Causes of aortic valve disease
There are many pathological conditions that over time can lead to degenerative changes, calcium deposition on the aortic valve leaflets, and changes in its function.
The human heart is able to compensate for poor circulation for some time. Sooner or later, clinical manifestations occur: dizziness, shortness of breath, fainting, palpitations, angina syndrome. Conservatively (with the help of medications) it is possible to partially compensate for the patient’s condition, but it will not change it radically and will not improve the quality of life.
How is aortic valve replacement performed at the CELT clinic?
An effective method for treating aortic valve disease is replacing the valve with an artificial biological prosthesis. There are two methods of such an operation - traditional surgical and endovascular (without incisions in the chest).
Traditionally, surgery to replace the aortic valve has been performed for many years in an open manner, that is, by opening the chest cavity. The operation is performed by excluding the patient's heart from the circulatory system and using a cardiopulmonary bypass machine (ACB). However, technology in medicine does not stand still. A few years ago, a new minimally invasive technique appeared: transcatheter aortic valve implantation (TAVI). In some cases, it is possible for a patient to undergo aortic valve replacement using this method.
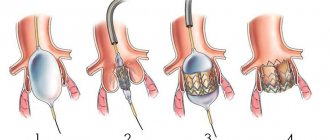
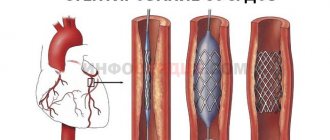
The principle of endovascular aortic valve replacement surgery is similar to stenting operations and consists in the fact that a special catheter with a folded artificial valve (bioprosthesis) placed at its end is accessed through the femoral artery to the site of the damaged aortic valve. Before installing this artificial valve, the lumen of the own aortic valve is expanded using a special balloon. The TAVI bioprosthesis is a specially machined tricuspid valve made from bovine pericardium supported by a stent (metal frame). The operation is painless for the patient as it is performed under anesthesia. There are two types of valves: balloon-expanding and self-expanding.
Typically, TAVI is the treatment choice for patients over 75 years of age with severe aortic stenosis who have contraindications to open surgery, but the indications have now expanded to include patients at low to moderate surgical risk.
Correction or replacement (prosthetics) of heart valves
Indications
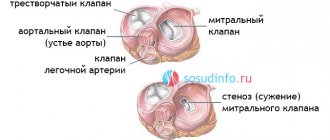
There are 2 valves that separate the upper chambers of the heart (atria) from the lower ones (ventricles) - mitral (bicuspid) and tricuspid (tricuspid), as well as 2 valves that separate the lower chambers of the heart from the large arteries - aortic and pulmonary.
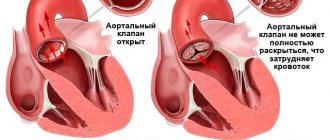
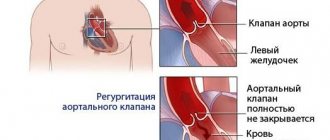
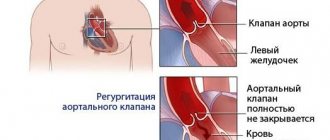
Each of these four valves is responsible for the unidirectional flow of blood during contraction of the atria and ventricles - so that the blood entering the heart enters the large arteries and then throughout the body. If one of the valves is damaged - whether due to leaky closure of the leaflets (valvular insufficiency) or severe narrowing of the opening (stenosis), blood flow is disrupted, and as a result, the function of the entire heart is impaired. In the first case, during ventricular contraction, the valve is unable to prevent retrograde blood flow from the ventricle into the atrium (so-called regurgitation occurs). In the second case, the valve does not open enough to allow free blood flow from the atrium to the ventricle or from the ventricle to the aorta/pulmonary trunk.
Valvular insufficiency can be the result of rupture of several chordae tendineae connecting each of the valve leaflets with the papillary muscles - this is possible with some heart diseases, with connective tissue diseases (including congenital), as well as with traumatic lesions. Stenosis often occurs against the background of rheumatic diseases.
Correction or replacement of heart valves is indicated in both cases - both with valvular insufficiency and with stenosis. The need for surgical intervention is decided depending on the severity of the insufficiency/stenosis.
For valve insufficiency, various types of surgical interventions are used. Here are some of them:
1) installation of synthetic chords instead of broken ones;
2) triangular/quadrangular resection of the valve;
3) transplantation of own chords;
4) edge-to-edge plastic surgery using the Alfieri method;
5) in case of mitral valve insufficiency due to coronary heart disease, annuloplasty or implantation of a biological prosthesis without removing the own valve is used.
Upon completion of the correction, transesophageal echocardiography is performed to assess the effectiveness of the intervention. If the results are unsatisfactory, either additional correction or valve replacement is performed.
Various types of surgical interventions are also used for valve stenosis. Including:
What are the advantages of transcatheter aortic valve implantation over open surgery?
With intravascular aortic valve replacement, there are no large incisions, which significantly reduces the duration of postoperative rehabilitation, reduces pain and eliminates the development of complications associated with inflammation (suppuration) of postoperative sutures. Other important advantages of TAVI include:
- Ability to perform surgery on patients at high surgical risk, elderly patients and patients with severe comorbidities
- Short operation time, reduced surgical trauma.
- The operation is performed under local anesthesia (in some cases under general anesthesia)
- There is no need to connect to a heart-lung machine and “turn off” the patient’s heart
- Reducing length of stay in hospital
- Low percentage of postoperative complications and rapid adaptation after discharge from hospital
The development of cardiac surgery over the past decades has significantly expanded the indications for correction of heart defects and improved the survival of patients in the long term after surgery. The number of operations for valvular heart disease performed under artificial circulation (CPB) has increased significantly. Due to a number of objective reasons, the number of repeated interventions is also increasing, which is associated with relapse of the defect after reconstructive plastic surgery, dysfunction of the mechanical prosthesis caused by endocarditis or inadequate anticoagulant therapy, degeneration of biological prostheses, etc. At the same time, repeated operations on heart valves are technically much more difficult and are considered by many authors as a risk factor [1, 3, 8].
The purpose of the study was to analyze the immediate results of repeated surgical interventions in patients who had previously undergone surgery for valve pathology under cardiopulmonary bypass.
Material and methods
For the period from 2003 to 2012, in the department of surgery of heart defects of the Federal State Budgetary Scientific Institution Russian Scientific Center for Surgery named after. acad. B.V. Petrovsky repeated interventions on the heart valve apparatus were performed in 90 (6.8%) patients. In 51 (56.7%) patients, the primary operation was performed in other medical institutions. The age of the patients ranged from 18 to 70 years (mean 54.6±9.5 years). Among the patients there were 33 (36.7%) men and 57 (63.3%) women (Table 1)
.
The study included patients admitted for re-intervention after successful primary correction. Patients who required reintervention as a result of a complication during their hospital stay after the primary operation were excluded from the study. The time between the first operation and re-intervention ranged from 2 months to 36 years, on average 11.6±8.5 years. Three patients in our study underwent heart valve surgery for the third time; the interval between the second and third interventions was 9, 12, and 15 years.
Among the primary interventions, reconstructive plastic surgery on the mitral valve was performed in 34 (37.8%) patients; isolated mitral valve replacement — 32 (35.6%); isolated aortic valve replacement — 20 (22.2%); mitral and aortic heart valve replacement was performed in 4 (4.4%). In 50 (89.2%) patients, mechanical prosthetic heart valves were used during the primary operation; biological prostheses were implanted in 6 (10.8%).
Indications for reoperation are presented in table. 2
.
Recurrence of heart disease in most cases (43 patients, 47.8%) was caused by the progression of the rheumatic process with fibrous deformation and calcification of the valve leaflets, subvalvular structures and fibrous ring. Over the past years after surgery, 10 patients developed a defect of rheumatic etiology on the unoperated valve. Among the causes of dysfunction of mechanical prosthetic valves, the following were identified: thrombosis (see figure on the color insert)
- 16 (17.8%) cases, pannus - 14 (15.6%), paraprosthetic fistulas - 13 (14.4%).
Figure 1. Mechanical heart valve obstruction by thrombus. In 10 patients, the formation of paraprosthetic fistulas was associated with prosthetic infective endocarditis; in 3, no signs of endocarditis were detected.
Resternotomy was performed using a standard technique with removal of wire ligatures and the use of an oscillating saw. This stage of the operation was greatly facilitated if the entire length of the pericardium was sutured during the primary operation. An adhesive process in the pericardium of varying severity was observed in all patients. Cardiolysis was carried out by blunt and acute methods. First of all, the structures necessary to connect the IR apparatus were identified. The IR apparatus was connected according to the aorta-vena cava scheme. In case of hemodynamic disturbances, cardiolysis was continued on parallel cardiopulmonary bypass and was carried out only to the extent necessary for the main stage of the operation, which made it possible to minimize blood loss at this stage of the operation.
The main stage was carried out under conditions of hypothermic perfusion, with cooling to 28-30 °C, and pharmacocold cardioplegia with a solution of Consol or Custodiol. The cardioplegic solution was delivered through the roller pump of the CPB device antegrade to the aortic root, selectively to the ostia of the coronary arteries, or retrograde through the coronary sinus. Cell Saver autologous blood return systems were used to reduce blood loss. To access the mitral valve, a left atriotomy was used (14 cases) along the old suture parallel to the interatrial groove, and for large sizes of the right atrium, preference was given to access through the right atrium and the interatrial septum (47).
And only in 5 cases was the Dubost approach performed, which has maximum visibility of the mitral valve in conditions of incomplete cardiolysis, but at the same time is the most traumatic. To access the aortic valve, an aortotomy was performed in a typical location.
During re-intervention, valve replacement was used in all cases after reconstructive plastic surgery. Isolated re-replacement of the mitral or aortic heart valve was performed in 36 (40%) patients, suturing of a paraprosthetic fistula with De Vega tricuspid valve repair was performed in 2 (2.2%). Repeated interventions on the mitral and aortic valves were performed in 14 (15.6%) patients, of which 6 (6.7%) underwent mitral and aortic valve replacement after primary repair. Replacement of the mitral and tricuspid heart valves was performed in 1 (1.1%) patient, replacement of the mitral and aortic valves - 2 (2.2%), replacement of the mitral valve and replacement of the aortic valve - 3 (3.3%), replacement of the aortic valve and mitral valve replacement — 2 (2.2%) patients. In addition, 3 (3.3%) patients additionally underwent coronary artery bypass surgery, 31 (34.4%) underwent tricuspid valve plastic surgery according to De Vega.
When replacing heart valves, bioprostheses were used in 11 (12.2%) patients, mechanical prostheses were implanted in 79 (85.9%).
CPB time averaged 113.6±34.7 minutes (from 50 to 212 minutes), aortic cross-clamp time was 80.4±29.3 minutes (32-172 minutes). Intraoperative blood loss averaged 1024.5±110.2 ml (400-2000 ml). The use of the Cell Saver system for the return of autologous blood, as well as the preoperative procurement of autoplasma, made it possible to avoid the use of donor blood components in more than half of the cases.
results
The average time in hospital was 15.5±9.4 days (8-45 days), the average length of stay in the intensive care unit was 2.5±1.4 days (from 1 to 16 days).
Mortality rate was 2.2% (2 patients). The causes of death were, in the first case, massive blood loss due to spontaneous rupture of the left ventricular myocardium on the 1st day after replacement of the mitral and aortic heart valves; in the second case, pulmonary embolism and arrhythmogenic cardiac arrest on the 1st day after replacement of the aortic and mitral heart valves .
Among non-lethal complications in the immediate postoperative period (Table 3)
Heart rhythm disturbances were the most common - in 35 (38.9%) patients.
In 6 (6.5%) patients, due to persistent junctional rhythm, a temporary pacemaker was installed for more than 10 days.
Atrial flutter developed in 9 (10%) cases, and cardioversion was successfully performed in 8 of them. In 1 patient, atrial flutter was refractory to electrical pulse therapy, and radiofrequency ablation was subsequently performed. Installation of a permanent pacemaker was performed in 5 patients; the indications were complete transverse block, nodal bradycardia, sinus node dysfunction and atrial fibrillation with pauses of more than 2.5 s. The second place in terms of development frequency was taken by heart failure, which was observed in 30 (33.3%) patients. In 10 (11.1%) patients, severe heart failure, which required cardiotonic support for more than 2 days, was due to the initial severe condition of the patients, the volume or the combined nature of the reintervention.
Respiratory failure was observed in 10 (11.1%) patients. Complications from the central nervous system in the immediate postoperative period were noted in 4 (4.4%) patients: encephalopathy was diagnosed in 3, acute cerebrovascular accident in the left middle cerebral artery system on the 2nd day after mitral valve replacement - in 1 (1 ,1%). Transient liver failure occurred in 2 (2.2%) patients, and renal failure occurred in 3 (3.3%) patients; in one observation, hemodialysis sessions were required. Infectious complications occurred in 2 patients: in one case, mediastinitis was diagnosed, in the second - suppuration of the soft tissues of the postoperative wound. Postoperative bleeding requiring resternotomy developed in 1 (1.1%) patient.
Discussion
The number of reoperations on heart valves increases every year. Repeat mitral valve interventions account for approximately 10% of all mitral valve surgeries in the United States [9]. In our center in 2012, repeated operations on valves accounted for 6.8% of all operations on the valve apparatus.
Revision cardiac surgery remains a challenge for cardiovascular surgeons. There are many risks associated with resternotomy beyond the surgery itself. Many surgeons prefer to perform thoracotomy to reduce the incidence of complications and reduce the length of stay of the patient in the intensive care unit and the overall length of hospitalization, especially in patients who have previously undergone open heart surgery [9].
Another controversial approach is reoperation through a minimally invasive approach. According to some authors [8], it allows one to avoid massive bleeding and injury to large vessels. The minimally invasive approach is mainly used for isolated re-operations on the mitral valve and after previous coronary artery bypass surgery. The goal of a minimally invasive approach is to reduce the risk of major bleeding during surgical access. A. Vleissis et al. [11] preferred to use a minimally invasive approach for repeated interventions on the mitral valve. According to them, in a group of 22 repeated operations on the mitral valve through a minimally invasive approach, there were no deaths or cases of postoperative wound infection.
F. Casselman et al. [1] published the results of a study of 80 patients who underwent endoscopic revision mitral valve surgery through a mini-thoracotomy approach without rib spread. The mortality rate in their series was 3.8%.
According to a study by R. Umakanthan et al. [10], including 90 patients, minimally invasive right thoracotomy without aortic cross-clamping may be a good alternative to traditional resternotomy when performing repeated interventions on the mitral valve. In their work, the authors drew conclusions about the safety and effectiveness of minimally invasive access, reducing surgical mortality in high-risk patients.
M. Ghoreishi et al. [5] prefer the resternotomy approach for repeated mitral valve interventions, but perform preoperative computed tomography of the chest to determine the risk of injury to large vessels during resternotomy. In a series of reoperations on the mitral valve, they divided patients into two groups based on the results of computed tomography: low risk (large vessels were located at a distance of more than 1 cm from the posterior surface of the sternum) and high risk (large vessels were located at a distance of less than 1 cm from the posterior surface of the sternum ). According to the authors, injury to large vessels during sternotomy has a significant negative impact on the results of surgical intervention. Intraoperative mortality was significantly higher when large vessels were injured. Patients in the high-risk group underwent cannulation of peripheral vessels before resternotomy to allow emergency transfer to CPB.
In the present study, we used only the transsternal approach. Most patients had concomitant heart valve pathology: in addition to aortic or mitral valve dysfunction, there was tricuspid valve insufficiency or a combination of aortic and mitral valve dysfunction. The minimally invasive approach for recurrent valve surgery has limitations for the simultaneous performance of concomitant cardiac interventions. When using a minimally invasive approach, it becomes difficult to perform interventions on two or three valves simultaneously, or “maze” surgery in patients with atrial fibrillation. To perform such operations, in our opinion, it is more justified to perform a standard resternotomy, which provides access to all structures of the heart to the greatest extent. To reduce the risk of injury to the heart and large vessels during resternotomy, we routinely suture the entire pericardium during the initial operation.
According to P. Ellman et al. [2], installation of an IR before resternotomy did not reduce the risk of intraoperative injury to large vessels. In the study of repeated heart valve surgeries, we did not perform peripheral vascular cannulation before resternotomy, and there were no cases of major bleeding during resternotomy.
According to the results of M. Murzi et al. [8], the volume of blood transfusion during repeated operations on heart valves is higher when using a minimally invasive approach compared to standard resternotomy (4.1 units versus 2.7 units). Surgical mortality with minimally invasive access was 5.7%, with resternotomy - 5.9%. The rate of conversion to standard resternotomy during minimally invasive valve reintervention was 1.7%. In the present study, when traditional resternotomy was used exclusively, the mortality rate was 2.2%.
In a 10-year single-center study, H. Vohra et al. [13] identified risk factors for mortality and postoperative complications, the most significant of which were: left ventricular ejection fraction less than 50%, the need for multivalve correction, and the urgency of the intervention.
In a study by P. Vogt et al. [12], which focused on reoperations for degeneration of biological aortic valve prostheses, risk factors for emergency reoperation were: active infective endocarditis before the primary operation, postoperative pneumonia after the primary operation, long-term dysfunction of the biological prosthesis, acute regurgitation on the bioprosthesis, and pulmonary hypertension. The independent risk factors for mortality during biological valve replacement surgery are: emergency surgery, high transvalvular gradient during the primary operation, and two- or three-vessel coronary artery disease. According to our data, repeat operations in the first 3 years after the primary operation were performed in 18 patients; they were associated with endocarditis before the primary operation, prosthetic valve thrombosis, paraprosthetic fistula and a high transvalvular gradient before the primary operation. Overall mortality within 30 days after aortic valve replacement was 5.2% (9 out of 172 patients). Moreover, the mortality rate in the group of planned reprosthetics was only 1.4% (2 out of 141), while emergency reprosthetics was accompanied by a mortality rate of 22.6% (7 out of 31) [12]. In our study, there were no deaths during emergency valve reinterventions. Both deceased patients underwent two-valve replacement.
According to research [5], simultaneous operations on two valves have a higher mortality rate. The main cause of early mortality in simultaneous mitral and aortic valve replacement was severe heart failure with low ejection fraction [7]. According to our data, the first cause of death was spontaneous rupture of the left ventricular myocardium of the 2nd type, while cardiolysis of the left parts of the heart was not performed, and there were no prerequisites for myocardial injury in the rupture zone. The cause of the second death was pulmonary embolism.
Paraprosthetic fistula was the reason for reoperation in 13 (10.9%) patients. In 10 of them, a paraprosthetic fistula occurred after mitral valve replacement and in 3 after aortic valve replacement. In 2 patients, after mitral valve replacement, it was possible to perform suturing of the paraprosthetic fistula, although the size of the paraprosthetic fistulas was more than 1 cm.
N. Fukunaga et al. [4] conducted a retrospective analysis of 118 reoperations of mitral valve replacement performed over 20 years. Worsened survival after repeat mitral valve surgery was observed in patients with tricuspid regurgitation grade 2+ or greater. Based on the results of the study, it was concluded that it is necessary to maintain the level of tricuspid regurgitation in the postoperative period no higher than 2+ to improve long-term survival. In our series of 90 recurrent heart valve surgeries, we performed De Vega tricuspid valve annuloplasty at the time of reoperation in 31 patients. Tricuspid valve annuloplasty was performed in patients with grade 2 or higher tricuspid regurgitation at the time of reoperation.
Conclusion
Repeated heart surgeries are one of the most difficult categories of surgical interventions, accompanied by high mortality and a large number of complications. In our group of patients, we were able to obtain good results in terms of mortality and the number of postoperative complications. We attribute these successes primarily to the coordinated work of all services providing surgical intervention and postoperative care. From a surgical point of view, the choice of access technique for visualizing cardiac structures is individual, however, we most often give preference to standard resternotomy, which has a number of undeniable advantages: it provides the most complete overview of all cardiac structures, allows for concomitant interventions on the heart and has a high level of safety with the correct technique its implementation.
In the 3-year period after the primary operation, the most common reasons for reoperation were: infective endocarditis, thrombosis of a mechanical prosthesis, and paraprosthetic fistula.