Heart valves and their defects
The heart has four chambers: two atria and two ventricles. From the atria, blood enters the ventricles, and then through the valves, with the help of contractions of the heart muscle, enters the arteries. Valves ensure blood flow in the desired direction and quantity. If they close or do not open completely, this prevents normal blood circulation.
As a result, the heart gradually increases in volume and stretches, compensating for blood deficiency and working under constant overload. Exhaustive work of the heart can cause the development of serious cardiovascular diseases, such as arrhythmia or heart failure. In addition, heart valve defects can cause complications due to certain ongoing infectious diseases.
Most often, heart defects are diagnosed in patients over sixty years of age. The reason is that with age, the valves of the valve apparatus lose their elasticity, and the heart increases in size. As a result, blood flow decreases and it unevenly fills the cavities of the heart - heart failure develops.
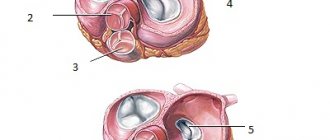
There are four types of heart valves, and each has a specific function:
- Aortic : prevents blood from flowing from the aorta into the left ventricle of the heart.
- Mitral : prevents the flow of blood from the left ventricle of the heart into the left atrium at the moment when the heart muscle contracts and blood is pushed into the vessels.
- Pulmonary : Prevents the flow of blood from the pulmonary artery into the right ventricle of the heart.
- Tricuspid : valve between the right ventricle of the heart and the right atrium.
If the valves are enlarged, narrowed, loose or torn, it becomes difficult for them to close, and blood flows back with each contraction of the heart. As a result, the heart experiences enormous stress and eventually loses its performance.
Depending on the form of the disease, heart valve defects can manifest as:
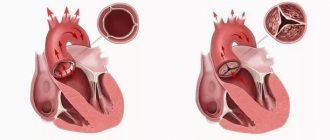
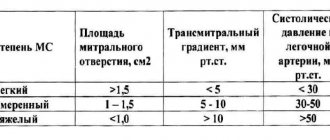
- stenosis – narrowing of the lumen (opening) of the vessels through which blood flows. This significantly increases the load on the heart, as it makes it more difficult for blood to be pumped out.
- insufficiency - damage to the heart valve leaflets, resulting in their inability to close completely. In such cases, the blood flows back.
- a combination of stenosis and insufficiency - the affected valves form an obstacle to the passage of blood. In this case, some of the blood passes through the hole, but returns back into the next phase of the cardiac cycle.
Publications in the media
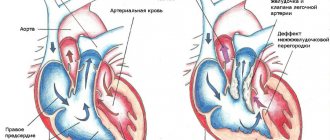
Single ventricle of the heart (SVC) is the absence of a septum between the ventricles of the heart. The main symptom is the communication of both atria through the mitral and tricuspid valves with a common ventricle. The structure of the heart is three-chambered. Frequency : 1.7% of all congenital heart defects (CHD).
Classification • Type A - single right ventricle (no inflow part of the right ventricle) • Type B - single left ventricle (no inflow part of the left ventricle) • Type C - undivided or common ventricle (no interventricular septum) • Type D - anatomy of the EVC is uncertain ( the inflow parts of both ventricles and the interventricular septum are absent).
Hemodynamics. In the single ventricular chamber, mixing of arterial and venous blood occurs. In the aorta and pulmonary artery (PA), which extend directly from the ventricle, the pressure is the same, which leads to the development of pulmonary hypertension.
Clinical picture • Complaints - shortness of breath, tachycardia, frequent respiratory diseases, more pronounced in the absence of PA stenosis • Examination •• Cyanosis, symptoms of “watch glasses” and “drumsticks”, more pronounced in PA stenosis •• “Heart hump” •• Systolic trembling along the left edge of the sternum •• Auscultation - II sound over the PA is accentuated in the absence of its stenosis; systolic murmur in the III–IV intercostal spaces; systolic murmur over the apex of the heart (relative mitral insufficiency); rough systolic murmur over the base of the heart according to the level of stenosis.
Research methods • ECG (deviation of the EOS to the right, hypertrophy of the EVC: the presence of ECG signs of hypertrophy of both the right and left ventricles) • X-ray examination of the chest organs (increase in the diameter of the heart shadow, increased pulmonary pattern) • Cardiac catheterization (high pressure in the EVC cavity, equal to the pressure in the aorta; increased oxygenation of blood in the ventricular cavity compared to the right atrium) • An angiocardiographic study allows you to determine the anatomical structure of the EVC, the location of the great vessels, and identify associated defects • EchoCG helps to identify the absence of an interventricular septum and the presence of two atrioventricular valves that open in EZhS.
Differential diagnosis • Tetralogy of Fallot • Open common AV canal • VSD • Transposition of the great vessels.
TREATMENT
Drug treatment - see Heart failure.
Surgery. Indications: all patients with EWS.
• Palliative operations are performed in young children and patients with the impossibility of radical correction due to many concomitant defects •• Surgical narrowing of the PA is performed with sharply increased pulmonary blood flow and high pulmonary hypertension •• Aortopulmonary anastomosis according to Blalock-Taussig is performed with severe PA stenosis and severe hypoxemia.
• Radical surgery is necessary when there is an increasing deterioration in the patient’s condition due to a progressive increase in the size of the heart and pulmonary vascular resistance. Radical correction is based on the principle of anatomical restoration of the structure of the heart - the creation of a septum dividing the EVC into arterial and venous chambers, and the elimination of associated heart defects. Necessary conditions for a successful operation are a sufficiently large size of the EVC cavity, correctly formed atrioventricular valves, normal location of the great vessels or synistrotransposition of the aorta in relation to the pulmonary trunk. Due to the fact that the implanted septum does not grow with the heart, the optimal age for surgery is 10–13 years. If radical correction is not possible (due to a seated tricuspid valve or due to insufficiency, hypoplasia or atresia of one of the atrioventricular valves) or is associated with a high risk, a hemodynamic Fontan correction is performed (see Tricuspid valve atresia).
Complications • Heart failure • Pulmonary hypertension • Cardiac rhythm disturbances • Postoperative complications (rhythm disturbances; renal failure; partial separation of the patch separating the UVC).
Course and prognosis. The prognosis is unfavorable. In the first year of life, 75% of patients die. The average life expectancy in the natural course of the defect is 6–7 years. Mortality after radical correction is up to 40%, after hemodynamic correction, subject to all Schuss criteria (see Tricuspid valve atresia) - up to 10%. Long-term mortality is 8–10%.
Synonyms • Common ventricle • Three-chamber heart with a single ventricle • Single-ventricle heart • Three-chamber heart with two atria
Abbreviations • EVC - the only ventricle of the heart • PA - pulmonary artery
Causes and symptoms
Heart valve defects can be congenital or acquired. The main causes of the development of heart valve defects are rheumatism, infections, myocardial and cardiovascular diseases.
Congenital heart valve defects develop before birth and depend on how the pregnancy progressed. Congenital heart valve defects are an extremely rare diagnosis, diagnosed only in 1% of cases. Congenital defects include defects of the aortic and pulmonary valves, which are treated by surgical intervention in the first years of the patient’s life.
Purchased . Acquired heart valve defects include transformations of the valve structure due to infections, inflammation, previous heart attacks, etc. Most of them arise as a result of a gradual change in the structure of the heart; in some cases, rheumatism leads to the defect. All congenital and acquired defects have related symptoms that can appear at any age:
- increased heart rate,
- dyspnea,
- swelling,
- other manifestations of heart failure.
Initially, they appear during physical activity, but as pathologies develop, they will begin to appear in a calm state. Among the types of heart valve defects, mitral valve prolapse is the most common. It occurs during heart contractions when the valve leaflets in the left atrium sag. The walls of the valve lose elasticity and it “leaks”. Prolapse can be primary or secondary:
- Primary prolapse refers to congenital valve defects. Connective tissue pathologies in this case are a genetic predisposition.
- Secondary prolapse is an acquired defect. It occurs due to chest injury, rheumatism or myocardial infarction.
Prolapse does not have serious health consequences, and its symptoms do not interfere with life. However, they may not appear for a long time and most often bother people in old age, which is why they are written off as “age-related.” If you do not pay attention to the symptoms in time, complications may arise, such as arrhythmia and heart failure.
Symptoms also include complaints of pain in the heart area. They arise against the background of anxiety, are not associated with physical activity and are not relieved with medication. The pain is not intense, but long-lasting, accompanied by anxiety and rapid heartbeat.
Arrhythmogenic right ventricular dysplasia
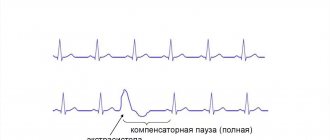
According to the clinical picture, there are 4 typical forms of this disease: a latent form, in which SCD due to ventricular fibrillation is the first manifestation of the disease; arrhythmic form, characterized by the presence of documented symptomatic ventricular tachyarrhythmias (ventricular extrasystole and ventricular tachycardia) with a QRS complex configuration similar to left bundle branch block; “paicisymptomatic form” – a form with symptoms of moderate severity, such as palpitations, pain in the heart area; a form manifested by heart failure (HF), predominantly right ventricular, with the presence or absence of cardiac arrhythmias.
The most common manifestations of ARVD are ventricular arrhythmias with ECG morphology like left bundle branch block, changes in depolarization and repolarization of the ventricular myocardium detected in the right precordial leads, as well as disturbances in global and/or local contractility of the RV and changes in the structure of its myocardium according to electrocardiography ( EchoCG), magnetic resonance imaging (MRI). It should be noted that there are many cases of asymptomatic disease, when the first and perhaps only manifestation is sudden death.
In a significant proportion of patients, ARVD remains unrecognized, despite the available clinical and instrumental signs. The disease can progress and lead over time to involvement of the LV myocardium in the pathological process. The clinical picture in such patients is dominated by signs of circulatory failure along with ventricular arrhythmias. Islands of fibro-fatty tissue found in ARVD form an arrhythmogenic substrate that carries the electrophysiological conditions for the development of re-entry of the excitation wave (re-entry), which underlies malignant ventricular tachyarrhythmias (VT).
Arrhythmias are induced by adrenergic stimulation, such as catecholamine infusion or exercise. 80% of patients demonstrate the appearance of ventricular extrasystole or VT during isoproterenol infusion. When analyzing Holter monitor ECG recordings of patients with sustained VT, an increase in the frequency of sinus rhythm is noted that precedes the paroxysm of VT, as a reflection of the activation of the sympathoadrenal system. Ventricular tachycardia in patients with ARVD usually has the ECG morphology of left bundle branch block, which indicates the origin of the arrhythmia from the RV myocardium. Several morphologies of VT are often recorded, since with this disease multiple arrhythmogenic foci can form. Patients with ARVD and their relatives often have a history of syncope of unspecified etiology. Fainting, as a manifestation of severe arrhythmic events, can occur long before the development of characteristic clinical and instrumental signs of ARVD. Subsequently, in these cases, progressive changes in the ECG and EchoCG parameters characteristic of this disease are observed.
Sudden death may be the first and only manifestation of ARVD, especially in young adults and athletes. All patients with known or suspected ARVD should be considered to be at increased risk of sudden death, even in the absence of documented ventricular arrhythmias. According to American authors, ARVD is posthumously diagnosed in approximately 5% of cases of sudden death among persons under 65 years of age and in 3–4% of cases of death of young athletes during competitions or training.
In the Veneto region of Italy, which is endemic for this pathology, ARVD causes sudden death in 20% of cases in people under 35 years of age. The annual incidence of VS in ARVD reaches 3% without treatment and can be reduced to 1% with primary or secondary prevention using pharmacotherapy. In the vast majority of cases, the mechanism of VS is the acceleration of the VT rhythm and its transformation into ventricular fibrillation. Peters S. et al. analyzed data from 121 patients with a verified diagnosis of ARVD and identified the following markers of increased risk of developing life-threatening ventricular arrhythmias and VS: male gender, maximum duration of the QRS complex in the right precordial leads >110 ms, increased size of the RV according to echocardiography, radiocontrast ventriculography, signs of involvement in the pathological process of the myocardium of the left ventricle (LV), dispersion of the JT interval in the left precordial leads > 30 ms, inversion of T waves in the precordial leads of the ECG beyond V3, dispersion of the duration of the QRS complex 50 ms. Identification of these signs seems most significant for asymptomatic patients with ARVD. Timely administration to such patients of means to prevent the development of fatal arrhythmias can significantly improve their life prognosis. Patients with ARVD may present with symptoms of isolated right ventricular or, less commonly, biventricular HF, which usually appear between the ages of 40 and 50 years. ARVD is a primary myocardial disease leading to right ventricular failure in the absence of pulmonary hypertension. The pathogenesis of the development of right ventricular failure in this pathology consists of dilatation, thinning of the wall and a progressive decrease in contractility of the RV myocardium due to its atrophy. Involvement of the papillary muscles in the process is accompanied by the development of tricuspid regurgitation.
Signs of right ventricular failure manifest, as a rule, 4–8 years after the onset of complete blockade of the right bundle branch - one of the characteristic electrocardiographic markers of damage to the RV myocardium and its structures of the cardiac conduction system. The dysplastic process can also involve the LV myocardium, leading to a decrease in its contractility, however, left ventricular failure is less characteristic of this disease. The development of left ventricular failure can create preconditions for diagnostic errors. For example, often instead of ARVD, idiopathic dilated cardiomyopathy (DCM), or DCM, is diagnosed as a result of viral myocarditis.
Left ventricular dysfunction in ARVD reflects biventricular dysplasia and must be differentiated from any other primary myocardial diseases that involve both ventricles in the pathological process and are manifested by the development of dilatation and decreased contractility. Reliable diagnosis of ARVD is of fundamental importance due to the high predisposition of patients with this pathology to the occurrence of malignant VTs, which are often resistant to drug therapy and require, in a significant proportion of cases, the use of special non-drug treatment methods.
In some cases, additional diagnostic difficulties in patients with ARVD are created by pain in the heart area, which does not always occur during exercise, but can sometimes be relieved by the use of nitrates. In such patients, histological studies reveal media proliferation with almost complete obstruction of the distal coronary bed, embedded in adipose tissue. These changes may explain the development of angina syndrome in ARVD.
CURRENT AND FORECAST
An important clinical feature of the disease that determines the prognosis is ventricular arrhythmias and right ventricular failure. According to Sagi et al. (1993), with effective antiarrhythmic therapy, as well as with the use of surgical treatment methods (ablation of arrhythmogenic zones), the 10-year survival rate is 77%. Lecleroq et al. (1991) report that the mortality rate for arrhythmogenic dysplasia of the right ventricular myocardium is 9% over 5 years. With effective antiarrhythmic therapy, according to serial electrophysiological testing, patients have a good long-term prognosis with continued use of the antiarrhythmic drug and the absence of progression of the pathological process in the right ventricle or right ventricular failure. With uncontrolled empirical therapy, mortality increases to 20% in the first 10 years (2.5% per year). The main cause of death in patients is ventricular fibrillation.
Diagnosis and treatment
If you or your loved ones are experiencing the symptoms described above, we recommend getting tested. During diagnosis, the doctor monitors heart parameters at rest and during exercise.
The patient is prescribed:
- daily ECG monitoring,
- echocardiography (ECHO-CG),
- chest x-ray,
- CT and MRI using special equipment that allows you to examine the heart virtually between beats.
Such diagnostics are carried out not only during the initial examination of patients with suspected disease, but also in dispensary groups of patients with an already confirmed diagnosis.
Depending on the diagnostic results, the doctor prescribes the necessary treatment: therapy or surgery.
- Therapy is aimed at preventing, preventing and alleviating relapses of the disease that became the root cause of the defect, as well as treating heart failure.
- Surgical intervention is an extreme necessity, which due to age or complications may not be prescribed for all patients.
Typically, heart valve disease is a mechanical problem that can only be solved with surgery performed by a surgeon. For stenosis, an operation is indicated to separate the fused valve leaflets and widen the atrioventricular opening - commissurotomy. If there is insufficiency, prosthetics are performed: replacement with a biological or mechanical analogue.
The only ventricle of the heart
Causes of the defect
Normally, the heart has two well-developed ventricles (right and left), which work as pumping chambers, expelling blood into the small (to the lungs) and large (to other organs) circulation circles. In the case when one ventricle is not developed correctly or is of insufficient size (hypoplastic), a well-developed ventricle takes over its function and works on both circuits of blood circulation, which leads to its overload and rapid exhaustion. In this case, venous (oxygen-poor) and arterial (oxygen-rich) blood mix, and all organs receive blood with insufficient oxygen content in the blood, which determines the presence of cyanosis in these children.
Diagnostics
Patients with a single ventricle of the heart require an integrated approach in the diagnosis and treatment of such a complex cardiac pathology. These patients require a complete examination of the entire cardiovascular system to assess the anatomy of the defect and its hemodynamic characteristics.
Patients suspected of having this pathology undergo a diagnostic study using the latest technologies and necessarily include transthoracic echocardiography (if necessary, transesophageal echocardiography), cardiac catheterization with angiocardiography and direct tensiometry, MSCT.
Treatment methods
All children diagnosed with a single ventricle of the heart require surgical treatment, the main goal of which is to create conditions that allow the single ventricle to work only in the systemic circulation and deliver oxygenated blood to all organs, while blood bypasses the pulmonary vessels hearts.
Rice. Bidirectional cavopulmonary anastomosis (Glenn procedure)
The first stage is the formation of a bidirectional cavo-pulmonary anastomosis (DCPA) (Glenn procedure), directing part of the venous blood coming from the superior vena cava directly to the pulmonary arteries. This reduces blood flow to the single ventricle, reduces the load on it and ensures better oxygen saturation of the blood.
Rice. Operation Fontan
The next step is to create a complete cavo-pulmonary anastomosis ( Fontan procedure ), moving venous blood from the inferior vena cava to the pulmonary arteries using a vascular prosthesis. As a result, all venous blood immediately flows to the lungs, where it is saturated with oxygen, bypassing the heart. From the lungs, oxygen-rich blood enters the chambers of the heart, and a single ventricle releases it into the systemic circulation to all organs and vital systems of the body. This procedure allows you to minimize the load on the single ventricle and ensures good oxygen saturation of the blood (no mixing of blood).
Thus, the Fontan operation
consists of hemodynamic correction, which allows you to direct venous, non-oxygenated blood directly into the pulmonary artery.
Observation of patients in the postoperative period
Maintaining a balanced pulmonary and systemic blood flow is the key to a good result of the operation, therefore, after performing each of the stages of treatment (DCPA, PCPA), all patients need regular monitoring and a complete examination to identify problems with the single ventricle and pulmonary vessels.