Availability of cardiac and vascular implants and grafts (Z95)
Excludes: complications due to cardiac and vascular devices, implants and grafts (T82.-)
Excludes: installation and adjustment of artificial cardiac pacemaker (Z45.0)
Presence of a coronary artery prosthesis
Condition after coronary angioplasty NOS
Presence of intravascular prosthesis NEC
Condition after peripheral angioplasty NOS
ICD-10 alphabetical indexes
External Causes of Injury - The terms in this section are not medical diagnoses, but rather a description of the circumstances under which the event occurred (Class XX. External Causes of Morbidity and Mortality. Heading Codes V01-Y98).
Medicines and chemicals - table of medicines and chemicals that have caused poisoning or other adverse reactions.
In Russia, the International Classification of Diseases
10th revision (
ICD-10
) was adopted as a single normative document for recording morbidity, reasons for the population’s visits to medical institutions of all departments, and causes of death.
ICD-10
introduced into healthcare practice throughout the Russian Federation in 1999 by order of the Russian Ministry of Health dated May 27, 1997 No. 170
The release of the new revision (ICD-11) is planned by WHO in 2022.
Abbreviations and symbols in the International Classification of Diseases, 10th Revision
NOS
- without further clarification.
NEC
— not classified in other categories.
†
— code of the main disease. The main code in the dual coding system contains information about the underlying generalized disease.
*
- optional code. An additional code in the double coding system contains information about the manifestation of the main generalized disease in a separate organ or area of the body.
Source
general information
Short description
Protocol name: Supportive rehabilitation “Supportive rehabilitation”, profile “Cardiology and Cardiac Surgery”, (adults). Protocol code
ICD-10 code(s): I10 Essential [primary] hypertension I11.0 Hypertensive [hypertensive] disease with predominantly cardiac involvement with (congestive) heart failure I11.9 Hypertensive [hypertensive] disease with predominantly cardiac involvement without (congestive) heart failure I12 Hypertensive [hypertensive] disease with predominant kidney damage I12.0 Hypertensive [hypertensive] disease with predominant kidney damage with renal failure I12.9 Hypertensive [hypertensive] disease with predominant kidney damage without renal failure I13 Primary arterial hypertension and renal hypertension I13.0 Hypertensive [hypertensive] disease with predominant damage to the heart and kidneys with (congestive) heart failure I13.1 Hypertensive [hypertensive] disease with predominant damage to the kidneys with renal failure I13.2 Hypertensive [hypertensive] disease with predominant damage to the heart and kidneys with (congestive) heart failure and renal failure I13.9 Hypertensive [hypertensive] disease with predominant damage to the heart and kidneys, unspecified I05 Rheumatic diseases of the mitral valve I05.0 Mitral stenosis I05.1 Rheumatic mitral valve insufficiency I05.2 Mitral stenosis with insufficiency I05.8 Other mitral diseases valve I05.9 Mitral valve disease, unspecified I06 Rheumatic diseases of the aortic valve I06.0 Rheumatic aortic stenosis I06.1 Rheumatic aortic valve insufficiency I06.2 Rheumatic aortic stenosis with insufficiency I06.8 Other rheumatic diseases of the aortic valve I06.9 Rheumatic aortic valve disease, unspecified I07 Rheumatic diseases of the tricuspid valve I07.0 Tricuspid stenosis I07.1 Tricuspid insufficiency I07.2 Tricuspid stenosis with insufficiency I07.8 Other diseases of the tricuspid valve I07.9 Tricuspid valve disease, unspecified I08 Lesions of several valves I08.0 Combined lesions of the mitral and aortic valves I08 .1 Combined lesions of the mitral and tricuspid valves I08.2 Combined lesions of the aortic and tricuspid valves I08.3 Combined lesions of the mitral, aortic and tricuspid valves I08.8 Other multiple valve diseases I08.9 Multiple valve lesions, unspecified I21 After acute myocardial infarction I20.0 Unstable angina I20.1 Angina with documented spasm I20.8 Angina, functional class II–II (after hospital treatment) I22 Repeated myocardial infarction I42.0 Dilated cardiomyopathy I42.1 Obstructive hypertrophic cardiomyopathy I42.1 Other hypertrophic cardiomyopathy I42.3 Endomyocardial (eosinophilic) disease I42.4 Endocardial fibroelastosis I42.5 Other restrictive cardiomyopathy I42.7 Cardiomyopathies Q21.0 Acute transmural infarction of the anterior wall of the myocardium Q21.1 Acute transmural infarction of the inferior wall of the myocardium Q25.0 Patent ductus arteriosus Q25.8 Other congenital anomalies of large arteries Q21.2 Primary pulmonary hypertension/Secondary pulmonary hypertension
Q20-28 Non-rheumatic heart valve defects Z95 After heart surgery (coronary artery bypass grafting, coronary angioplasty and stenting)
Abbreviations used in the protocol: AH - arterial hypertension ALT - alanine aminotransferase AST - aspartate aminotransferase AMI - acute myocardial infarction HF - heart failure SRBP - C-reactive protein TSH - thyroid-stimulating hormone FC - functional class ECG - electrocardiography EchoCG - echocardiography PROBNP - natriuretic B-type peptide
Date of development of the protocol: 2014.
Patient category: adults.
Source
Publications in the media
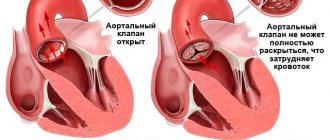
Aortic valve stenosis is a heart defect in the form of narrowing of the aortic opening due to pathology of the aortic valve and perivalvular structures.
Frequency • Isolated aortic valve stenosis - 1.5–2% of acquired valve defects; Aortic valve stenosis in combination with other defects - 23% • Congenital aortic stenosis - 3–5.5% of all congenital heart defects; in combination with other congenital disorders - 13% • The predominant gender is male.
Genetic aspects. Elastin gene defects (130160) in Williams syndrome (see Miscellaneous syndromes) and Eisenberg syndrome (*185500, supravalvular stenosis of the aorta, pulmonary arteries, peripheral arteries).
Etiology • Congenital anomalies, including cusp number anomalies and congenital isolated aortic valve calcification syndrome (61.3%) • Rheumatism (12.6%) • Atherosclerosis (24.2%) • Adult idiopathic calcification (1.9%).
Pathophysiology • Increased afterload ® increased left ventricular (LV) wall tension ® concentric LV hypertrophy ® decreased muscle fiber tension [according to Laplace's law: wall tension = (pressure´ radius)/(2 ´ wall thickness)] • This mechanism maintains systolic function, despite an increase in systolic pressure in the left ventricle • Further myogenic dilatation leads to decompensation and rapid progression of circulatory failure • A sharp decrease in cardiac output leads to insufficient blood supply to the brain, which is manifested by transient or permanent ischemia • If aortic valve insufficiency is associated with aortic valve stenosis, to the increased afterload, an increase in preload is added, which leads to an even greater increase in LV wall tension and a decrease in effective ejection • As a result of the discrepancy between perfusion pressure in the coronary bed and, accordingly, the volume of coronary blood flow to the needs of the hypertrophied myocardium, relative coronary insufficiency develops • With aortic atherosclerosis, the frequency of concomitant IHD, which makes diagnosis difficult • All of these factors lead to a high risk of sudden death • Late manifestations of the defect - hypertension in the pulmonary circulation.
Classification . Depending on the severity (determined by the systolic pressure gradient [SPG] between the LV and the aorta and the area of the valve orifice), four degrees of stenosis are distinguished: degree I - moderate stenosis, GSD <50 mm Hg, valve orifice area >1 cm2 ( norm 2.5–3.5 cm2); II degree - severe stenosis, GSD 50–80 mm Hg, valve opening area 1–0.7 cm2; III degree - severe stenosis, GSD >80 mm Hg; IV degree - critical stenosis, GSD above 80 mm Hg, valve opening area 0.7–0.5 cm2.
Clinical picture and diagnosis
• Complaints: pain in the heart (angina), fainting and shortness of breath are classic symptoms of aortic stenosis.
• Peripheral symptoms are caused by small output syndrome •• Pallor of the skin •• Tachycardia •• Low slow pulse •• “Heart hump” (with the development of the defect in childhood).
• Valvular symptoms •• Systolic trembling in the sternum, in the jugular and supraclavicular fossae •• Weakening of the aortic component of the II tone with severe calcification and decreased mobility of the valves •• With preserved mobility of the valves - increased II tone or click •• With relative mobility of the valves following I tone is used to listen to the systolic ejection sound, which occurs when the movement of the valves stops •• A rough, loud, high-frequency fusiform ejection noise, very well carried to the shoulders, shoulder blades, back of the head and, especially, to the great vessels (the volume of the noise is sometimes so high that it is even carried out on the chair on which the patient is sitting) •• The duration of the noise reflects the severity of the obstruction to a greater extent than its intensity •• Sirotinin-Kukoverov symptom - increased noise when raising the arms up •• With severe calcification of the valves, high-frequency components of the noise can be carried out in the axillary region or to the apex of the heart in the form of a soft mid-systolic murmur, simulating the murmur of mitral regurgitation - Galawarden's symptom.
• Left ventricular symptoms are caused by hypertrophy, dilatation and insufficiency of the pumping and contractile functions of the LV •• Long-lasting, but not diffuse apical impulse, shifted to the left, and a palpable IV tone are almost always present •• An increase in the area of relative dullness of the heart to the left •• Auscultatory signs of congestion in the lungs - diffuse moist rales of various sizes, best heard in the basal regions.
• Symptoms of the underlying disease: rheumatism, atherosclerosis, congenital heart disease.
Special studies
• ECG: signs of hypertrophy and overload of the left sections, primarily the LV; signs of myocardial ischemia.
• Sphygmography: a prolonged low pulse waveform with a difficult-to-define dicrotic notch and jagged apex—a symptom of a “cockscomb.”
• X-ray of the chest organs •• Cardiomegaly (cardiothoracic index exceeds 50%) due to expansion of the LV arch •• Signs of pulmonary congestion •• Expansion of the aortic arch due to its poststenotic dilatation •• Calcification of the leaflets and fibrous ring of the aortic valve.
• EchoCG •• Expansion of the cavity and hypertrophy of the LV myocardium •• Violation of local and global systolic, as well as diastolic functions of the LV •• Poststenotic expansion of the ascending aorta •• Damage to the cusps and apparatus of the aortic valve (defects, vegetations, abnormalities in the number of cusps, increased echogenicity and acoustic shadow with calcification, decrease in the amplitude of movement of the valves) •• In Doppler mode, high-speed flow is recorded through the narrowed opening of the aortic valve and the peak systolic and average pressure gradients between the LV and the aorta are determined •• The area of the aortic valve opening is measured in Doppler and B-modes (as a basis For diagnosis, the worst indicator is taken).
• Catheterization of the left and right ventricles and aorta: direct pressure measurement; see also Aortic valve insufficiency.
• Left ventriculography, ascending aortography •• The presence and degree of stenosis is determined by the diameter of the jet of contrast agent expelled from the LV •• The presence of zones of hypo- and akinesia of the LV indicates myocardial ischemia •• Combined valvular lesions are also diagnosed.
• Coronary angiography: see Aortic valve insufficiency.
TREATMENT
• Drug therapy •• The possibilities of conservative treatment are limited, since it has little effect on the functional class and mortality •• Regardless of the severity of aortic stenosis, infective endocarditis is prevented (see Infectious endocarditis) •• LV systolic dysfunction with congestive heart failure - cardiac glycosides, diuretics , treatment of atrial fibrillation, dual-chamber pacemaker, vasodilators - in small doses and under monitor control (can increase GDM) •• For refractory heart failure - IV inotropes •• Intra-aortic balloon counterpulsation is used as a backup method of hemodynamic stabilization in preparation for surgery •• Termination of pregnancy is indicated.
• Surgical treatment •• Indications ••• Aortic valve stenosis grade III–IV, accompanied by clinical symptoms ••• Asymptomatic aortic stenosis with LV dysfunction ••• There is no consensus on whether surgical treatment is indicated for severe asymptomatic aortic stenosis with normal function LV •• Contraindications: see Aortic valve insufficiency •• Methods of surgical treatment ••• Balloon valvuloplasty - radical treatment for a congenital unicuspid or bicuspid aortic valve, preparation for replacement in case of cardiogenic shock, patient refusal or impossibility of replacement in the near future (for example, with pregnancy, in elderly and debilitated patients) ••• Aortic valve replacement with a mechanical artificial heart valve under conditions of artificial circulation ••• Biological prostheses are not used.
Specific postoperative complications • Restenosis • Angina pectoris • Hemolytic anemia • Secondary infective endocarditis of the prosthesis • Thromboembolism • Aneurysms of the ascending aorta when using disc prostheses with a small opening angle.
Course and prognosis • In the natural course, the 5-year survival rate does not exceed 25%, and the 10-year survival rate does not exceed 10% • The average life expectancy after the onset of angina attacks is 3.5 years, after the onset of syncope episodes - 2 years, after the onset of LV failure - 1.5 years, after the onset of generalized heart failure - 7 months • Sudden death is recorded in 15–20% of patients with clinical manifestations • With GDM above 50 mm Hg. 17% of patients survived 3 years • With surgical treatment, hospital mortality is 3–8%, 9-year survival exceeds 85% with initially preserved function and 10–25% with LV dysfunction • In general, the prognosis after aortic valve replacement with its stenosis is better, than with insufficiency • After valvuloplasty for isolated calcification and rheumatism, despite a decrease in GDM and the area of the aortic valve orifice, in most cases severe aortic valve stenosis persists, postoperative mortality reaches 6%, mortality within a year is 25%, restenosis rate is 60% within 6 months.
Synonym. Aortic stenosis, aortic stenosis.
Abbreviations • GSD - systolic pressure gradient • LV - left ventricle.
ICD-10 • I06 Rheumatic diseases of the aortic valve • I35 Non-rheumatic lesions of the aortic valve
general information
Short description
Approved by the Joint Commission on the Quality of Medical Services of the Ministry of Health of the Republic of Kazakhstan dated July 13, 2020 Protocol No. 111
Congenital heart defects are abnormalities in the structure and (or) function of the cardiovascular system , resulting from a violation of its embryonic development.
Protocol title: CONGENITAL HEART DEFECTS IN ADULTS
| ICD-10 | |
| Code | Name |
| Q20 | Congenital anomalies [malformations] of the heart chambers and connections |
| Q20.0 |
|
| Q20.1 |
|
| Q20.2 |
|
| Q20.3 |
|
| Q20.4 |
|
| Q20.5 |
|
| Q20.6 |
|
| Q20.8 |
|
| Q20.9 |
|
| Q21 | Congenital anomalies [malformations] of the cardiac septum |
| Q21.0 |
|
| Q21.1 |
|
| Q21.2 |
|
| Q21.3 |
|
| Q21.4 |
|
| Q21.8 |
|
| Q21.9 |
|
| Q22 | Congenital anomalies [malformations] of the pulmonary and tricuspid valves |
| Q22.0 |
|
| Q22.1 |
|
| Q22.2 |
|
| Q22.3 |
|
| Q22.4 |
|
| Q22.5 |
|
| Q22.6 |
|
| Q22.8 |
|
| Q22.9 |
|
| Q23 | Congenital anomalies [malformations] of the aortic and mitral valves |
| Q23.0 |
|
| Q23.1 |
|
| Q23.2 |
|
| Q23.3 |
|
| Q23.4 |
|
| Q23.8 |
|
| Q23.9 |
|
| Q24 | Other congenital anomalies [malformations] of the heart |
| Q24.0 |
|
| Q24.1 |
|
| Q24.2 |
|
| Q24.3 |
|
| Q24.4 |
|
| Q24.5 |
|
| Q24.8 |
|
| Q24.9 |
|
| Q25 | Congenital anomalies [malformations] of large arteries |
| Q25.0 |
|
| Q25.1 |
|
| Q25.2 |
|
| Q25.3 |
|
| Q25.4 |
|
| Q25.5 |
|
| Q25.6 |
|
| Q25.7 |
|
| Q25.8 |
|
| Q25.9 |
|
| Q26 | Congenital anomalies [malformations] of large veins |
| Q26.0 |
|
| Q26.1 |
|
| Q26.3 |
|
| Q26.4 |
|
Protocol development/revision date: 2021 (2021 revision)
Users of the protocol: cardiac surgeons, therapists, cardiologists, arrhythmologists, general practitioners.
Patient category: adults.
Level of evidence scale:
| A | A high-quality meta-analysis, systematic review of RCTs, or large RCTs with a very low probability (++) of bias, the results of which can be generalized to an appropriate population. |
| IN | High-quality (++) systematic review of cohort or case-control studies, or high-quality (++) cohort or case-control studies with very low risk of bias, or RCTs with low (+) risk of bias, the results of which can be generalized to an appropriate population . |
| WITH | Cohort or case-control study or controlled trial without randomization with low risk of bias (+). The results of which can be generalized to the relevant population or RCTs with very low or low risk of bias (++ or +), the results of which cannot be directly generalized to the relevant population. |
| D | Case series or uncontrolled study or expert opinion. |
Clinic automation: fast and inexpensive!
300 clinics from 4 countries connected
— 800 RUB / 4500 KZT / 27 BYN — 1 workplace per month
Clinic automation: fast and inexpensive!
- 300 clinics from 4 countries connected
- 1st place - 800 RUB / 4500 KZT / 27 BYN per month
I'm interested! Contact me
Minimally invasive aortic valve replacement surgery
Return to section:
Cardiology department
The blood expelled by the heart enters the aorta. The aorta is the largest vessel in the human body, through which blood is directed to organs and tissues. The aorta is connected to the heart by the aortic valve. By opening, this valve facilitates the outflow of blood from the heart; when closing, it prevents blood from penetrating back into the heart chambers.
Surgery to replace (prosthetic) the aortic valve may be required if dysfunction (malfunction) of the valve occurs. This, in turn, may be due to two reasons:
- The aortic valve narrows and does not allow the required amount of blood to pass through, i.e. Blood flow to the body decreases - this is called “aortic stenosis.” Among the main causes are age-related degenerative changes and rheumatism.
- The valve “leaks”, does not hold blood, and blood leaks back into the heart cavity - this is called “aortic regurgitation”. The main causes: congenital genetic defects, age-related enlargement of the aorta and valve ring, aortic diseases and bacterial damage to the valve.
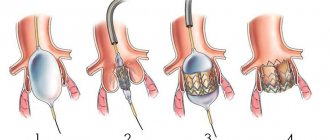
Aortic valve replacement surgery involves removing the failed valve and replacing it with an artificial one. The operation is performed under conditions of artificial circulation, i.e. in a stopped heart, when the function of blood supply to organs and tissues is taken over by a special device. During the operation, the patient is under anesthesia and does not feel pain.
The first operation of aortic valve replacement with an artificial valve was performed in the early 1960s. Traditionally, the operation is performed through a 20 cm long midline incision in the sternum. To reach the heart, it is necessary to cut through the skin, soft tissue, the bone located in the middle of the chest, called the sternum, and the special lining of the heart, the pericardium. The next step is to connect the heart-lung machine by sequentially connecting the aorta and cardiac veins to it, and then temporarily stop the heart. Next, the surgeon dissects the aorta in a transverse direction to see the aortic valve. If the valve is severely damaged, it is removed and a new, artificial heart valve is sewn in its place. Artificial valves are divided into two types:
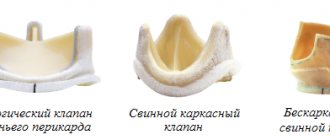
- Mechanical (metal-carbon): durable, can work effectively for decades, but requires lifelong use of drugs that reduce blood clotting (anticoagulants), which increases the risk of bleeding;
- Biological (created from animal or human tissue): more physiological, but less durable, lasting no more than 20 years, but does not require lifelong use of anticoagulants.
| Modern types of artificial heart valves: on the left – mechanical; on the right is biological. | |
After installing a new valve, the integrity of the aorta, sternum, soft tissue of the chest and skin is restored in turn. The duration of the operation is 2.5 – 3 hours.
| Operational access scheme. Full median sternotomy (left), J-shaped ministernotomy (right). | |
Modern knowledge and experience make it possible to reduce the size of the chest wound to perform aortic valve replacement surgery. Such operations are called minimally invasive, i.e. low-traumatic - through reduced access. The use of small accesses was proposed in the mid-1990s.
The technique of surgical intervention remains the same, but the length of the incision is only 7 centimeters, and you can do without a complete dissection of the sternum (full median sternotomy), which can significantly reduce the trauma of the operation, the severity of pain in the postoperative period, preserve the skeletal function of the chest, and speed up the recovery of the respiratory system. functions and return to normal life while maintaining high effectiveness of treatment.
The undoubted advantage for the patient with this operation is the cosmetic result.
Unlike traditional surgery, minimally invasive surgeries take slightly longer (3 – 3.5 hours).
Rice. 3 . Appearance of the chest wound after traditional (left) and minimally invasive surgery (right).
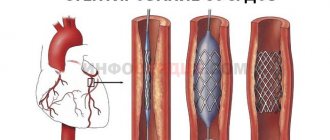
Over the past decade, a new, unique method of aortic valve replacement has been developed: Transcatheter Aortic Valve Implantation (TAVI).
This procedure is performed in a cath lab with a team consisting of cardiologists, cardiac surgeons, and x-ray surgery specialists. Most importantly, this procedure is performed on a beating heart, thus avoiding the risks associated with cardiopulmonary bypass.
An incision 3 cm long is made on the side of the chest as shown in Figure 4. In this case, the intervention is called transapical catheter valve implantation.
Rice. 4 . Access scheme for transapical catheter implantation of the aortic valve (Cheung, Lichtenstein, Annals of Cardiothoracic Surgery, 2012).
Two special hemostatic sutures are placed in the area near the apex of the heart, through which a high-tech device is passed - a delivery system, the diameter of which is only 8 mm. This system contains a biological valve in a “collapsed” state.
The valve is positioned at the required level under the control of an X-ray machine, which can rotate around the patient on a special arc, making it possible to obtain X-ray images from different angles.
Next, the artificial valve opens, occupying a predetermined position in the aortic root. The valve can be opened either using a balloon inflated with helium (see Fig. 5), or independently - for valves with a frame made of shape memory metal called “Nitinol” (an alloy of nickel and titanium).
| Opening an artificial valve using a balloon | View of the valve installed in the aortic position during transapical catheter implantation |
| (Cheung, Lichtenstein, Annals of Cardiothoracic Surgery, 2012). | |
Rice. 6 . Biological valve for implantation in the aortic position using a transcatheter method.
It should be noted, however, that there are strict indications for such an operation. Transcatheter valve implantation is performed for those patients who are contraindicated for traditional surgical intervention due to high risk (they may not tolerate traditional surgery): these are patients with critical aortic valve stenosis, usually elderly (over 80 years old), patients who have undergone coronary artery bypass surgery, patients with diseases of the aorta, which create technical difficulties when accessing the heart and valve from the classical approach.
The transcatheter implantation operation takes about 40 - 60 minutes, and the recovery period after it is much shorter compared to traditional surgery.
At the Department of Cardiac Surgery of City Hospital No. 40, all considered types of correction of acquired aortic valve defects are used.
← Back
Classification
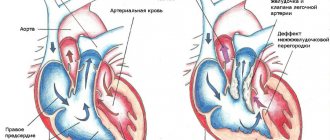
Several classifications of congenital heart defects have been proposed, common to them is the principle of subdividing defects according to their effect on hemodynamics. The most general systematization of defects is characterized by combining them, mainly according to their effect on pulmonary blood flow, into the following 4 groups. I. Defects with unchanged (or slightly changed) pulmonary blood flow: anomalies in the location of the heart, anomalies of the aortic arch, its coarctation, aortic stenosis; pulmonary valve insufficiency; mitral stenosis, atresia and valve insufficiency; triatrial heart, defects of the coronary arteries and conduction system of the heart.
II. Defects with hypervolemia of the pulmonary circulation: 1) not accompanied by early cyanosis - patent ductus arteriosus, atrial and ventricular septal defects, Lutambashe syndrome, aortopulmonary fistula; 2) accompanied by cyanosis - tricuspid atresia with a large ventricular septal defect, patent ductus arteriosus with severe pulmonary hypertension and blood flow from the pulmonary trunk to the aorta.
III. Defects with hypovolemia of the pulmonary circulation : 1) not accompanied by cyanosis - isolated stenosis of the pulmonary trunk; 2) accompanied by cyanosis - triad, tetralogy and pentade of Fallot, tricuspid atresia with narrowing of the pulmonary trunk or small ventricular septal defect, Ebstein's anomaly (displacement of the tricuspid valve leaflets to the right ventricle), hypoplasia of the right ventricle.
IV. Combined defects with disruption of the relationship between various parts of the heart and large vessels: transposition of the aorta and pulmonary trunk (complete and corrected), their origin from one of the ventricles, Taussig-Bing syndrome, common truncus arteriosus, three-chambered heart with a single ventricle, etc.
Diagnostics
DIAGNOSTIC METHODS, APPROACHES AND PROCEDURES
Diagnostic criteria: Clinical manifestations depend on the type and severity of the heart defect. Clinical manifestations of congenital heart disease can be combined into 4 syndromes:
- Cardiac syndrome (complaints of pain in the heart, shortness of breath, palpitations, interruptions in heart function, etc.; upon examination - pallor or cyanosis, swelling and pulsation of the vessels of the neck, deformation of the chest like a cardiac hump; palpation - changes in blood pressure and characteristics of the peripheral pulse, changes in the characteristics of the apex impulse with hypertrophy/dilatation of the left ventricle, the appearance of a cardiac impulse with hypertrophy/dilatation of the right ventricle, systolic/diastolic “cat purr” with stenosis; percussion - expansion of the boundaries of the heart according to the expanded sections; auscultation - changes in rhythm, strength , timbre, solidity of tones, the appearance of noise characteristic of each defect, etc.).
- Heart failure syndrome (acute or chronic, right or left ventricular, dyspnea-cyanotic attacks, etc.) with characteristic manifestations.
- Chronic systemic hypoxia syndrome (growth and development retardation, symptoms of drumsticks and watch glasses, etc.)
- Respiratory distress syndrome (mainly with congenital heart disease with enrichment of the pulmonary circulation).
Differential diagnosis
Differential diagnosis and rationale for additional studies
Additional laboratory tests:
- Tests for hepatitis B, C, HIV, microreaction (to exclude infectious pathology).
Additional instrumental studies:
- Daily ECG monitoring (Holter monitoring) Standard Holter ECG monitoring has diagnostic meaning only in the presence of symptoms, probably associated with the presence of arrhythmias (subjective sensations of interruptions, accompanied by dizziness, fainting, history of syncope, etc.).
- Multislice CT angiography/MRI is an additional noninvasive imaging modality if echocardiography results are inconclusive. Direct visualization of the defect and pulmonary veins is possible, RV volume and function can be measured, and shunt volume can be assessed.
- An exercise test may be useful to determine a patient's exercise capacity when symptoms are inconsistent with clinical findings and to document changes in oxygen saturation in patients with pulmonary arterial hypertension.
- Coronary angiography is used to identify coronary anomalies or arterial obstruction, and men 35 years of age and older, premenopausal women 35 years of age and older with risk factors for atherosclerosis, and postmenopausal women should undergo cardiac catheterization and coronary angiography to detect CAD before cardiac surgery.
| Diagnosis | Rationale for differential diagnosis | Surveys | Diagnosis exclusion criteria |
| BEAM | High pulmonary hypertension | angicardiography | |
| Coronary pulmonary fistulas | angicardiography | ||
| Rupture of sinus of Valsalva | ECHOCG |
Aortic stenosis
The prevalence of aortic stenosis (AS), according to various authors, ranges from 3–4 to 7%. With age, the frequency of detection of this defect increases, amounting to 15–20% in people over 80 years of age. Moreover, with increasing life expectancy, the incidence of AS in the population will also increase. It is more often observed in males (2.4:1), but in the older age subgroup women predominate. Over the past 30 years, the etiology of aortic valve disease has changed. While the prevalence of post-rheumatic lesions of the aortic valve (AV) decreased from 30 to 18%, the frequency of surgical correction of bicuspid aortic valves - from 37 to 33%, there was an increase in the frequency of calcified aortic stenosis (CAS) from 30 to 46%, especially in individuals over 65 years old. This circumstance causes difficulties in diagnosis, consisting in the blurring of clinical symptoms (due to the presence of associated diseases, especially in the elderly) and difficulties in interpreting the results of instrumental studies. At the same time, timely detection of symptoms of the disease is the cornerstone of the management of patients with AS, since their appearance sharply accelerates the progression of the defect, aggravates the condition of patients and significantly reduces the average life expectancy and quality of life. Also, the appearance of AS symptoms is an absolute indication for cardiac surgical correction of the defect.
Classification of aortic stenosis
AS is divided by origin into congenital and acquired, by the volume of damage - into isolated and combined, by localization - into valvular, supravalvular, subvalvular or caused by hypertrophic cardiomyopathy.
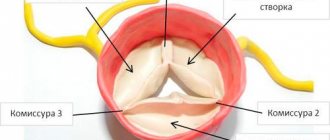
Congenital malformations of aortic valves may include unicuspid, bicuspid, or tricuspid valves or the presence of a dome-shaped diaphragm.
- The unicuspid valve causes severe obstruction already in infancy and is the cause of death in children under 1 year of age.
- Stenosis of the congenital bicuspid valve leads to the appearance of turbulent blood flow, traumatic to the valve leaflets, which subsequently leads to fibrosis, increased stiffness and calcification of the leaflets and narrowing of the aortic opening in adults.
- A congenitally altered tricuspid valve is characterized by the presence of leaflets of uneven size with signs of fusion along the commissures. However, turbulent blood flow caused by a mild birth defect can lead to fibrosis and ultimately to calcification and AS.
Among the acquired forms of AS there are:
- Rheumatic AS as a consequence of the inflammatory process, accompanied by fusion of commissures, vascularization of the leaflets and annulus fibrosus, which leads to the development of marginal fibrosis. Rheumatic valve disease is characterized by the presence of both AS and regurgitation. Other signs of the rheumatic process are often found in the heart, in particular damage to the mitral valve.
- CAS, which develops in the elderly, is caused by both mechanical wear of the valve and the presence of long-term inflammation with infiltration of the leaflets by macrophages and T-lymphocytes, followed by deposition of calcium pyrophosphate crystals in the fibrous ring, leading to narrowing of the aortic opening and spreading to the valve leaflets. Among the causes of the inflammatory response, the most frequently mentioned are oxidized LDL (by analogy with atherosclerosis) and infectious agents (Chlamydia pneumoniae), which can serve as triggers of the “response to injury” and form primary “nests of calcification.” Under the influence of activation of osteogenesis markers (constitutively expressed) and collagen remodeling in the AC leaflets, myofibroblasts acquire osteoblastic functions. Another source of osteogenesis of the endochondral type may be pluripotent mesenchymal cells circulating in the bloodstream and penetrating into the thickness of the AV valves through damage in the endothelial layer. Under these conditions, macrophages and T-lymphocytes serve as factors of neo-osteoclastic resorption. Additional regulators of the processes occurring are vitamin D, parathyroid hormone (PTH) and the state of bone metabolism, which undergo significant changes in old age and lead to D deficiency, hyperparathyroidism and osteoporosis. All of the above contributes to the formation of mature bone tissue with the presence of microfractures, functioning bone marrow and signs of bone remodeling in the thickness of the AC valves and allows us to consider AC calcification in patients with CAS as a regenerative rather than a degenerative process.
Pathophysiology
In response to the occurrence of mechanical obstruction of blood expulsion and an increase in systolic tension of the LV wall, its concentric hypertrophy develops, which allows creating an additional pressure gradient on the AV without reducing cardiac output, expanding the LV cavity, and is not accompanied by clinical symptoms. Over time, given the heterogeneous nature of hypertrophied myocytes and the increase in the severity of mechanical obstruction, left ventricular failure occurs due to the expansion of the chambers of the left chambers of the heart and the development of venous congestion in the pulmonary circulation. In the later stages of the disease, there is a decrease in cardiac output, stroke volume, and, accordingly, the pressure gradient.
Patients with AS are characterized by a negative correlation between systolic wall stress and ejection fraction (EF), which causes a reflex decrease in the latter in some patients due to “uncoordinated afterload.” In other cases, the reason for the decrease in EF is a decrease in LV contractility. Thus, increased afterload and altered contractility contribute to worsening LV systolic function.
Along with an increase in the collagen content in the myocardium, which is characteristic of many cardiac diseases, in AS there is a change in its transverse striation, which leads to an increase in myocardial mass, an increase in diastolic stiffness and impaired diastolic function, as a result of which greater intracavitary pressure is required to fully fill the LV chambers. Clinically, this is associated with the sudden development of episodes of pulmonary edema in patients with AS without obvious precipitating factors.
Often in real clinical practice, when conducting an ECG study, signs are revealed that indicate the presence of “myocardial ischemia”, consisting of ST segment depression and/or T wave inversion without concomitant arterial hypertension (AH), which causes overdiagnosis of coronary heart disease (CHD) . This largely depends on the characteristics of coronary blood flow in patients with AS, manifested by an increase in its absolute values, however, the relative parameters (calculated for the mass of the hypertrophied LV) are within normal limits.
Further progression of LVH can lead to impaired myocardial oxygenation in patients with critical AS, even in the absence of significant changes in the coronary arteries. The main substrate of myocardial ischemia in AS, as in other heart diseases, is an imbalance between oxygen consumption and the possibility of its delivery.
The increase in myocardial oxygen demand is due to:
- increase in myocardial mass due to LV hypertrophy;
- increased systolic tension of the LV wall;
- prolongation of the time of expulsion of blood from the LV cavity.
Impaired oxygen delivery through the coronary arteries is caused by:
- excess pressure compressing the coronary arteries from the outside over the perfusion pressure inside the coronary vessels;
- shortening of diastole.
Additional factors that reduce LV myocardial perfusion are:
- relative decrease in capillary density;
- an increase in end-diastolic pressure in the LV cavity, leading to a decrease in perfusion pressure in the coronary arteries.
Clinical manifestations of AS
During AS in adults, there is a long latent period, during which a gradual increase in obstruction and pressure overload of the LV myocardium occurs in the complete absence of any symptoms. Cardiac manifestations of acquired AS usually appear in the fifth or sixth decade of life and are presented in the form of angina, syncope, shortness of breath and, ultimately, heart failure.
Angina pectoris occurs in approximately 2/3 of patients with critical AS (about half of whom have severe coronary artery obstruction). The clinical picture is similar to the manifestations of angina pectoris within the framework of coronary artery disease. Attacks occur during physical activity and stop at rest.
In the absence of stenosing coronary sclerosis, angina pectoris in patients with AS occurs with a certain combination of three factors:
- decrease in diastole duration;
- increased heart rate;
- decrease in the lumen of the coronary vessels.
However, for AS there is a specific “substrate” for the development of angina, which consists in the presence of reverse (reverse) blood flow through the coronary arteries during systole, caused by the excess of pressure in the LV cavity over the pressure in the aorta.
Convincing evidence of this phenomenon is the fact that angina disappears immediately after aortic valve replacement (AVR).
Syncope (fainting) is the second classic sign of severe AS. In this case, syncope refers to a transient loss of consciousness caused by inadequate perfusion of the brain with blood enriched with a sufficient amount of oxygen. Often in patients with AS, the equivalent of syncope is dizziness or attacks of unexplained weakness. There are several reasons for the development of fainting states (dizziness) with AS:
- obstruction of the LV outflow tract;
- rhythm and conduction disturbances;
- decreased vasomotor tone;
- carotid sinus hypersensitivity syndrome;
- hyperactivation of LV mechanoreceptors;
- age-related decrease in the number of pacemaker cells (PAC).
Dyspnea in AS is presented in two variants: paroxysmal nocturnal dyspnea due to a decrease in sympathetic and increased parasympathetic tone of the autonomic nervous system (calcification of the conduction system, decrease in the number of pacemaker cells with age), as well as attacks of cardiac asthma/alveolar pulmonary edema that occur suddenly, often at night, without other manifestations of chronic heart failure (unspecified neurohumoral mechanisms). Since cardiac output in severe AS remains at a sufficient level for many years, symptoms such as fatigue, weakness, peripheral cyanosis and other clinical manifestations of the “low cardiac output” syndrome, as a rule, remain mild until the later stages of the disease.
A rare associated symptom of AS is gastrointestinal bleeding, both idiopathic and due to vascular angiodysplasia of the intestinal submucosa, described by Heyde in 1958. The most common source of bleeding is the ascending colon. A feature of these bleedings is their disappearance after surgical correction of the defect.
Diagnostics
Objective signs of severe AS are: diffuse apical impulse, slowing and decreasing pulse in the carotid arteries, decreasing intensity of the aortic component in the formation of the 2nd heart sound with its possible paradoxical splitting, systolic ejection murmur over the aorta.
Systolic murmur in AS is characterized by a rough character, appearing shortly after the first sound, increasing in intensity and reaching a peak in mid-systole, followed by weakening and disappearing immediately before the 2nd sound. The murmur is best heard at the base of the heart. It works well on the vessels of the neck.
Features of systolic murmur with calcified AS in elderly people are:
- reducing its intensity;
- changing the timbre from rough to soft;
- displacement of the auscultatory maximum to the apex of the heart (Galaverden's symptom).
The latter circumstance leads the general practitioner to an erroneous interpretation of the auscultation picture as a manifestation of relative mitral regurgitation as part of CHF.
Electrocardiography. The main ECG sign of AS is LV myocardial hypertrophy. At the same time, its absence does not exclude the presence of even critical AS, especially in the elderly. T wave inversion and ST segment depression are often observed in leads with a vertical position of the ventricular complex (the cause of which is described above). ST segment depression of more than 0.2 mV is often detected, which is an indirect sign of concomitant LVH. Occasionally, infarct-like ECG changes may be observed, consisting of a decrease in the amplitude of the R wave in the right chest leads, which also causes overdiagnosis of coronary artery disease.
Atrial fibrillation and/or various types of intraventricular blocks indicate concomitant mitral valve calcification.
Chest radiography usually reveals an aortic configuration of the heart, calcification of the aortic valve, and post-stenotic dilatation of the aorta, more typical of bicuspid AS. In later stages, dilatation of the LV cavity and signs of pulmonary congestion are noted. With concomitant damage to the mitral valve, dilation of the left atrium is observed.
The basic method for diagnosing AS is a duplex echocardiographic study (2DEchoCG), the purposes of which are (class I):
- diagnosis and assessment of severity of AS (level of evidence B);
- assessment of the severity of LVH, chamber size and LV function (level of evidence B);
- dynamic examination of patients with established AS as the severity of clinical signs or symptoms changes (level of evidence B);
- assessment of the severity of the defect and LV function in patients with established AS during pregnancy (level of evidence B);
- dynamic monitoring of asymptomatic patients: annually for severe AS; every 1–2 years for moderate AS and every 3–5 years for mild AS (level of evidence B).
The severity of AS is assessed according to the criteria presented in Table 1.
Natural course
The rate of progression of AS is quite variable. The average increase in transaortic gradient per year is 7 mm Hg. Art., the peak velocity of the transaortic flow is 1 m/s, and the average decrease in the area of the aortic opening ranges from 0.02 cm2 to 0.3 cm2 per year. CAS has a significantly faster rate of progression in contrast to “rheumatic” or bicuspid AK. The main predictors of rapid progression are the presence of concomitant ischemic heart disease, hypertension, hyperlipidemia, as well as old age and smoking. The study of the natural course of the disease in symptomatic patients made it possible to establish that the prognosis is influenced not only by the fact of the appearance of symptoms, but also by their combination and the rate of increase in severity, which is accompanied by a sharp increase in cases of sudden death.
Treatment
Treatment goals:
- prevention of sudden death and heart failure;
- relief of disease symptoms and improved quality of life.
Drug treatment
Prescribed to inoperable patients due to concomitant pathology. The choice of conservative tactics in patients with CAS is limited and is aimed at reducing the severity of clinical symptoms. The following classes of drugs are used:
- b-blockers (with aortic valve opening area >0.8 cm2) and nitrates (with caution) – for angina pectoris. The most preferred drugs are bisoprolol, carvedilol and metoprolol;
- digoxin (for atrial fibrillation and/or ejection fraction 25–30% or lower);
- diuretics (with caution in CHF);
- ACE inhibitors (careful dose titration).
If pulmonary edema occurs, administration of sodium nitroprusside in an intensive care unit is indicated to reduce congestion and improve LV function. Class III antiarrhythmic drugs are prescribed when atrial fibrillation occurs after ineffective cardioversion to control the ventricular rate of the heart.
Indications for surgical treatment
The leading method of surgical correction of AS is AVR.
The absolute indication for AVR is the presence of proven severe AS according to 2D echocardiography in combination with clinical symptoms and/or LV systolic dysfunction, planned CABG or other surgical interventions on the aorta and/or other heart valves (class I).
In other cases, the determination of indications is approached individually, taking into account not only the severity of the defect and the clinical picture, but also the severity of valve calcification, the presence of factors for the rapid progression of the disease (age, associated conditions, including coronary artery disease), as well as the availability of high-tech cardiac surgery (class II) .
Unfortunately, most general practitioners believe that left ventricular failure, advanced age, associated conditions, and improvement in physical status after the start of conservative therapy are contraindications for OVR. This approach cannot be considered justified, since timely surgical intervention, regardless of age, significantly increases life expectancy and improves its quality.
Antithrombotic therapy in patients with CAS
The survival of patients, their functional status, the function of the valve apparatus and the frequency of complications after AVR depend on a number of factors, including those related to the patient (liver condition, diet, compliance, etc.), parameters of intracardiac hemodynamics, type of surgical intervention, type prosthesis and associated diseases. The highest risk of thrombotic complications, regardless of the type of prosthesis, is observed in the first 3 months, especially in the first days after surgery, i.e., until complete “endothelialization” of the artificial heart valve. The risk of thrombotic complications is also increased in the presence of certain clinical conditions, including atrial fibrillation, a history of thromboembolic events, LV dysfunction, and hypercoagulable conditions.
All patients with mechanical prostheses require lifelong warfarin therapy (Table 2). Acetylsalicylic acid (ASA) is recommended for all patients with artificial heart valves, including in the form of monotherapy in patients with biological valves without risk factors and in combination with warfarin in patients with mechanical valves, as well as in patients with biological prostheses with presence of risk factors. If there is a high risk and it is impossible to take ASA, it is recommended to add clopidogrel (75 mg/day) to warfarin. However, even while taking anticoagulants in operated patients, the lifetime risk of thromboembolism remains within 1 to 2% per year, which is significantly lower than in the absence of warfarin therapy. With biological prostheses, the thrombogenic risk averages 0.7% per year.
Thus, the prescription of antithrombotic therapy requires appropriate patient education and should be individualized in each specific case (Table 2).
In case of AS, prevention of infective endocarditis is carried out according to standard regimens exclusively for 2 categories of patients: after undergoing AVR with implantation of an artificial heart valve and having previously suffered infective endocarditis.
Management of postoperative patients
A. First medical examination
Along with anamnesis and objective examination, it includes: ECG, chest x-ray, 2DECHO, clinical blood test, urea nitrogen/creatinine, LDH and INR (if necessary). The purpose of the examination is to evaluate the function of the artificial valve, identifying infection, myocardial infarction, conduction disorders or valvular disorders.
B. Follow-up management of patients with an uncomplicated course of the postoperative period
Examination intervals are determined by the needs of the patient. In an uncomplicated course, they are once a year and include the collection of complaints, an objective examination, and also, in certain cases, an ECG and chest x-ray. Additional tests include hemoglobin concentration, hematocrit and LDH. In the absence of signs of LV dysfunction, dysfunction of artificial or other heart valves, 2DECHOCG is not indicated. However, as soon as a new heart murmur is suspected or the patient’s clinical status changes, 2-ECHOCG is performed every 3-6 months.
B. Follow-up management of patients with a complicated course of the postoperative period
Patients with LV systolic dysfunction following heart valve surgery should receive appropriate standard therapy. Treatment should be continued even if the symptoms of LV systolic dysfunction are leveled or reduced.
LV dysfunction or overt signs of heart failure after heart valve replacement may result from:
- LV dysfunction existing before surgery, the manifestations of which may remain unchanged or decrease only partially in the future;
- perioperative myocardial damage;
- progression of diseases of other heart valves;
- complications associated with artificial heart valves;
- concomitant heart diseases such as ischemic heart disease and hypertension.
For patients who do not experience improvement or have a deterioration in functional status after surgery, appropriate tests are indicated to identify the cause, including echocardiography, and, if necessary, transesophageal echocardiography and cardiac catheterization with angiography. Patients with postoperative LV systolic dysfunction, even in the absence of symptoms, should receive standard therapy, which should not be discontinued once functional status improves. In this case, patients should undergo a full range of primary and secondary prevention measures to reduce the risk of cardiovascular events in the future.
Taking into account the difficulties that arise for practicing physicians and the features of the ICD X revision, below we provide examples of the formulation of the clinical diagnosis of various variants of AS.
I 35.0 Calcified aortic (valve) stenosis of mild (moderate, severe) degree, asymptomatic (decompensated) form. NK IIA, FC III (NYHA).
I 06.2 Rheumatic heart disease: combined aortic disease with a predominance of stenosis (or insufficiency) of the aortic valve. NK I, FC II (NYHA).
Q 23.1 Congenital bicuspid aortic valve with stenosis (and/or insufficiency), mild (moderate, severe) stenosis, asymptomatic (decompensated) form. NK IIA, FC III (NYHA).