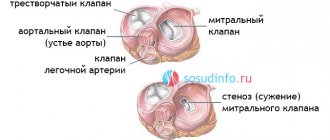
1.Heart valve replacement
Heart Valve Replacement
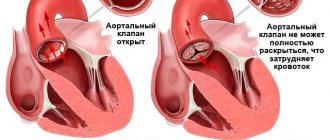
– currently this is a very effective procedure with a low risk of complications. It is indicated for patients with symptoms of aortic valve stenosis. For some patients, refusing this advanced surgical technology means a significant reduction in life expectancy or the risk of sudden death. If there are no obvious contraindications to surgery (for example, coronary heart disease, previous heart attack), then this operation is usually prescribed and recommended for all patients with a degree of stenosis above average. With severe symptoms (chest pain, fainting, breathing problems), the life expectancy of many patients without heart surgery does not exceed 5 years, since the disease progresses over time.
Heart valve replacement surgery can be performed using an open procedure.
or
minimally invasive.
Although this is a rather complex procedure, extensive experience with such surgical treatment has now significantly reduced the possible risks. All stages are worked out to the smallest detail. During valve replacement, the possibility of complications and emergency situations is taken into account, therefore the operating room and medical staff are prepared taking into account the different developments of the situation during the operation.
Heart valve replacement occurs in eight stages.
Before this, the patient undergoes
preparation for the operation
(outpatient or in a hospital), following certain instructions and taking a number of medications. In some cases, it is necessary, on the contrary, to discontinue regularly taken medications.
A must read! Help with treatment and hospitalization!
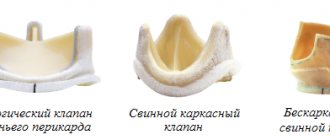
Various types of prostheses are widely represented in modern aortic root surgery. When replacing the aortic valve (AV) with a mechanical prosthesis, there is a need for lifelong use of anticoagulants, a high risk of thromboembolic complications, and acoustic discomfort. Aortic valve replacement with frameless prostheses or complete replacement of the aortic root with an auto- or xenograft is not always justified in elderly and senile patients due to the technical complexity of such an operation. From these positions, frame bioprostheses occupy an intermediate position, practically no different in implantation technology from mechanical prostheses; this type of xenovalves makes it possible to refuse the prescription of oral anticoagulants 3-6 months after surgery, which, of course, is a significant fact in elderly and senile patients.
The paper presents 3-year results of implantation of a low-profile frame prosthesis treated with glutaraldehyde BioLAB KA/PT of domestic production, which has been used in the clinic of our institute since 2008. There are studies that contain recommendations considering this xenovalve as the prosthesis of choice in patients of the older age group [ 2]. However, these works did not analyze the factors that form the transprosthetic pressure gradient, nor did they analyze the influence of the hemodynamic characteristics of the prosthesis on long-term results. In addition, not a single case of bioprosthesis dysfunction at various times after surgery has been described. Our experience, describing both positive and negative impressions from the clinical use of this bioprosthesis, can be useful not only to cardiac surgeons and cardiologists, but also to researchers directly related to the development and production of xenovalves.
Material and methods
Between December 2008 and December 2011, 166 patients were implanted with low-profile xenopericardial bioprostheses BioLAB CA/PT in the aortic position. The average age of the patients was 69.3±4.5 years (60-82 years), of which 75 (45%) were men and 91 (55%) women. On average, chronic heart failure in patients corresponded to functional class III. Stage I circulatory failure was diagnosed in 5 (3%) patients, stage IIA - in 122 (74%), stage IIB - in 39 (23%). The cause of the formation of aortic disease in 113 (68%) patients was degenerative changes, in 35 (21%) - chronic rheumatic heart disease, in 8 (5%) - pathology of congenital bicuspid aortic valve, in 10 (5%) - primary infective endocarditis. The average body surface area was (according to DuBois) 1.7±0.1 m2 (1.3-2.2 m2). The dimensions of the implanted prostheses were determined before surgery based on ultrasound data and intraoperative assessment of the size of the fibrous ring. The bioprosthesis, selected according to the size of the fibrous ring of the AC, was washed for 90 minutes in an isotonic sodium chloride solution with the latter being changed three times every 30 minutes. The next step was to implant the bioprosthesis into the aortic position with separate U-shaped sutures using 2-0 polyester sutures. In order to implant a prosthesis of maximum diameter, preference was given to the supraannular position.
Prostheses No. 20 were implanted in 53 (32%) patients, prostheses No. 22 - 76 (46%), prostheses No. 24 - 34 (20%), prostheses No. 26 - 2 (1%). In 94 (60%) cases, correction of the aortic defect was performed as part of a combined cardiac intervention (Table 1)
.
results
Already by the time of discharge from the clinic, the majority of patients had significantly decreased the symptoms of circulatory failure in the systemic and pulmonary circulation, and their tolerance to physical activity increased.
At the hospital stage, 5 (3%) cases of death after surgery were recorded. In 4 (2.4%) patients, the cause of death was heart failure, in 1 (0.6%) - acute cerebrovascular accident.
During the hospital stay, 2 (1.2%) cases of prosthetic dysfunction were noted. In 1 patient, on the 25th day after surgery, dysfunction of the aortic prosthesis was diagnosed with the formation of a peak transprosthetic pressure gradient (PTPG) of 72 mmHg. This patient successfully underwent declination of the biological AC prosthesis. During the operation, a mixed thrombus was found on the ventricular surface of the prosthesis in the projection of the commissures. In the second patient, on the 26th day after surgery, according to echocardiography from the level of the prosthesis leaflets, a pronounced regurgitation jet was observed, occupying 50% of the volume of the left ventricle (LV). This patient underwent successful revision of the aortic valve replacement. The operation revealed sagging of the left coronary cusp into the ventricular cavity with impaired coaptation in the central part of the prosthesis (see figure, a)
.
Figure 1. BioLAB KA/PT bioprostheses removed during re-operation.
a — sagging of the left coronary cusp into the ventricular cavity with impaired coaptation in the central part of the prosthesis. One patient was diagnosed with type II aortic dissection according to De Bakey on the 30th day after surgery. The cause of intimal detachment was pronounced atherosclerotic changes in the vascular wall in the area of the aortotomy access. This patient successfully underwent supracoronary replacement of the ascending aorta. Other complications that did not require reoperation are presented in Table. 2
.
To identify the factors that have the greatest influence on the formation of the pressure gradient on the BioLAB KA/PT bioprosthesis, a correlation analysis was carried out, having previously excluded outliers (“outliers”). The analysis included indicators of PCGD, prosthesis size, body surface area (BSA), body mass index (BMI), LV stroke volume (SV) after surgery, postoperative indicators of LV end-diastolic volume, LV end-diastolic size and myocardial mass indexed to BSA, LV ejection fraction.
The analysis revealed that PCGD is in direct and statistically significant dependence on BMI ( r
=0.55) and LV SV (
r
=0.35), inversely - on the size of the prosthesis (
r
= –0.27). The remaining parameters either had a weak connection with PCGD or did not have one at all. To identify the nature of this relationship, a nonlinear regression analysis was performed. When modeling the relationships, the function of the dependence of PPGD on the indicated values was as follows: PPGD = 40.3 + 0.34 · BMI + 0.1 · LV SV - 1.65 · (prosthesis no.).
According to the data obtained, the functional state of the myocardium, expressed in the value of the LV ejection fraction, as well as the degree of cardiac muscle hypertrophy do not have a statistically significant effect on the formation of transprosthetic resistance to blood flow in patients of this group.
The duration of stay in the intensive care unit was 2.5±1.8 days, in the general ward - 21±10 days.
In the absence of indications for continuous use of oral anticoagulants (atrial fibrillation, history of cardiac thrombosis, reduced myocardial contractility, atriomegaly), anticoagulant therapy was prescribed for the period necessary for endothelialization of the prosthesis braid (6 months).
The completeness of patient follow-up over a three-year period was 81% (128 patients), the average follow-up period was 28.9 ± 7.4 months (4-48 months).
In the long-term period, 2 (1.2% of those discharged) patients died. The cause of death in one case was uncontrolled gastrointestinal bleeding secondary to severe sepsis and disseminated intravascular coagulation. The patient died 4 months after surgery. A pathoanatomical study was not performed, but according to repeated control ultrasound examinations of the heart, no signs of dysfunction of the bioprosthesis in the aortic position were revealed. In another case, the immediate cause of death was prosthetic dysfunction caused by acute prosthetic endocarditis with tissue necrosis of the aortic root, tearing of the prosthesis at ⅔ of the circumference of the fibrous ring, which necessitated repeated surgical intervention for health reasons 4 months after surgery. The patient's death was confirmed intraoperatively, and the cause of death was found to be uncontrolled bleeding.
Thus, the actuarial survival rate, calculated using the Kaplan-Meier method, at the end of the 1st and 3rd years of observation was 98 ± 2%.
During dynamic observation, positive dynamics of physical status in the form of a decrease in FC (NYHA) was noted in surviving patients. The average FC during follow-up in 92% of patients was II ( p
<0.001 compared to the value before surgery). The main changes in FC occurred during the first year after surgery, subsequently remaining at an almost unchanged level.
In the group of patients we studied, 5 patients (3% of those discharged) required re-operation on AV within 4 to 40 months after surgery, and 1 (0.6%) patient was re-operated 16 months after the initial operation for dysfunction of the mitral prosthesis. In 5 out of 6 cases, the cause of dysfunction was prosthetic endocarditis. In one case, the dysfunction of the prosthesis was caused by sagging of one of the valves, similar to the case presented above. The second patient was diagnosed with a paraprosthetic fistula extending 1 cm with a pronounced volume of discharge from the level of the prosthesis. In 2 patients, prosthetic endocarditis led to structural dysfunction of the prosthesis. In the first case, wrinkling of the leaflets and separation of one of the leaflets from the prosthesis stand were observed, which led to a violation of coaptation (see figure, b)
.
Figure 1. BioLAB KA/PT bioprostheses removed during re-operation.
b - wrinkling of the valves and separation of one of the valves from the prosthesis stand. In the second case, 40 months after surgery, the cause of dysfunction was calcium degeneration of biological tissue. Except for the described fatal case, all patients were successfully re-operated. Phenomena of prosthetic endocarditis in the early postoperative period (up to 1 month) were observed in 5% of patients, but they did not lead to changes in the implanted xenovalve or paravalvular structures. All of these cases of endocarditis were successfully treated at the time of discharge. Thus, we consider the rate of 5% in the early postoperative period to be quite good. But we regard the development of prosthetic endocarditis within 1 year after surgery in 8% of patients discharged in a stable condition, without clinical, laboratory or instrumental signs of active infection, as a fairly high level of risk. Perhaps this complication is associated with the presence of a synthetic braid of the prosthesis, which becomes a place for pathogenic microflora to settle and multiply. The highest risk of developing prosthetic endocarditis is observed in the first 12 months after surgery and amounts to 0.8-2.4%. In the future, the risk of developing prosthetic endocarditis is reduced to 0.1-0.2%. Given the data obtained regarding the risk of early prosthetic endocarditis, transesophageal echocardiography should be recommended for any suspicion of intracardiac infection activity, regardless of transthoracic ultrasound findings.
In 1 patient, 2 months after surgery, acute thrombosis of the axillary artery was diagnosed, despite a constant level of International Normalized Ratio within the range of 2.5-3.5. This case was the only one, and the number of patients without thromboembolism and thrombosis (excluding thrombosis of the prosthesis in the early postoperative period) was 99±1% by the end of the 3rd year of observation.
“Minor” bleeding (nasal bleeding, bleeding gums) was observed in 2 (1.2% of discharged) patients.
Thus, the actuarial frequency of absence of the need for repeated surgical intervention in the long-term period was 97±1% at the end of the 1st year of observation and 92±4% at the end of the entire observation period, the frequency of absence of structural dysfunction was 98%.
Both at discharge and during follow-up, all patients underwent control echocardiography, according to which a statistically significant regression of LV myocardial hypertrophy was noted (Table 3)
.
The dynamics of echocardiographic parameters depending on the size of the prosthesis are presented in Table. 4
.
Discussion
The current stage of development of surgery for acquired heart defects is characterized by an increase in the level of safety and effectiveness of surgical treatment of heart disease, the development of new economical and effective technologies, an expansion of the range of correctable pathologies and a reduction in the number of contraindications to the operation. Improvements in surgical techniques, instruments, methods of artificial circulation and myocardial protection have led to a significant improvement in the immediate results of surgical treatment of aortic defects. The immediate results of surgical treatment of the defect are determined both by its etiology, the initial severity of the disease and the severity of changes in the valve, as well as the correct choice of method and the adequacy of correction [5]. Implantation of mechanical prosthetic heart valves is still associated with such disadvantages as the need for constant use of anticoagulants, a high risk of thromboembolic complications, which significantly reduces the quality of life of patients. Alternatively used biological prostheses provide a better quality of life, form a blood flow structure close to physiological, and in most patients do not require anticoagulants [3, 4].
According to foreign authors [7], interest in AC replacement with frame bioprostheses does not wane. And the reason for this is new methods of processing and storing xenotissue, which have increased the service life of bioprostheses, as well as an increase in the number of elderly and senile patients. In patients over 60 years of age with an implanted biological prosthesis, the risk of bleeding is much lower than with mechanical prostheses. In 2002, for the first time, global sales of biological replacement valves exceeded sales of mechanical valves by US$100 million, with a total of US$800 million [6]. In Russia, the share of bioprostheses is also increasing every year: in 2007, the number of implanted bioprostheses was only 4% of the total, and in 2009 it increased to 10.5% [1]. This is due, on the one hand, to the dissatisfaction of surgeons with the long-term results of the use of mechanical prostheses, in particular the number of specific complications and the quality of life of patients, and on the other hand, to the development of new promising designs and technologies for the manufacture of bioprosthetic heart valves.
AK replacement with a xenopericardial prosthesis is accompanied by low mortality in both early and long-term periods, and a low incidence of specific complications. Good hemodynamic characteristics, resistance to infection, low risk of thrombosis and “major” complications caused by taking anticoagulants, according to many authors, make frame bioprostheses a real alternative to modern mechanical prostheses. The fairly low thrombogenicity of frame bioprostheses allowed us to abandon prolonged or lifelong anticoagulant therapy in most patients, which is especially important in patients of the older age group, in whom it is undesirable. At the same time, we adhered to the generally accepted methodology, according to which constant use of anticoagulants under strict monitoring of the state of the blood coagulation system was recommended for all patients whose main rhythm was atrial fibrillation, patients with low LV ejection fraction and a history of thrombosis of the heart cavities.
conclusions
The magnitude of the peak blood pressure gradient on the BioLAB CA/PT prosthesis is determined by the formula PHGD = 40.3 + 0.34 · BMI + 0.1 · LV SV - 1.65 · (prosthesis number). During dynamic observation for up to 3 years, the value of the peak transprosthetic pressure gradient does not change statistically significantly.
In patients with aortic stenosis, by the end of the 1st year after implantation of the BioLAB CA/PT frame bioprosthesis, there was a decrease in the mass of the LV myocardium by an average of 31% (from 212±0.14 to 155±38 g/m2; p
<0.001).
The severity of the processes of reverse development of LV myocardial hypertrophy does not depend on the size of the implanted prosthesis ( p
>0.05).
The main complication caused by the prosthesis after implantation of the BioLAB KA/PT xenovalve is prosthetic endocarditis. The highest risk of developing prosthetic endocarditis is observed in the first 12 months after surgery and amounts to 0.8-2.4%. In the future, the risk of developing prosthetic endocarditis is reduced to 0.1-0.2%.
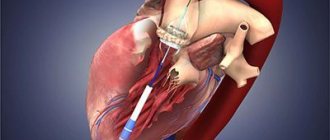
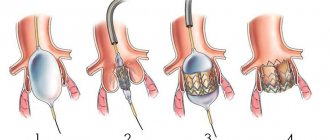
2.Stages of operation
At the first stage
the patient is connected to cardiac monitors, the chest is processed, breathing tubes are inserted from an artificial respiration apparatus, which is connected only after the anesthesia begins to take effect (i.e., the only discomfort for the patient may be some soreness and soreness in the throat after the operation).
The anesthesiologist puts the patient under general anesthesia. From this moment on, the patient does not feel anything.
An ultrasound device is placed through the esophagus into the heart area, transmitting an image of the heart to a monitor throughout the operation.
Second phase
- This is the opening of the chest. After marking, the heart surgeon makes an incision on the chest from the top of the rib cage to the navel. With the minimally invasive method, the incision length is two-thirds shorter.
The purpose of the third stage
is connection to a machine that provides artificial blood circulation. During the operation, the blood is enriched with oxygen outside the lungs, then it returns to the aorta and moves through the systemic circulation.
At this stage, the surgeon stops the heart, washes it and places it in a special solution that maintains its viability outside the circulation.
Fourth and fifth stages
- This is actually replacing the valve. The aorta is cut and the diseased valve is removed. If part of the aorta is also affected, it is also removed and a graft is placed. The valve hole is measured to select a new valve of the required size. The new valve is sewn on, then it is necessary to check that there are no leaks through it.
Sixth stage
- this is a disconnection from the artificial blood circulation machine. The aorta is sutured, and the trapped air is removed from the heart cavity. The heart begins to beat under the current of its own blood. If an uneven rhythm is observed, an electric shock is applied, which restores an even pulse.
Seventh stage
- chest closure. The bones and tissues are stitched together, and the sternum is closed with a suture that remains visible for life.
The entire operation usually lasts from 2 to 5 hours.
is important for the outcome of treatment .
It starts in the intensive care ward. Discharge from the hospital occurs 5-9 days after surgery. Postoperative rehabilitation continues on an outpatient basis.
Visit our Cardiology page
Consequences and rehabilitation in the postoperative period
After the operation, the patient is in the intensive care unit. Doctors monitor the patient’s condition, as negative consequences are possible and he will need rehabilitation . On the first day, there may be complications such as internal bleeding, thromboembolism, cardiac arrhythmias, infective endocarditis, and heart attack (in less than 5% of cases). After two days, the patient is allowed to get up and walk. At this time, he may complain of chest pain and increased fatigue. The patient is discharged home within 4 to 10 days, where he is under the supervision of a doctor.
The postoperative period lasts 3 weeks, during which temporary disturbances in sleep, vision, appetite, and swelling of the legs are possible. A month later the patient undergoes a full examination. The cardiologist calculates adequate physical activity, diet, and prescribes supportive treatment. It is important to perform physical therapy to strengthen the heart muscle.
4. Lifespan of heart valves
Today, two types of heart valve implants are used in heart surgery: artificial and biological.
The first ones are durable, but their installation is associated with lifelong use of a number of drugs to eliminate the risk of blood clots. Biological valves are closest to living tissues, but wear out faster. Cardiologists are constantly working to develop and introduce into medical practice new types of valves that combine the best properties of both types - durability and maximum acceptability for the body. The service life of artificial valves is 10-15 years. Newer materials are still being studied. Widespread practice of their use in the near future will provide cardiologists with more accurate information about their service life.
Telemedicine consultation at the Bakulev Center
Based on the results of a consultation at the telemedicine department of the Bakulev National Center for Cardiovascular Surgery, the patient was recommended for surgical treatment under artificial circulation - aortic valve replacement.
Polyclinic NCSS
Two weeks after the telemedicine consultation, an examination was carried out in the clinic of the National Center for Cardiovascular Surgery, based on the results of which the diagnosis was clarified:
“Calcified aortic valve disease is a critical stenosis. Relative mitral insufficiency. Relative tricuspid insufficiency. Pulmonary hypertension. Permanent form of atrial fibrillation. Atherosclerosis of the coronary arteries. NK 2A. FC 3".
A decision was made to hospitalize him.
Preparing for the TAVI procedure
A few days before your therapeutic catheterization, you will undergo a diagnostic catheterization, during which the medical team can visualize the condition of your valve and the entire heart muscle as a whole. You will also be asked to undergo a blood test to check your general blood counts, coagulation factors, etc. On the day of the procedure itself, you will be asked to fast for several hours, even if local anesthesia or sedation is planned.