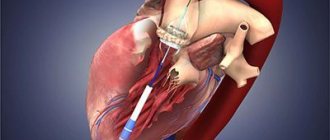
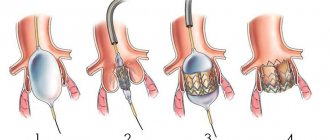
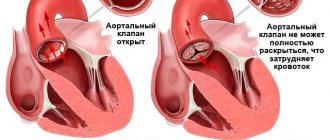
Heart valves are the basis of the internal frame of the heart, which are folds of connective tissue. Their functions boil down to delineating the volumes of blood in the atria and ventricles, allowing these chambers to alternately relax after pushing out blood at the time of contraction.
If the valve for some reason cannot perform its function, intracardiac hemodynamics, or internal blood flow, is disrupted
. Because of this, the heart muscle gradually wears out and heart failure develops. In addition, blood can no longer circulate normally throughout the body, since the pumping function of the heart is impaired, and blood stagnation occurs in the internal organs - kidneys, liver, brain. Sooner or later, if left untreated, congestion leads to dystrophy of all human organs, and, as a consequence, to death. Therefore, valve pathology is a fairly serious problem, in some cases requiring cardiac surgery.
There are two types of valve surgery – valve surgery and valve replacement. In the first case, the valve is reconstructed on a support ring and is used for heart valve insufficiency. The second type of operation involves complete replacement of the valve with a prosthesis. Heart valve replacement will be discussed in more detail below. Most often, the mitral and aortic heart valves are replaced.
Indications for surgery
The main indication for replacing a valve in the heart is its severe organic damage with the formation of heart disease,
having a significant effect on hemodynamics. Valve defects can develop as a result of rheumatic fever (rheumatism) - a form of streptococcal infection characterized by damage to the joints and heart (usually occurs as a result of frequent tonsillitis, chronic tonsillitis).
The need for valve replacement is taken into account based on the stage of heart failure, as well as according to data obtained from ultrasound of the heart (echocardioscopy).
valvular structure of the heart and an example of mitral valve stenosis requiring replacement
So, clinical indications for surgery:
- Fainting, chest pain, shortness of breath in patients with aortic valve stenosis,
- Clinical manifestations of aortic stenosis in patients who have undergone coronary artery bypass grafting,
- Severe stages of chronic heart failure - severe shortness of breath at the slightest household activity and/or at rest, significant swelling of the limbs, face, whole body (anasarca) in patients with moderate or severe mitral valve stenosis,
- Initial signs of heart failure (shortness of breath during significant physical exertion, heart rhythm disturbances) in patients with mild mitral valve stenosis,
endocarditis is one of the causes of valve damage
Infectious, or bacterial endocarditis - vegetation of bacterial inflammation on the inner lining of the heart, including the valves.
Echocardioscopy data:
- Severe (critical) aortic stenosis, even in the absence of clinical manifestations - the area of the aortic valve opening is less than 1 cm2,
- Reduced ejection fraction (the volume of blood thrown into the aorta with each contraction of the left ventricle) less than 50%,
- The area of the mitral annulus is less than 1.5 cm2,
- Ejection fraction with mitral stenosis is less than 60%.
A new approach to assessing cardiac remodeling during mitral valve replacement
SonoAce Ultrasound Magazine
Contains current clinical information on ultrasonography and is aimed at ultrasound doctors, published since 1996.
The diagnostic contribution of each of the structural and geometric indicators of cardiac remodeling used today is sufficiently informative, but reflects only one of the many aspects of this complex process. The role of an indicator that can comprehensively and fully reflect the dynamics of cardiac remodeling, in our opinion, can be performed by the ratio of the volumes of the heart cavities, called the volume remodeling index (VRI) of the cardiac cavities. IRO reflects the ratio of ventricular volume to atrium volume. It can be used separately to assess remodeling of the right and left hearts. Since a specially conducted study showed that remodeling of both the left and right chambers of the heart occurs according to an identical algorithm, it is considered most appropriate to assess the degree of cardiac remodeling by changes in the remodeling index of the volumes of the left chambers [1, 2].
Mechanical disc prostheses disrupt the physiological structure of blood flow: they divide a single blood flow into two eccentric flows, unequal in volume and speed (main and small), which leads to a violation of the homogeneity of the flow, exceeding flow rates above physiological values and the formation of zones of slow blood flow. Typically, surgeons orient these prostheses with the large opening toward the posterior wall of the left ventricle (LV). Deviation of the direction of flow may require additional time for adaptation of the LV cavity to the altered blood flow and longer periods of restoration of the geometry of the shape. Flow eccentricity (displacement of the center of flow from the center of the orifice of the disc prosthesis) is their key hemodynamic characteristic. Bicuspid prostheses are characterized by a central flow pattern (the center of the flow coincides with the center of the prosthesis passage), which is obviously more favorable for restoring the geometry of the LV cavity. The symmetry of the location of the obturator elements facilitates diastolic filling. The structure of the blood flow on these prostheses is more uniform, the deviation of the axis of blood flow movement is significantly less than that of disk models [1-10].
In general, disc prostheses for mitral replacement are not inferior to bicuspid types of ACL in terms of efficiency and safety of operation, patient survival rates and the development of complications within 10 years after surgery [7]. But the ability of disc types of ACLs to create the necessary physiologically similar flow in the LV cavity after mitral replacement is more limited, whatever the orientation of the large hole of the prosthesis in the LV cavity (Fig. 1).
Rice. 1.
EchoCG image of a disc prosthesis in the mitral position.
A)
Apical access.
b)
Apical access, with magnification.
V)
Along the short axis.
G)
With color mapping of transprosthetic flow.
Bicuspid prostheses, having a lower profile height compared to disc ACLs, meet the surgical requirements for partial or complete preservation of the LV chordal apparatus during mitral valve replacement [1-2]. The nature of the flow behind the bicuspid valve is more consistent with the organization of a transprosthetic flow close to the physiological type, which contributes to a more effective restoration of the geometry of the LV cavity (Fig. 2).
Rice. 2.
EchoCG image of a bicuspid prosthesis in the mitral position.
A)
Apical access.
b)
Apical access, with magnification.
V)
Along the short axis.
G)
With color mapping of transprosthetic flow.
Numerous studies have established that mitral valve replacement with mechanical prostheses of both types leads to a significant improvement in the geometric, structural and morphological parameters of all cavities of the heart. Both disc and bicuspid prostheses contribute to changes in the geometry of the heart cavities, which lead to improved intracardiac hemodynamics and an increase in the patient’s quality of life. Studies have proven that the design features of prostheses reliably influence the processes of remodeling of the heart cavities. Implantation of bicuspid prostheses creates more favorable conditions for restoring intracardiac hemodynamics and cardiac geometry [1, 2, 5].
The blood flow in biological prostheses is closest to native: the flexible leaflets of the bioprosthesis in diastole ensure freedom of the lumen of the valve passage and create a much smaller obstacle to transprosthetic blood flow. The blood flow through the biological prosthesis is predominantly laminar, oriented along the long axis of the LV to its apex (Fig. 3).
Rice. 3.
EchoCG image of a biological prosthesis (Sorin Perikarbon) in the mitral position.
A)
Apical access.
b)
Apical access, with magnification.
V)
Along the short axis.
G)
With color mapping of transprosthetic flow.
When the prosthesis is opened, the planes of the xenopericardial valves attached to the struts create a kind of short vessel, with a length equal to the height of the prosthesis frame struts (which is 18-22 mm), beyond which small paraflow vortices arise around the laminar diastolic transprosthetic flow, which practically do not distort the laminar nature transvalvular flow, its hemodynamic characteristics and direction [1, 2, 5].
In comparison with mechanical prostheses, in the long term after prosthetics, in patients with biological prostheses, the structural and geometric parameters of the heart are closer to normal than in patients with mechanical prostheses [5], and positive remodeling of the heart cavities after implantation of biological prostheses begins already in the early postoperative period .
It has also been established that with mitral valve replacement, restoration of atrial geometry significantly lags behind the restoration of the ventricles. Therefore, a complete restoration of the normal ratio of the heart cavities does not occur, but a significant improvement occurs, as evidenced by the dynamics of the values of the ratio of the volumes of the heart cavities. IRO made it possible to assess the degree of pathological remodeling of the cardiac chambers in acquired mitral valve defects before surgery and at all stages of surgical treatment. The statistical analysis we carried out earlier [1] confirmed the good quality and high predictive reliability of the proposed model for assessing the degree of pathological remodeling of the cardiac chambers using the IRO index. Three variants of the scenario for the restoration of the geometry of the heart chambers were proposed, depending on the initial level of IRO, reflecting the degree of pathological remodeling with a prognostic assessment of the restoration of the geometry of the heart [1]:
- Type I (mild degree) - area of favorable prognosis (ROI ≥ 0.9);
- Type II (moderate degree) - area of incomplete recovery (IRO 0.6-0.8);
- Type III (severe degree) is an area of poor prognosis (ROI ≤ 0.5).
This classification was tested on 368 patients, divided into 3 groups depending on the type of prosthesis implanted in the mitral position. In a comparative analysis of the characteristics of cardiac remodeling in these groups, a correlation was reliably established between the nature of changes in postoperative cardiac remodeling and the type of mitral prosthesis (Fig. 4).
Rice. 4.
Changes in the type (severity) of remodeling in patients:
A)
Group 1 with bicuspid dentures (%).
b)
Group 2 with disc prostheses (%).
V)
3rd group with biological prostheses (%).
Analysis of the heart geometry in the study groups according to the type of remodeling before surgery showed that in group 1, in patients with bicuspid valves, the proportion of prognostically favorable type I was 38.9%, which is almost 3.0% less than in group 2 patients with disc prostheses, and 13.9% more than in the 3rd group of patients with biological prostheses. The proportion of patients with prognostically unfavorable type III was the largest in the group of patients with biological prostheses and amounted to 50.0%. This turned out to be 22.2% higher than in the group with bicuspid prostheses, and 15.7% higher than in the group with disc prostheses.
The restoration of intracardiac hemodynamics and, accordingly, the geometry of the heart cavities in all studied groups began almost immediately after the operation (see Fig. 4).
In the early stages after mitral valve replacement, the proportion of patients with type I remodeling increased slightly: in the 1st group of patients with bicuspid prostheses - by 3.9%, in the 3rd group of patients with biological prostheses - by 16.6%. In the 2nd group of patients with disc prostheses, the proportion of people with type I decreased by 22.7%. In all groups, the proportion of people with prognostically unfavorable type III remodeling significantly decreased: in the 1st group - by 8.7%, in the 2nd group - by 9.3%, and in the 3rd group the most pronounced positive dynamics were noted (in early after surgery, the proportion of patients with unfavorable type III remodeling decreased by 41.7%).
In the long term after surgery, in group 1, the proportion of patients with type I increased by another 23.9%, in group 2 - by 33.1%, and in group 3 - by 47.3%. As a result, the proportion of patients with type I in group 1 was 66.7%, in group 2 - 52.2% and in group 3 - 88.9%. The proportion of patients with unfavorable type III decreased compared to the early postoperative period in the 1st group by another 12.4%, in the 2nd - by 16.3%, and in the 3rd group there were no patients with type III. In the groups with mechanical prostheses, the proportions of patients with type III were almost equal: 6.7% and 8.7% in groups 1 and 2.
The proportion of patients with II, moderately favorable type of remodeling in group 1 before surgery was 33.3%, in group 2 - 23.8% and in group 3 - 50.0%. In the early stages after surgery, the proportion of patients with type II increased significantly in all groups: in group 1 - by 4.8%, in group 2 - by 32.1%, and group 3 - by 25.0%. In the long term after surgery, it decreased in all groups: in the 1st group - by 11.4%, in the 2nd - by 16.5%, in the 3rd - by 38.9%. As a result, compared with the initial data, the proportion of patients with type II remodeling in group 1 decreased by 3.3%, in group 3 - by 13.9%, and in group 2 it increased by 15.6%, most likely due to improved cardiac geometry in patients with type III remodeling (see Fig. 4).
The structure of transprosthetic blood flows, design and hemodynamic features of various types of prostheses have a direct impact on the intensity of postoperative restoration of cardiac geometry. Therefore, the decrease in the proportion of patients with type I and the increase with type II in the early postoperative period can be explained by the fact that the hemodynamics of disc prostheses are less consistent with the requirements for rapid restoration of intracardiac hemodynamics and the geometry of the heart cavities than that of bicuspid prostheses. Therefore, in the early postoperative period, in the group of people with disc prostheses, adaptation to the altered transprosthetic blood flow apparently occurs, as a result of which some regression of the geometric parameters of the heart cavities is noted. Nevertheless, the use of disk-type prostheses for the mitral position of prosthetics is completely justified, since over the course of 5 years the heart gradually adapts to the specific functioning of the disk prosthesis and the geometry of the heart chambers is gradually restored, as a result of which the proportion of patients with type III, a prognostically unfavorable type of remodeling, is almost The proportion of patients with bicuspid prostheses is approaching. The structure of blood flow through a biological prosthesis is as close as possible to the hemodynamic characteristics of the native valve, creating the most favorable conditions for restoring the geometry of the heart chambers. It was in the 3rd group of patients with biological prostheses that the most noticeable positive dynamics in cardiac remodeling according to the RBI index was noted. In addition to the hemodynamic characteristics of the transprosthetic blood flow, such pronounced positive dynamics were facilitated by the fact that in this group there were few patients with rheumatic lesions of the mitral valve, but all patients with separation of the chordae from the mitral valve leaflets were in this group.
In the group with biological prostheses before surgery, the proportions of patients with types I and II were equal (25% each), the number of patients with type III was 50%. Thus, there were 26.3% more patients with III, the most unfavorable type of remodeling in group 3 than in the group with bicuspid prostheses, and 15.7% more than in the group with disc prostheses. There is an explanation for such a significant number of patients with a prognostically unfavorable type of remodeling: it was in the group with biological prostheses that the largest number of patients with class IV (62.8%) appeared. The severity of positive dynamics in the remodeling of the cardiac chambers was noted almost immediately after the operation. Finally, the study noted a pronounced positive trend in patients with type III remodeling, the proportion of which decreased by 41.7% already in the early stages after surgery, and in the long term there were no patients with this type of remodeling in the group with biological prostheses. This is quite consistent with the patterns of remodeling of all structural and geometric parameters of the heart in this group. The dynamics of the values indicates that the hemodynamic characteristics of the transprosthetic blood flow through the biological prosthesis are closest to native ones and create conditions that are closest to natural ones, which creates conditions that are most favorable for the restoration of intracardiac hemodynamics and the geometry of the heart chambers (see Fig. 4, c) .
Conclusion
This study using clinical material confirmed that all types of prostheses implanted in the mitral position contribute to the effective restoration of intracardiac hemodynamics. The nature and intensity of restoration of cardiac geometry is reliably influenced by the features of transprosthetic blood flow, determined by the design characteristics of the prosthesis. The presented research results convincingly prove that mitral valve replacement with mechanical prostheses in the long term after surgery ultimately leads to identical results, but the nature and timing of restoration of the heart cavities differ significantly. Thus, in patients with disc prostheses in the early stages after surgery, there was a significant regression in the geometry of the heart, which can be regarded as a reaction to the specificity of eccentric transprosthetic blood flow with impaired homogeneity and high flow velocity, with the formation of zones of slow flow, etc. Nevertheless, the disc prosthesis significantly improves intracardiac hemodynamics, which gradually leads to an improvement in the geometry of the cardiac chambers. The structure of the transprosthetic blood flow of a bicuspid prosthesis, separated by two symmetrical leaflets, is distinguished by a more physiological character. Therefore, already in the early stages after surgery, positive dynamics are noted in the remodeling of the cardiac chambers, which ultimately leads to a significant improvement in the geometry of the heart. As a result, there were 14.5% more patients with I, the most favorable type of remodeling in the group with bicuspid prostheses than in the group with disc prostheses, and 2% fewer with III, the most unfavorable type. But in the group with biological prostheses in the long term after surgery, there were no patients with type III left at all. The completely open free passage of the biological prosthesis does not create an obstacle to transprosthetic blood flow, maintaining its structure similar to the structure of blood flow on the native human mitral valve, which contributes to the rapid restoration of heart geometry and the transition to a more favorable type of remodeling already in the early stages after surgery. In the long term, the most favorable type I of remodeling in this group was 88.9%, and type II, moderately favorable, was 11.1%. The absence of prognostically unfavorable type III in the group with biological prostheses in the long term after surgery reliably indicates how important the nature of transprosthetic blood flow is in restoring the geometry of the heart. The closer the nature of transprosthetic blood flow is to physiological parameters, the faster and more fully the geometry of the heart is restored. Therefore, one of the productive directions for improving the structural form of a mechanical prosthesis is to find a solution that provides maximum freedom of passage opening.
conclusions
- Restoration of cardiac geometry in the long term after surgery can be determined by the dynamics of changes in the RRI index, which reflects the transition of the state of cardiac geometry from one type of remodeling to another.
- After mitral valve replacement, the geometry of the heart improves, but the degree and nature of recovery reliably depend not only on the nature and severity of the initial pathology, but also on the characteristics of transprosthetic blood flow, which influences the processes of remodeling of the heart cavities.
- Bicuspid prostheses are the most effective of all modern mechanical prostheses in the mitral position for restoring the geometry of the cardiac chambers. Disc prostheses, to a greater extent than bicuspid prostheses, distort the normal physiological structure of blood flow, which leads to disruption of the uniformity of flow, exceeding flow rates above physiological values, and the formation of zones of slow blood flow. This may require additional time for the cardiac chambers to adapt to the altered blood flow and contributes to a moderate regression of the IRO of the cardiac chambers in the early stages after surgery, increasing the overall duration of time for restoration of the geometric shape of the heart.
- The most pronounced positive dynamics in restoring the geometry of the heart cavities was noted when biological prostheses were implanted in the mitral position. The completely open free passage of the biological prosthesis does not create an obstacle to transprosthetic blood flow, maintaining its structure similar to the structure of blood flow on the native human mitral valve, which contributes to the rapid restoration of heart geometry and the transition to a more favorable type of remodeling already in the early stages after surgery.
Literature
- Bockeria L.A., Kosareva T.I., Makarenko V.N. Types of pathological cardiac remodeling in patients with acquired mitral valve defects // Clinical Physiology of Blood Circulation. 2011 (in print).
- Bockeria L.A., Kosareva T.I., Makarenko V.N. and others. Assessment of the ratio of the volumes of the heart cavities as an index of remodeling in acquired mitral valve defects. Clinical Physiology of Blood Circulation. 2010. N1. pp. 22-30.
- Bockeria L.A., Kosareva T.I., Makarenko V.N. and others. Remodeling of the cardiac cavities depending on the type of mechanical prosthesis after mitral replacement // Bulletin of the Scientific Center for Cardiovascular Surgery named after. AN. Bakulev "Cardiovascular diseases". 2010. N6. pp. 54-62.
- Ivleva O.V. Assessment of left ventricular remodeling processes in patients with sinus rhythm and atrial fibrillation before and after mitral valve replacement. Diss. ...cand. honey. Sci. M., 2008. 202 p.
- Kosareva T.I., Makarenko V.N., Muratov R.M., Fadeev A.A. Echocardiographic assessment of remodeling of the heart cavities during mitral valve replacement with mechanical and biological prostheses // Clinical Physiology of Blood Circulation. 2010. N2. pp. 28-35.
- Abhayaratna WP, Barnes ME, Langins AP Reversal of left atrial remodeling in patients with “isolated” left ventricular diastolic dysfunction // J Am Coll Cardiol. 2007. N49 (Suppl A). P. 91A (abstr).
- Fiore AC, Swartz MT, Sharp TG Double-Valve Replacement with Medtronic-Hall or St. Jude Valve // Ann. Thorac. Surg. 1995. V. 59. P. 1113-1119.
- Greffe G., Henaine R., Metton O. et al. Choice of echocardiography method for postoperative evaluation of mitral valve replacement with a mechanical prosthesis // Arch. Cardiovasc. Dis.; 2008; 101(4): 204-212.
- Machler H., Gert R., Mathias P. et al. Influence of a tilting prosthetic mitral valve orientation on the left ventricular flow — an experimental in vivo magnetic resonance imaging study // Eur J Cardiothorac Surg. 2007. V. 32. P. 102-107.
- Machler H., Perthel M., Reiter G. et al. Influence of bileaflet prosthetic mitral orientation on left venticular flow — an experimental in vivo magnetic resonance imaging study // Eur J Cardiothorac Surg. 2004. V. 26. P. 747-753.
SonoAce Ultrasound Magazine
Contains current clinical information on ultrasonography and is aimed at ultrasound doctors, published since 1996.
Contraindications for surgery
Heart valve replacement surgery is contraindicated for the following diseases and conditions:
- Acute myocardial infarction,
- Acute cerebrovascular accidents (stroke),
- Acute infectious diseases, fever,
- Exacerbations and worsening of chronic diseases (diabetes mellitus, bronchial asthma),
- Extremely severe heart failure with an ejection fraction of less than 20% with mitral stenosis, in which case the attending physician should decide whether a heart transplant is necessary.
Prosthetic heart valves – what are they?
Since the 1970s, the configuration of prosthetic valves has undergone some changes. Valves based on ball prostheses are considered one of the most outdated.
Later, valves based on hinged disc prostheses began to be used.
The most modern valves are those based on bicuspid hinged prostheses, which are currently used.
In addition, in patients with an increased risk of thrombosis, models obtained from the pig heart are used - biological prostheses, or xenografts.
The disadvantage of mechanical prostheses is the high rate of formation of blood clots on the valve leaflets
, which is associated with a high risk of pulmonary embolism, ischemic stroke, thrombosis of the femoral arteries with possible amputation of a limb, etc. In this regard,
in elderly people (over 65 years old), it is preferable to undergo valve replacement surgery with a biological prosthesis.
It is also possible to have an operation with prosthetic replacement of the aortic valve with the patient’s own pulmonary valve with simultaneous replacement of the latter with a biological prosthesis.
The disadvantage of biological prostheses is the high risk of re-development of bacterial inflammation on the installed porcine valve.
The service life of the valves in the absence of complications is from 10 to 15 years; if the valve wears out, it is possible to perform a second operation to replace it.
Patient Information
- CELL TRANSPLANTATION: real achievements and opportunities for clinical application
- Choosing a prosthesis in Russian conditions
- Coronary artery bypass surgery without artificial circulation - what should be considered when choosing a clinic to perform it?
- “With a whip of a butt... you’ll kill it!”, or the truth about coronary artery bypass grafting and stenting
- Delays in issuing clopidogrel prescriptions to patients after stenting are a common and deadly problem.
The traditional type of surgical treatment of damaged heart valves in our country and abroad is their replacement with various prostheses.
At the early stage of the development of cardiac surgery, doctors tried to use valve devices based on biological tissues of xenogeneic (i.e., borrowed from animals) or allogeneic (homogeneous) (i.e., borrowed from humans) origin as replacement material. The main disadvantage of these devices is the limited service life of the valve due to the gradual destructive effect on biological tissues from the recipient's body. Since in those years (60-70) any heart surgery was perceived as a difficult test for the patient (and also for the surgeon), the primary task was to develop a type of prosthesis, the implantation of which would be final in the surgical treatment of valve disease. Bioprostheses clearly did not meet this condition. The search began for materials and models for creating synthetic, mechanical prostheses. More than several dozen models have been created, of which only two design types have modern worldwide recognition and distribution: a prosthesis with one folding round disk (Fig. 1) and a prosthesis with two wings in the shape of a semicircle (Fig. 2-1, Fig. 2-2 ).
| Fig. 1 Domestic single-disk prosthesis “LIKS-2” | Fig. 2-1 Domestic double-leaf prosthesis “Carbonics-1” | Fig. 2-2 Bicuspid prosthesis from Sulzer Carbomedics (USA) |
Implantation of mechanical prostheses from a very early stage was accompanied by frequent cases of the development of cerebral circulatory disorders with deep disability of patients or their death, and therefore the main condition for the life of a patient with a mechanical prosthesis was developed - taking drugs that disrupt the blood coagulation process by reducing the production of coagulation proteins in liver. Targeted selective suppression of liver function for this purpose was achieved by prescribing specific substances to the patient - coumarin derivatives. Treatment with coumarin derivatives refers to the so-called anticoagulant therapy, and the substances themselves are called indirect anticoagulants. Several decades of use of mechanical prostheses have not produced as satisfactory results as expected, regardless of the model of the prosthesis. The main problem after their implantation remains the high incidence of circulatory disorders in organs, including the brain, due to the separation of small blood clots from the synthetic moving parts of the prosthesis, the possibility of the formation of which exists even with diligent and excessive anticoagulant therapy. On the other hand, drug-induced aggravation of blood coagulation disorders increases the risk of bleeding of various locations, often fatal. Therefore, in patients with stomach and duodenal ulcers, severe hypertension with the risk of cerebral hemorrhages, etc., the use of anticoagulants is very dangerous.
At present, we can say with confidence that attempts to modify the mechanisms of fastening the valves of mechanical prostheses, giving them mobility, creating a prosthesis from flexible synthetic structures, etc. have completely exhausted their capabilities in reducing the incidence of thromboembolic circulatory disorders, and the use of anticoagulant therapy with all its possible complications is mandatory for any mechanical prosthesis.
Having exhausted the reserves for changing the models of mechanical prostheses in relation to reducing circulatory disorders, researchers and clinicians are trying to find ways to precisely control the action of indirect anticoagulants in the hope of being able to deepen anticoagulant therapy while minimizing the complications of the latter. Since the mid-80s, in the countries of Western Europe and North America, there has been a laboratory testing system for all reagents to perform an analysis of the depth of anticoagulant therapy (reagents for determining the so-called prothrombin index, time, ratio) in comparison with the World Health Organization standard ( WHO). Reagents whose sensitivity differs from the WHO standard by more than 20% are considered unsuitable for use. The remaining reagents are used with a correction factor to determine the modern form of expression for prothrombin time - the International Normalized Ratio (INR).
Finally, manufacturers of mechanical prostheses have created personal coagulometers based on the determination of INR using strips with dry reagents. The patient himself or with the help of relatives takes blood by piercing the fingertip, applies a drop of blood to a certain place on the strip with reagents, and then inserts the strip into the device, on the screen of which after 30-60 seconds. The INR value is given.
In the cardiac surgical literature, the impact of new methods of monitoring therapy with indirect anticoagulants on the incidence of cerebrovascular accidents and the life expectancy of patients with mechanical prostheses in general is now being assessed with hope.
From the above, we can conclude that despite all the measures taken, thrombus formation on a mechanical prosthesis remains highly probable, and anticoagulant therapy, which is unsafe in itself, is mandatory for a patient with any mechanical prosthesis.
Now let’s evaluate the Russian living conditions of a patient with mechanical prostheses.
Domestic single-disk prostheses are quite reliable and have shown a low frequency of breakdowns in the human body during long-term operation, which cannot be said about double-leaf domestic models, the dysfunction of which is of a purely technical nature (loss or fragmentation of the valves) is associated with a series of scandalous lawsuits in the mid-90s . Such breakdowns are clinically extremely difficult, and if the patient is far from a specialized cardiac surgery center, death is likely in almost 100% of cases. In fairness, it should be noted that all the world's major manufacturers of mechanical prostheses did not avoid cases of technical breakdowns of their models, although the frequency of their occurrence was low.
As for the problems of blood clotting on a prosthesis, all of the above points can be completely transferred to our Russian prostheses, but with some national characteristics, which are outlined below.
Firstly, in Russia there are real difficulties in accurately determining the prothrombin test in one form or another due to the purchase by hospitals of a wide variety of reagents, often very far from WHO standards. There is a vicious practice of long-term storage of the prepared reagent in order to save money, as a result of which the digital expression of the prothrombin test completely misinforms both the patient and the attending physician. The definition of the modern form of expression for prothrombin time, i.e. INR is performed in even fewer laboratories in the country. As a result, reliable analysis can be obtained only in hospitals where heart operations are performed and great attention is paid to verification of the technique. The second feature follows from this.
Secondly, if we consider that there are only a few dozen such hospitals and centers in Russia, then one can imagine the obstacles in obtaining analysis, which are of a territorial nature.
Thirdly, indirect anticoagulants are not produced in Russia. Among the inexpensive drugs, there is the drug phenylin produced by the Kharkov Chemical Pharmaceutical Plant (KCP) and the Tallinn CPZ. Despite the same dosage, the effect of the drugs is noticeably different. Moreover, we noticed differences between different series of the drug from the same plant. All this again requires bringing the patient closer to a laboratory that can assess the effect of taking the drug. Fortunately, the highly standardized indirect anticoagulant warfarin from Nycomed is now on sale in Russia, and individual coagulometers from Sulzer are also imported into Russia, but the cost of these products is incomparable with the above-mentioned drugs, and they are inaccessible to most Russians.
Fourthly, the awareness of provincial Russian doctors about the principles of therapy with indirect anticoagulants is completely insufficient. Our practice provides many examples of a doctor’s complete misunderstanding of how the results of the analysis and the dosage of the drug relate, what tactics to choose when performing surgical procedures in a patient taking indirect anticoagulants, etc. As a result, the patient himself must have a deep understanding of this subject, i.e. The principle that works is “saving drowning people is the work of the drowning people themselves.”
Over the years of mechanical prosthetic fascination, years of hope and disappointment, cardiac surgery has advanced, reducing and reducing the mortality associated with the operation, with the result that repeated cardiac surgery has become commonplace, performed with risks no different from the initial operation.
In the new conditions, it is necessary to reassess our views on valve prosthesis, which consists in the fact that the quality of life of a patient with a prosthesis should be more significant for us than the fear of a second operation, that the apparent reliability and durability of a mechanical prosthesis results in the replacement of one disease with another, sometimes more more dangerous than the first. That is why we offer patients to return to implantation of prostheses made from biological tissues. These prostheses do not require therapy with indirect anticoagulants, modern methods of processing biological tissue make it possible to ensure normal operation of the prosthesis for up to 10-12, and sometimes up to 20 years, the biological degradation of the prosthesis occurs gradually, without the occurrence of critical situations, like a natural human valve affected by a pathological process. Let's look at the instructions for the biological prosthesis Sulzer Mitroflow Synergy in the “Indications” section: “... the valve is proposed for use in those patients who do not want long-term anticoagulant therapy for various reasons, such as living in remote areas, the presence of diseases of the gastrointestinal tract or other potential sources of bleeding in whom surgery in other areas is expected, in elderly patients, and in other situations where difficulties in anticoagulant therapy are expected for social or medical reasons.” Are there any patients who are candidates for implantation of a mechanical prosthesis? Who can know what awaits a patient with a mechanical prosthesis in the future, whether he will develop a stomach ulcer, whether he will need surgery, whether a young woman will want to give birth to a child? Life with a mechanical prosthesis is full of dangers and limitations.
That is why we have in our arsenal and offer patients a large selection of valve replacements created from biotissues. Among them are xenoarterial (Fig. 3) and xenopericardial (Fig. 4) prostheses from the Brazilian company BRAILE, biological prostheses from Sulzer (Fig. 5-1), (Fig. 5-2), and domestic bioprostheses "KemKor" (Kemerovo) (Fig. 6) and, finally, donor homografts of the original production (Fig. 7).
| Rice. 3 Biological xenoaortic prosthesis BRAILE (Brazil) | Fig.4 Biological xenopericardial prosthesis BRAILE (Brazil) | Rice. 5-1 Biological xenopericardial prosthesis Mitraflow Synergy (USA) |
| Rice. 5-2 Biological xenoaortic prosthesis “LABCOR” (USA) | Fig.6. Domestic biological xenoaortic prosthesis “KemKor” | Fig.7 Homoaortic graft (homograft, allograft) |
Preparing for surgery
Once a diagnosis of heart disease or infective endocarditis has been made, the decision regarding the need to replace the affected valve should be made as soon as possible. After this, the patient undergoes the required minimum of clinical studies and is referred by the attending physician to the cardiac surgery center. Typically, surgery can be performed within a few months of diagnosis. If a patient submits an application to the regional health department for a quota (budgetary allocations from the federal budget to provide high-tech assistance to the population), then a response to the quota can be received within 20 days.
For admission to the cardiac surgery department, the following documents and examinations are required:
- Passport, insurance policy, SNILS,
- Referral from the treating cardiologist or therapist,
- Extract from the previous place of hospitalization (department of cardiology, therapy) with the examination methods performed,
- If the patient has not been hospitalized, it is necessary to perform on an outpatient basis general clinical blood and urine tests, a biochemical blood test, determination of blood group and blood coagulation ability, ultrasound of the heart, ECG, 24-hour monitoring of ECG and blood pressure, chest x-ray, exercise tests (treadmill test, bicycle ergometry),
- You may need to consult an ENT doctor, gynecologist, urologist and dentist to exclude foci of chronic infection.
Operation technique
The patient is given general anesthesia. After opening the chest, the patient is connected to a heart-lung machine. Mitral valve replacement involves opening the left atrium, and aortic valve replacement involves opening the aortic wall. The doctor removes the affected valve and sews in a new one. The duration of the operation is 3 - 6 hours.
If such surgery is contraindicated for the patient, aortic heart valve replacement can be performed endovascularly or minimally invasive surgery can be used.
How is the operation performed?
Preoperative preparation is limited to the prescription of sedatives and hypnotics. The operation is performed under general anesthesia on the same or the next day after hospitalization using a heart-lung machine, which performs the functions of pumping blood throughout the body during manipulations.
After putting the patient into deep sleep, a median sternotomy is performed - a longitudinal incision of the skin and sternum. Next, an incision is made in the left atrium for mitral valve replacement and in the aortic wall for aortic valve replacement. After this, the prosthesis ring is fixed with continuous sutures and the dissected part of the heart is sutured.
After installing the prosthesis, electrodes for temporary cardiac stimulation must be applied, and the surgical wound is sutured. Wire sutures are used to fuse the edges of the sternum.
In the early postoperative period, the patient is in the intensive care unit with artificial ventilation, the cessation of which is possible only when the patient is completely stabilized and spontaneous breathing is restored.
The operation time is from three to six hours, and the hospital stay is determined by the general condition of the patient and ranges from two to four weeks.
In addition to open heart operations, it is currently possible to perform minimally invasive operations, in particular, with a mini-access from an intercostal incision on the right or left without dissecting the sternum, as well as with endovascular intervention.
minimally invasive aortic valve replacement
The latter is used only for aortic valve replacement and is carried out by introducing a biological prosthesis through the femoral vein into the right and then into the left atrium with further location in the aorta.
Endovascular heart valve replacement is primarily preferred for individuals for whom open heart surgery is contraindicated.
Video: report on valve replacement surgery
Cardiac surgery for mitral valve defects
Return to section:
Cardiac surgery department
The mitral valve is located between the left atrium and the left ventricle.
Normally, it opens in diastole, allowing arterial blood to pass from the left atrium into the left ventricle, and closes in systole under the influence of blood pressure during contraction of the left ventricle, preventing the reverse flow of blood from the left ventricle into the left atrium.
There are 3 types of mitral valve defects:
1) Mitral insufficiency. With pathology of connective tissue or changes in the heart muscle, a disturbance in the structure of the mitral valve occurs, which leads to “bending” of its valves into the cavity of the left atrium during contraction of the left ventricle, part of the blood flows back into the atrium.
2) Mitral stenosis. Due to infectious diseases such as rheumatism, the MV narrows, which leads to disruption of the flow of blood from the left atrium to the left ventricle.
3) Combined MV defect (insufficiency + stenosis).
Currently, in modern cardiac surgery for mitral valve defects, reconstructive (valve-preserving) operations are performed, as well as operations to replace (prosthetics) valves with a mechanical or biological prosthesis. Reconstructive operations are performed both for stenosis and mitral valve insufficiency.
Reconstructive surgeries
| Fig. 1 . Mitral annuloplasty on a support ring. |
An example is annuloplasty, which involves restoring the function of the affected valve using a rigid or elastic support ring.
It is fixed to the walls of the heart at the level of the opening connecting the atrium to the ventricle. As a result of sewing in such a support ring, the diameter of the atrioventricular orifice decreases, which ensures more complete closure of the valves and normalizes intracardiac blood flow.
Another option for reconstructive surgery on heart valves is surgery using various suture techniques.
During this procedure, the surgeon performs plastic surgery on the valve leaflet tissue, removes calcified deposits, or restores the structure of the altered chordae tendineae, which control the movement of the leaflets.
Rice. 2 .
Suture plasty of the mitral valve. A, B – resection of the altered portion of the posterior mitral valve leaflet; C, D, E – stages of eliminating the gap in the valve.
MK prosthetics.
A radical method of treating the mitral valve is its replacement. Modern mechanical (artificial heart valves) and biological valves are used.
| Mechanical prosthesis MK | Biological prosthesis MK |
Fig.3 . Mechanical (left) and biological (right) mitral valve.
In the case of pronounced morphological changes in the patient’s own valve, when it is no longer possible to preserve it, the valve is removed followed by prosthetics. Surgical replacement of the mitral valve is performed on a non-functioning heart using a heart-lung machine.
Mechanical (artificial heart valves) are very reliable, last a lifetime and do not need to be replaced, but require constant use of special medications to reduce blood clotting.
Biological valves (of animal or human origin) can deteriorate over time, and the lifespan of these valves is highly dependent on the age of the patient and his concomitant diseases. With age, the process of destruction of biological valves slows down greatly. The decision about which valve is the best option in a particular situation is made individually before surgery in a mandatory conversation between the surgeon and the patient.
Technical progress in cardiac surgery, namely the introduction into daily surgical practice of new instruments that make it possible to modify surgical approaches to the heart, inevitably puts forward the goal of performing them with minimal intraoperative trauma for the patient.
Choosing the most rational access to various parts of the heart is one of the necessary conditions for solving this problem.
Online access should ensure the solution of the main tasks:
- provide the surgeon with sufficient space for ease of manipulation in the surgical area;
- surgical access should preferably be less traumatic for the patient.
The traditional surgical approach to the heart is a longitudinal median sternotomy (Fig. 4).
| Diagram of median sternotomy | Type of postoperative scar |
On the one hand, it allows the surgeon to perform the necessary surgical procedures on the heart for various forms of its pathology and is as convenient as possible for connecting a heart-lung machine.
On the other hand, this access may not be optimal. This is due to a number of reasons:
- Greater trauma, the integrity of the chest is compromised, which requires a longer period of healing of the postoperative wound.
- High risk of postoperative complications (sternal instability). These complications are especially dangerous in elderly patients.
- Questionable cosmetic effect.
Fig.5 . View of the surgical field during reconstructive interventions on the mitral valve from a minimally invasive approach to the heart.
All cardiac surgeons will agree that mitral valve reconstruction through a minimally invasive approach (right minithoracotomy) should be performed on the patient with the same skill and quality as when performing operations through a median full sternotomy.
Correction of the mitral valve from a right-sided minithoracotomy is performed in the 4th intercostal space, 6 cm long, in the projection of the 4th intercostal space on the right.
| Scheme for performing a right minithoracotomy | Type of postoperative scar |
Benefits of Minimally Invasive Valve Surgery
- Less pain syndrome. Since access to the heart is through a right lateral minithoracotomy, where the length of the skin incision is about 7 cm, the frame function of the chest is also preserved, i.e. its integrity is not compromised than with the traditional approach - median sternotomy, where the length of the skin incision is about 20 cm, and to perform surgical manipulation of the heart it is necessary to saw the sternum along the midline.
- Reduced risk of complications. Since the chest wall maintains its integrity, the risk of developing complications such as postoperative sternal instability is eliminated.
- Faster recovery and return to normal activities. Less surgical trauma and preservation of the frame function of the chest after minithoracotomy allows for early activation and rehabilitation of patients, which helps reduce the length of hospital treatment.
- The scar is less noticeable. Most patients are very satisfied with the cosmetic results after surgery.
← Back
Cost of the operation
In most cases, surgery to replace heart valves is performed free of charge, thanks to quotas from the Russian healthcare system under the compulsory medical insurance system. However, if for some reason it is not possible to obtain a quota, there is always the option of carrying out the operation at your own expense.
The cost of the operation itself, the prosthesis and rehabilitation in the early postoperative period ranges from 90 to 300 thousand rubles
, and the price is higher, the more complex the operation is, for example, the simultaneous replacement of the aortic valve and pulmonary valve is higher than one of them.
Heart valve replacement surgeries are carried out in all major cities of Russia, and now such interventions are not rare or inaccessible to the population.
Types of heart valve replacement surgeries
Surgeons perform 2 types of operations. Valve insufficiency is corrected using plastic surgery. When the valve narrows (stenosis), it is completely replaced. The most common surgery is to replace the heart's mitral or aortic valve.
Two types of prostheses are used - biological and mechanical. Biological, porcine valve, is installed in elderly patients. It does not provoke blood clots and lasts 8-15 years. The disadvantage of a porcine prosthesis is the high risk of bacterial inflammation. The mechanical valve has a service life of more than 20 years, but with it the person must take blood thinners for life.
A heart valve is replaced when it wears out. The cost of the operation is determined by the surgeon based on a complete examination of the patient.
Complications
The most serious complications after the introduction of a prosthesis are thromboembolic ones. Prevention of their development is lifelong antithrombotic therapy with the help of anticoagulants and antiplatelet agents - drugs that “thin” the blood. Such drugs include:
- Subcutaneous injections of heparin in the early postoperative period,
- Continuous use of warfarin under monthly monitoring of INR (international associated ratio) - an important indicator of the blood clot-forming system; normally it should be within 2.5 - 3.5,
- Constant use of aspirin (thromboAss, acecardol, aspirin Cardio, etc.).
No less dangerous consequences are the development or recurrence of infective endocarditis, the prevention of which is the rational prescription of antibiotics in the postoperative period, as well as their further use during any operations and minimally invasive interventions (tooth extraction, gynecological and urological manipulations, etc.).
Lifestyle
A person’s further life after surgery comes down to the following points:
- Regular visits to the doctor - monthly in the first year after surgery, every six months in the second year and annually thereafter, with constant monitoring of the functions of the cardiovascular system using ECG and echocardioscopy,
- Regularly taking prescribed medications (anticoagulants, antibiotics),
- Treatment of residual heart failure with the constant use of digoxin and diuretics (indapamide, veroshpiron, diuver, etc.),
- Adequate physical activity
- Compliance with the work and rest schedule,
- Following a diet - excluding fatty, fried, salty foods, eating large amounts of vegetables, fruits, dairy and cereal products,
- Complete elimination of bad habits.
Forecast
The prognosis after surgery is undoubtedly higher than without it,
since with heart defects severe heart failure develops, which not only impairs the tolerance of normal physical activity, but also leads to death. In patients after surgery, mortality is much lower, and is mainly associated with the development of thromboembolic complications (0.2% of deaths per year). Therefore, surgery to replace heart valves is an intervention that significantly prolongs the patient’s life and improves its quality.
MenuRKDC
What artificial heart valves are there?
Currently, there are two main types of artificial heart valves: mechanical and biological, which have their own characteristics, advantages and disadvantages.
Mechanical heart valves
These are prostheses that serve to replace the function of a person's natural heart valve. The human heart has four valves: tricuspid, mitral, pulmonary and aortic. The purpose of the heart valves is to ensure unhindered blood flow through the heart through the pulmonary and systemic circulation to organs and tissues. As a result of various diseases, the functioning of the valves (one or more) is disrupted, which is manifested by a narrowing of the valve opening or its insufficiency. Both of these processes can lead to the gradual development of heart failure and deterioration in health. Mechanical heart valves are designed to replace a diseased valve with a prosthetic valve to restore its function and thereby restore adequate heart function.
Types of Mechanical Heart Valves
There are three types of mechanical heart valves: ball, sloping disc and bicuspid - in various modifications.
The first artificial heart valve was a ball valve, which consists of a metal frame enclosing a silicone elastomer ball. When the blood pressure in the heart chamber exceeds the pressure outside the chamber, the ball is pushed against the frame and allows blood to flow. Upon completion of the contraction of the heart muscle, the pressure in the chamber decreases and becomes lower than behind the valve, so the ball moves in the opposite direction, closing the passage of blood from one chamber of the heart to another. In 1952, Charles Hufnagel implanted a ball heart valve in ten patients (six of whom survived the operation), marking the first successful long-term use of artificial heart valves. A similar valve was invented by Miles "Lowell" Edwards and Albert Starr in 1960 (in the literature it is called the silastic ball valve). The first human heart valve implant was performed on September 21, 1960. It consisted of a silicone ball enclosed in a frame created from the base of the valve. The ball valve has a high tendency to form blood clots, so such patients are forced to constantly take high doses of anticoagulants. ceased production of these valves in 2007.
Disc heart valves were soon created. The first clinically available artificial heart disc valve was the Bjork-Schily valve, which has undergone various significant changes since its invention in 1969. The disc valve consists of a single circular obturator, which is adjusted by a metal spacer. They are made from a metal ring coated with porous polytetrafluoroethylene. Mechanical prostheses are much more durable, but require lifelong anticoagulant therapy and are less physiological. In general, the problem of choosing a prosthesis is a really big problem for both the surgeon and the patient. Therefore, the motto “Every patient has his own prosthesis” is very relevant and is unlikely to lose relevance in the foreseeable future. In which threads are sewn to hold the valve in place. A metal ring with the help of two metal supports holds a disk that opens and closes while the heart performs its pumping function. The valve disc itself is usually made of an extremely hard carbon material (pyrolytic carbon) to allow the valve to operate without wear for many years. In the United States, the most popular model of disc heart valve is the Medtronic-Hall model. In some models of mechanical heart valves, the disc is divided into two parts that open and close like doors.
St. Jude Medical is a leader in bicuspid valves, which consist of two semicircular valves that rotate around a spacer attached to the base of the valve. This design was proposed in 1979 and, although they helped overcome some of the problems that were noted with some valves, bicuspid valves are susceptible to backflow of blood (regurgitation) and therefore cannot be considered ideal. However, bicuspid valves allow blood to flow more naturally than ball or disc valves. One of the advantages of these valves is that they are well tolerated by the patient.
Mechanical heart valves are the most reliable and trustworthy today and allow the patient to live a normal life. Most mechanical valves last a minimum of 20-30 years.
Hydraulics
Many complications associated with mechanical heart valves can be explained by hydraulics. For example, blood clot formation is a side effect of the cutting force created by the shape of the valves. An ideal artificial valve in the future should have minimal pressure on its components, be characterized by minimal regurgitation, minimal turbulence, and not separate the blood flow in the valve area.
Effect on blood
One of the main disadvantages of mechanical heart valves is that patients with such valves must constantly take blood thinners (anticoagulants). Blood clots that form as a result of the destruction of red blood cells and platelets can block the lumen of blood vessels, which leads to serious consequences.
All models of mechanical heart valves are susceptible to the formation of blood clots due to high stress activity, stagnation and separation of blood flow. The ball valve design results in stress on the walls, which damages cells and also separates the blood flow. The disc valve also suffers from separation of blood flow behind the valve strut and disc as a result of a combination of fast and slow flows. Bicuspid valves are characterized by high stress activity, as well as leakage and slowing of blood flow near the valve.
In general, blood cell damage is observed in both mitral and aortic prosthetic valves. Valvular thrombosis is most often characteristic of an artificial mitral valve.
Since mechanical heart valves are subject to stress, patients require constant use of anticoagulants. Bioprostheses are less susceptible to blood clots, but given their longevity, they are usually most useful in people over 55 years of age.
Mechanical heart valves can also cause hemolytic anemia and hemolysis of red blood cells as they pass through the valve.
Biological valves
Biological valves are valves that are created from animal tissue, such as pig heart valve tissue, and are pre-treated with some chemical treatment to make them suitable for implantation in the human heart. The thing is that the pig heart is more similar to the human heart than others, and therefore is best suited for use in replacing heart valves. Porcine heart valve implantation is a type of so-called. xenotransplantation.
Another type of biological valve uses biological tissue that is sutured to a metal frame. The tissue for such valves is taken from bovine or equine pericardium. Pericardial tissue is very suitable for valves due to its extraordinary physical properties. This type of biological valves is very effective for replacement. The tissue for such valves is sterilized, as a result of which they cease to be foreign to the body, and no rejection reaction is observed. These valves are flexible and durable.
Mechanical prostheses are much more durable, but require lifelong anticoagulant therapy and are less physiological. In general, the problem of choosing a prosthesis is a really big problem for both the surgeon and the patient. Therefore, the motto “Every patient has his own prosthesis” is very relevant and is unlikely to lose relevance in the foreseeable future.