fallot // J. Jap. Ass.Thorac.Surg. – 1980. – Vol. 28, No. 4. – P. 493-505.
- Mattila S., Luosto R., Ketonen P. et al. Total correction of tetralogy of Fallot in adults // Scand. J. Thor. Cardiovasc. Surg. – 1984. – Vol. 12. – P. 654-663.
- Oelert HR, Hetser J, Luhmer B et al. Criteria for and against primary correction of Fallots tetralogy // J. Thorac. Cardiovasc. Surg. – 1984. – Vol. 32. – No. 4. – P. 215-220.
- Pacifico AD, Kirklin JW, Blackstone EH Surgical management of pulmonary stenosis in tetralogy of Fallot // J. Thorac. Cardiovac. Surg. – 1977. – Vol. 74, No. 3. – P. 382-395.
- Presbitero P., Demarie D., Aruta E. et al. Results of total correction of tetralogy of Fallot performed in adults // Ann. Thor. Surg. – 1988. – Vol. 46, No. 3. – P. 297-301.
- Rizzoli G., Massucco A., Bramana T. et al. The risk of surgical treatment of tetralogy of Fallot; an appoisal // Int. J. Cardiol. – 1985. – Vol. 9, No. 1. – P. 7-22.
- Takauchi Y. The study on right to left ventricular peak systolic pressure ratio
Ukrkardio