The aortic valve allows blood to flow unidirectionally from the left ventricle to the aorta. When the heart contracts, the leaflets of the aortic valve open, freely allowing blood to pass through, and after contraction, the leaflets close hermetically, preventing the reverse flow of blood from the aorta into the left ventricle.
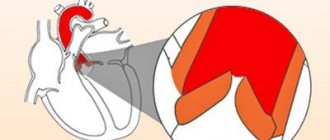
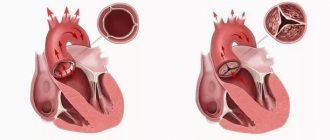
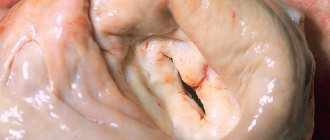
In the vast majority of people, the aortic valve consists of three leaflets. About 2% of the world's population have a bicuspid aortic valve.
The valve that has three valves turned out to be the most effective and durable.
The bicuspid aortic valve experiences a large mechanical load, as a result of which valve dysfunction is much more common with this structure.
Over a long period of time, the bicuspid valve can successfully perform its assigned function, and the person does not experience health problems.
But, as a rule, sooner or later the function of the bicuspid aortic valve is impaired, as its narrowing or, more rarely, insufficiency develops.
At CELT you can consult a cardiologist.
- Initial consultation – 3,500
- Repeated consultation – 2,300
Make an appointment
How is bicuspid aortic valve diagnosed?
Diagnosis of this pathology is usually not difficult. Ultrasound examination of the heart with Dopplerography (echocardiography or EchoCG) allows not only to identify this anatomical feature, but also to determine the degree of dysfunction of the valve and the severity of overload of the left side of the heart. Bicuspid aortic valve is often combined with coarctation of the aorta or other congenital heart defects, enlargement of the aortic root. Patients with bicuspid aortic valves are more likely to have a dissecting aortic aneurysm. These abnormalities are also diagnosed using cardiac ultrasound.
In our clinic, echocardiography is performed only by high-level specialists using expert-class ultrasound machines.
What is heart valve disease?
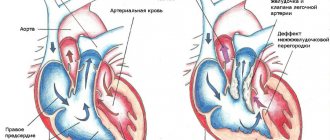
Each heart valve is a complex mechanism that, like gate leaves, opens and closes the flow of blood through the chambers of the heart and from the heart to the aorta and pulmonary artery. Valves allow blood to flow in only one direction.
The human heart consists of four cavities - two atria and two ventricles. Blood enters the atria of the heart through the veins, from the atria to the ventricles, from the ventricles to the large arteries (aorta and pulmonary artery). Along the path of its movement, at the junction of the atria into the ventricles and the ventricles into the arteries, there are heart valves - movable valves consisting of individual elements (cusps). If the heart valve does not work properly, problems with blood flow (in the form of backflow or obstructed blood flow) occur.
The nature of changes in the heart valves can be divided into two groups: - valves that do not close completely (valve insufficiency), which leads to regurgitation (backflow) of blood through the valve in the opposite direction (for example, from the aorta to the left ventricle) and - valves valves that do not open properly (valve stenosis), causing blood flow to become obstructed and restricted.
Valvular heart defects are relatively common, accounting for 20 to 25% of all organic heart diseases in adults. The most frequently detected defects are the mitral valve, the second most common are lesions of the aortic valve. In almost all cases in children and in 90% of cases in adults, the occurrence of the defect is associated with rheumatism. The second most common disease is bacterial endocarditis. Rare causes of the formation of the defect can be systemic lupus erythematosus, scleroderma, rheumatoid arthritis, and in adults - atherosclerosis, coronary heart disease.
Of particular note is a condition that often occurs in healthy people called mitral valve prolapse, or “click syndrome,” “slamming valve syndrome,” “click and murmur syndrome,” “mitral valve aneurysmal bowing syndrome,” “Barlow syndrome,” Engle’s syndrome. and others. Cuffer and Borbillon in 1887 were the first to describe the auscultatory phenomenon of systolic clicks (clicks) of the heart. The term "mitral valve prolapse", which is currently most widespread, was first proposed by J Criley.
The mitral valve blocks the reverse flow of blood from the left ventricle into the left atrium. Prolapse is a condition when the valve leaflets, at the moment of contraction of the ventricle, do not close the hole “tightly”, but bend into the cavity of the atrium, allowing blood to pass in the opposite direction. This is accompanied by a characteristic clicking sound or heart murmur. The amount of blood returning to the atrium can serve as a measure of the severity of the defect.
Depending on when the heart valve defect appeared, primary and secondary prolapse are distinguished: 1. Primary (idiopathic) valve prolapse is congenital, caused by a genetic defect in the structure of the connective tissue that makes up the valve leaflets. 2. Secondary (acquired) heart valve prolapse appears as a result of chest trauma, rheumatism, myocardial infarction and other causes. Today, some experts consider primary mitral valve prolapse to be just a variation of the norm, and not a disease at all.
What is the treatment for patients with bicuspid aortic valve?
If the patient is not bothered by anything, he is able to perform the physical activities he needs, and according to ultrasound there are no signs of valve dysfunction and heart overload, then there is no need for treatment. The patient should be regularly monitored by a cardiologist.
If the patient has characteristic complaints and cardiac ultrasound reveals a significant dysfunction of the valve (for example, high-grade insufficiency or critical stenosis) and signs of heart overload, then the doctor may decide to replace the aortic valve.
Make an appointment through the application or by calling +7 +7 We work every day:
- Monday—Friday: 8.00—20.00
- Saturday: 8.00–18.00
- Sunday is a day off
The nearest metro and MCC stations to the clinic:
- Highway of Enthusiasts or Perovo
- Partisan
- Enthusiast Highway
Driving directions
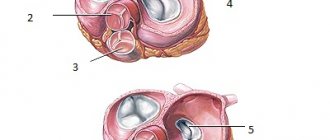
Leaf and semilunar valves
The leaflet valves are located around the right and left atrioventricular orifices. Those openings that connect the right atrium with the right ventricle, and the left atrium with the left ventricle.
Why casement?
Because each of these valves is a set of flat leaflets. The right atrioventricular has three valves, and the left has two.
Their valves are some kind of flat triangular petals. With their wide ends, these petals are tightly connected to a dense tendon ring surrounding the hole. And their narrow and movable ends hang freely into the cavity of the ventricle (this is in the opening position).
At the moment of closing, it is the flaps that close the opening, tightly adjacent to each other. You can read about each of the leaf valves in the articles:
- "Mitral valve"
- "Tricuspid valve"
The semilunar valves are located in the openings that connect the ventricles of the heart to the large arteries leaving the heart.
These are the aorta and pulmonary artery. The aorta comes from the left ventricle, and the pulmonary artery comes from the right.
Each hole is equipped with a valve consisting of three semicircular pockets.
Semilunar valves, like leaflet valves, are formed by folds of the inner layer of the heart wall - the endocardium. But the structure of these “folds” is different.
Right semilunar valve
Its second name is pulmonary valve. It regulates the flow of blood from the right ventricle to the pulmonary artery.
Its structure is no different from the structure of the aortic valve: it is also equipped with three beautiful “pockets”. But they got different names:
- front door
- back
- right
This valve is less dense than the aortic valve, which can be explained simply: the load on it is much less. The right ventricle is not as powerful as the left; the pulmonary circulation is called small because it is much shorter and less branched.
Valve Definition
The body, which is a complex mechanism, has many devices to direct flows in the right direction. Such devices are found in the heart muscle - they are more complex. They are also located in capacitive vessels of various calibers.
The valve apparatus is a set of anatomical structures that, when working together, prevent the reverse (retrograde) movement of blood.
Sinuses of the aortic valve and pulmonary valve
The sinuses of the aorta and pulmonary artery are the spaces between each of the semilunar valves and the wall of the vessel.
The height of the aortic sinuses in adults is 1.7-2 cm, their depth is from 1.5 to 3 mm. Deepening of the sinuses occurs with age. The spaces between the valves located next to each other have the shape of triangles, with the base facing the ventricles. The triangles consist of collagen and elastic fibers; they connect the valves and form the fibrous rings of the valves.
At the base of the aorta, an oval fibrous structure with three teeth forms, resembling a crown.
The pulmonary trunk usually consists of three sinuses: anterior, left and right. Sometimes there are two sinuses. The sizes of these sinuses vary significantly in different age groups, and also have individual characteristics. In adults, the left sinus is 19-32 mm wide, 12-16 mm high, the right sinus is 20-32 mm and 10-15 mm. Front 20-30 mm and 10-15 mm respectively.
Not everyone recognizes the existence of a fibrous structure at the base of the pulmonary trunk.
Medical Internet conferences
Introduction
Bicuspid aortic valve is the most common congenital heart defect. Remaining asymptomatic for a long time, it may eventually become complicated by the development of aortic stenosis or aortic insufficiency. Bicuspid aortic valve is also associated with aortic aneurysm and dissection. Given the high prevalence of this defect and the severity of complications, it is believed to cause more deaths than all other congenital heart defects combined [1].
Introduction
Bicuspid aortic valve is the most common congenital heart defect. Remaining asymptomatic for a long time, it may eventually become complicated by the development of aortic stenosis or aortic insufficiency. Bicuspid aortic valve is also associated with aortic aneurysm and dissection. Given the high prevalence of this defect and the severity of complications, it is believed to cause more deaths than all other congenital heart defects combined [1].
Introduction
Bicuspid aortic valve is the most common congenital heart defect. Remaining asymptomatic for a long time, it may eventually become complicated by the development of aortic stenosis or aortic insufficiency. Bicuspid aortic valve is also associated with aortic aneurysm and dissection. Given the high prevalence of this defect and the severity of complications, it is believed to cause more deaths than all other congenital heart defects combined [1].
Clinical case
Patient A., 51 years old, was admitted on March 4, 2021 with complaints of shortness of breath with difficulty inhaling and exhaling when going up to the 4th floor; dizziness when moving to a vertical body position; attacks of rapid heartbeat that occur in a lying position, lasting a few seconds, stopping on their own.
Considers himself sick for a year and a half (from the age of 49), when dizziness first began to bother him. Since March 2021, shortness of breath occurs during physical activity. Over the course of six months, an increase in blood pressure to 140 and 90 mm was repeatedly recorded. rt. Art. Since September 2021, attacks of rapid heartbeat occur occasionally at rest. An examination at a local clinic revealed a congenital heart defect: bicuspid aortic valve. Sent to the cardiology hospital.
From the life history it is known that the patient served in the army, coped well with physical activity, currently works as a mechanic at a factory, and previously smoked. As a child I had frequent sore throats. Among the operations undergone, he noted a tonsillectomy at the age of 12 years.
Upon examination, an asthenic physique attracted attention. On palpation, a high, intense, resistant, dome-shaped apical impulse was detected in the 5th intercostal space 1 cm outward from the left midclavicular line. During percussion, the left border of relative cardiac dullness was shifted to the left and coincided with the apical impulse. On auscultation, heart sounds were rhythmic, heart rate 72 per minute. At the top, the first tone was slightly weakened, but louder than the second tone. Above the aorta, the second sound was weakened, a rough systolic murmur was heard, carried through to the vessels of the neck and the entire precordial region. Blood pressure was 140 and 80 mm Hg. The radial artery pulse was the same in both arms, rhythmic with a frequency of 72 per minute.
There were no changes in general and biochemical blood tests as of 03/05/2019.
During electrocardiography on March 4, 2021 (Fig. 1), sinus rhythm was recorded with a frequency of 72 per minute. The electrical axis of the heart was located horizontally. Signs of left atrium enlargement were determined.
Holter ECG monitoring dated March 11, 2021, which lasted for 23 hours, showed the dominant rhythm was sinus. The average heart rate was 54 per minute. A paroxysm of ventricular tachycardia consisting of 5 contractions with a frequency of 129 per minute, 24 single ventricular extrasystoles was recorded. Supraventricular ectopic activity consisted of 288 contractions, of which 12 atrial groups lasting from 3 to 8 contractions with a frequency of 105 to 129 per minute, 12 episodes of paired atrial extrasystoles, including with aberration of intraventricular conduction, 201 single atrial extrasystoles. There were no diagnostically significant dynamics of the ST segment.
X-rays of the chest organs with a contrast-enhanced esophagus dated March 5, 2021 (Fig. 2) revealed signs of dilation of the ascending aorta, a slight enlargement of the left ventricle, and right-sided scoliosis of the thoracic spine.
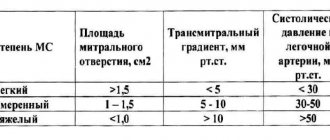
Echocardiography on March 5, 2021 (Fig. 3) visualized a bicuspid aortic valve and multiple calcifications at the base and distal parts of the leaflets. Slight aortic regurgitation was detected; aortic stenosis, the degree of obstruction is severe, the maximum gradient is 79 mm Hg, the average gradient is 46-50 mm Hg, slight expansion of the aortic root (diameter 4.2 cm). There was a pronounced expansion of the ascending aorta (diameter 4.6-4.8 cm), slight pulmonary hypertension: systolic pulmonary artery pressure 36 mm Hg. Slight left ventricular hypertrophy was determined: myocardial mass index 107 g/m², slight enlargement of the left atrium (end-systolic volume 74 ml). The left ventricular ejection fraction was 66%. A pseudonormal type of transmitral blood flow was recorded.
Coronary angiography on March 6, 2021 revealed stenosis of the right coronary artery in the second segment up to 80%.
The patient was diagnosed:
Congenital heart defect: bicuspid aortic valve. Combined aortic disease: aortic stenosis of III-IV degree. Aortic insufficiency degree I. Arterial hypertension stage III risk 4.
Complications: Chronic heart failure stage IIa, functional class 2.
Treatment was prescribed: acecardol 100 mg once a day in the evening, cozaar 50 mg once a day in the morning, bisoprol 2.5 mg once a day in the evening.
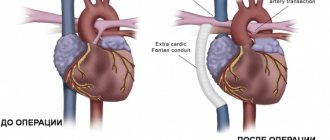
A council of cardiac surgeons recommended aortic valve replacement for the patient.
Discussion
The incidence of bicuspid aortic valve ranges from 0.6% to 2% [1]. The disease occurs 3 times more often in men than in women.
The frequency of detection of pathology in first-degree relatives of a patient is 9% [2]. In recent years, views on this pathology have changed significantly, and today, when talking about this defect, they mean valvulo- and aortopathy of hereditary origin, caused by molecular and histological changes in the connective tissue [3]. The genetic mechanisms leading to the formation of a bicuspid aortic valve are not well understood. Currently, NOTCH1 remains the only confirmed candidate gene whose mutation is associated with both familial and sporadic cases of bicuspid aortic valve with concomitant thoracic aortic aneurysm [4].
The clinical picture in patients with a bicuspid aortic valve can vary from severe manifestations of valve dysfunction in infancy to their complete absence in older people [1]. In the presence of aortic stenosis or aortic insufficiency, patients are bothered by headaches, fainting, and dizziness, as in our patient. Clinical manifestations may also be caused by dilation of the ascending aorta (aortopathy), coarctation of the aorta, and the development of infective endocarditis [2].
Signs of a bicuspid aortic valve can be detected by auscultation. In patients with a normally functioning bicuspid aortic valve, a systolic ejection murmur is heard at the apex of the heart and in the projection of the aorta. In children predominantly 7-11 years old, aortic systolic clicks can be detected in the 2nd intercostal space to the right of the sternum [5]. With aortic regurgitation, a diastolic murmur is heard above the aorta and at Botkin's point, and in the presence of aortic stenosis (as in our patient), a rough systolic murmur is heard above the aorta, conducted to the vessels of the neck.
The electrocardiogram is usually normal, but there may be signs of left ventricular hypertrophy. The mainstay of diagnosis is echocardiography [6].
Currently, the following types of surgical interventions can be performed in children with a bicuspid aortic valve due to its stenosis: balloon valvuloplasty, valve replacement with mechanical and biological prostheses, Ross operation and reconstructive plastic surgery on the aortic valve [7].
According to Petrushenko D. Yu. and co-authors, satisfactory results of treatment of critical aortic stenosis in newborns and children in the first months of life were reported both when performing balloon valvuloplasty and open reconstructive plastic surgery on the valve. Both procedures are palliative: sooner or later the patient will again need surgical treatment, since after balloon valvuloplasty valve insufficiency develops, and after open repair there is a risk of developing restenosis [7]. Aortic valve replacement is performed in children and adolescents in whom previous balloon valvuloplasty has resulted in aortic insufficiency. The operation is performed during the period when the growth of the aorta is completed [5]. Surgical treatment of bicuspid aortic heart disease in adults in tactical terms does not cause much discussion: unlike children, the issue of tactics and surgical techniques is much simpler to solve - the vast majority of patients require aortic valve replacement [3]. Patients with large aortas have a significant risk of mortality within 5 years due to aortic dissection or rupture, especially when the aortic diameter reaches 6 cm [8]. In case of bicuspid aortic valve, surgery on the ascending aorta is indicated in the case of: enlargement of the root or ascending aorta >55 mm; enlargement of the root or ascending aorta >50 mm and the presence of other risk factors; enlargement of the root or ascending aorta >45 mm during planned aortic valve replacement surgery. With planned replacement of the ascending aorta (including the aortic root), surgical mortality ranges from 1.6-4.8% [9].
It is known that in patients with Marfan syndrome, β-blockers and/or losartan can slow down the dilatation of the aortic root and reduce the risk of aortic complications. By analogy, it is common clinical practice to recommend these drugs to patients with a bicuspid aortic valve and enlargement of the root or ascending aorta [10]. Therefore, our patient was prescribed bisoprolol and cozaar.
In the presence of a bicuspid aortic valve, as with other heart defects, when performing invasive medical interventions accompanied by bacteremia, it is necessary to prescribe antibiotics to prevent infective endocarditis.
The prognosis for bicuspid aortic valve is generally favorable. However, more than half of all patients in the world who require aortic valve replacement are patients with this heart defect [5]. According to Pelekh D.M., who analyzed the outcomes of surgical treatment of patients with aortic stenosis, including patients with bicuspid valvulopathy, aortic valve replacement leads to good immediate and long-term results: a decrease in the size of the left chambers of the heart, a decrease in the severity of myocardial hypertrophy, and an improvement in myocardial contractility left ventricle and, consequently, the well-being of patients. Most patients progress to NYHA functional class 1–2. After surgery, acute cerebrovascular accidents, prosthesis thrombosis, and infective endocarditis may develop. Mortality in the early postoperative period ranges from 9.3 to 12.3% [11].
Conclusion
Bicuspid aortic valve is a common heart defect, the presence of which predisposes to severe complications. The disease may not appear clinically for a long time. To prevent the development of complications, it is necessary to timely identify this heart defect and carefully monitor the patient to timely determine indications for surgical treatment.
Structure and operation of semilunar valves
As I already said, semilunar valves are not flat petals, but three small semicircular pockets.
When the blood flow rushes from the ventricle into the artery (aorta or pulmonary artery), the empty and therefore flat “pockets” “fall” into the cavity of the vessel and allow the flow to pass in the desired direction: from the ventricle to the artery.
All this happens at the moment when the ventricle contracts, that is, at the moment of ventricular systole.
When the ventricle relaxes or its diastole occurs, blood from the artery tries to get back into the ventricular cavity. But, as soon as the blood flow reverses, the beautiful semilunar pockets fill with blood. They become large and voluminous, and therefore cover the hole. Thus, the path of blood in the opposite direction is closed.
Both the right and left semilunar valves have three “pockets”.
Human circulatory system
The structure of the human heart
What does a human heart look like?
Where is the human heart?
What's Below the Heart?
Heart cavity
How many valves are there in the heart?
Valve apparatus of the heart
Structure of the heart wall
Wall of the heart: pericardial sac
Heart wall, myocardium
General understanding of anatomical structures
The valve apparatus consists of many structures that prevent the retrograde movement of blood.
It also contains semilunar valves or flaps. Outwardly, they resemble pockets located on the inner walls of blood vessels. The semilunar valves are located at the exit of the pulmonary artery, as well as the aorta from the ventricles. As soon as blood is ejected into the pulmonary artery, aorta, these structures are pressed against the walls of the vessels.
The blood that has flowed into the pockets fills them, and they close tightly. Therefore, when the ventricles relax, blood does not return to the heart. In this way, the movement of matter is maintained strictly in one direction.
The pockets in question do not have chordae tendineae. Moreover, such connections are more reminiscent of vein pockets than atrioventricular valves.
Pathologies
If the integrity or function of the valves is compromised as a result of injury or inflammation, pathological conditions develop that must be recognized and proper therapy initiated (acute or long-term cardiac failure due to damage to the heart valves).
A well-known vein pathology associated with valve dysfunction is varicose veins of the legs, which is dangerous for its complications (thrombophlebitis, swelling of the legs, blockage of the pulmonary artery). Currently, modern medicine has a number of effective methods for restoring the normal functioning of the body, in particular, the valves in question. Naturally, in each case an individual approach to the sick person is determined.
Thus, we looked at the functions of the semilunar valves, their role in the overall functioning of the human body, the problems that can cause disorders and pathologies associated with the valves.
Operating principle
By definition, a crescent valve prevents blood from returning to the ventricles of the heart. When the myocardium contracts, blood in the ventricle area begins to move in several directions to the area:
- semilunar valves;
- atria.
As soon as the blood encounters the atrioventricular structures, the valves close. The pressure in the ventricles and on the falciform pouch increases. Therefore, the blood flow is directed to the aorta, and from the cavity of the right ventricle to the area of the pulmonary trunk. In this situation, the semilunar pouches will be open and the flap pouches will be closed.
When the blood flow is directed from the left stomach to the aorta, the fluid presses the semilunar pouches against the aortic wall.
After relaxation of the ventricles, the blood flow entering the artery tends to return to the left ventricle.
The arterial sinuses become filled with blood, and the crescent-shaped pouches of the aorta close. Therefore, blood flow does not flow back into the ventricle area.
The semilunar pulmonary recess functions according to the same scheme.
Types of heart valves
- The first group is the structures separating the ventricles and atria.
- The second group is the valves located at the junction of the aorta and the trunk of the pulmonary artery, in the area where these vessels depart from the ventricles of the heart.
The aortic and pulmonary valves have the following structures:
1. Semilunar valves (semilunar valves of the heart).
2. Spaces between the flaps (inter-flap triangles).
3. Sinuses.
4. Fibrous rings (the existence of which is debated).
Semilunar valves
Since only the flaps included in these valves have a semilunar shape, it is correct to call these valves the aortic valve and the semilunar valve of the pulmonary trunk. Both valves have three flaps. The aortic valve has right, left and posterior valves. And the pulmonary valve has an anterior instead of a posterior one.
The dimensions of the valves differ for people of different ages, and there are also individual differences. As a rule, the semilunar valves of the aorta are wider in width than the aortic sinuses, and, on the contrary, they are smaller in height. This structure helps them move down and close the valve when they are filled with blood. The openings of the coronary arteries are located in the aortic sinuses.
The semilunar valves are located near the annulus fibrosus. They are formed by a fold of the endocardium. There are front, left and right crescent flaps. Their ventral edges are connected to the ends of the sinuses. The valves and sinuses form the sockets. The crescent valves are slightly larger in size than the sinuses of the pulmonary trunk.
Vessel valves
Semilunar valves in blood vessels perform the same role as in the heart. They interfere with retrograde blood flow. Located in vessels that are not endowed with a well-developed muscular layer, these are lymphatic vessels and veins.
Most of these valves are found in the veins of the lower extremities. For example, the great saphenous vein of the leg contains more than 8 valves. Why? Because the veins are not endowed with a muscular membrane, blood under the influence of gravity will stagnate in the lower extremities. Semilunar valves prevent blood stagnation in the lower extremities, and not only in them.
Source