Home / Encyclopedia of diseases / Diseases on A / Aortic aneurysm
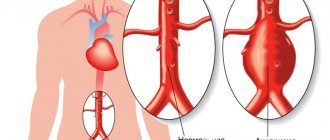
Aortic aneurysm is an enlargement of a section of the aorta caused by a pathological change in the connective tissue structures of its walls due to an atherosclerotic process, inflammatory lesion, congenital defect or mechanical damage.
Depending on the location of the aneurysm, aneurysms of the thoracic aorta, thoraco-abdominal (thoracic-abdominal) aorta and abdominal aorta are distinguished. In turn, thoracic aortic aneurysm can be divided into aortic sinus aneurysm, ascending aortic aneurysm, aortic arch aneurysm and descending aortic aneurysm.
In addition, a special category is a dissecting aortic aneurysm - a pathological cavity or canal formed in the thickness of the aortic wall as a result of its dissection with blood pumped from the lumen of the aorta through a defect in the intima (inner lining of the vessel) resulting from a pathological process or damage. Dissection of the aortic wall occurs inside its tunica media.
Symptoms of aortic aneurysm
Aortic sinus aneurysm may be accompanied by aortic valve insufficiency or narrowing of the lumen of the coronary arteries of the heart. Reaching a large size, such an aneurysm can compress the pulmonary trunk, right ventricle and right atrium, which leads to the formation of subacute right ventricular heart failure, characterized by liver enlargement, swelling of the jugular veins and the appearance of edema. Rapid compression of the pulmonary trunk by an aneurysm can lead to sudden death of the patient.
An aneurysm of the ascending aorta is usually manifested by dull retrosternal pain, which in some patients is accompanied by reflex attacks of shortness of breath. If the aneurysm reaches a large size, it can cause atrophy of the adjacent areas of the sternum and ribs, with pathological vascular pulsation appearing in the second or third intercostal space to the right of the sternum. Compression of the superior vena cava by an aneurysm or a breakthrough of the aneurysm into it leads to the development of superior vena cava syndrome, which in turn causes swelling of the neck, face, arms, and swelling of the jugular veins.
Aneurysm of the aortic arch most often manifests itself as shortness of breath (and, as a rule, it is more difficult to take a breath), caused by compression of the trachea and bronchi. Compression of the left main bronchus by an aneurysm can lead to atelectasis (collapse) of the left lung. Sometimes hemoptysis appears, which may precede the rupture of an aneurysm. Compression of the left inferior laryngeal nerve by an aneurysm is manifested by a dry cough, attacks of suffocation, and a change in voice timbre (hoarseness). The development of superior vena cava syndrome is possible. When an aneurysm compresses the brachiocephalic trunk, left subclavian and left common carotid arteries, symptoms of a gradually worsening disturbance of the blood supply to the upper extremities and head appear. A rupture of an aortic aneurysm into the esophagus or trachea is possible, which, as a rule, develops gradually, which is initially manifested by the appearance of scanty bloody vomiting or hemoptysis, but then massive bleeding develops.
An aneurysm of the descending aorta leads to compression of the nerve roots, vertebral bodies, esophagus and left lung. Compression of the nerve roots leads to intense pain that is resistant to the administration of the most powerful painkillers. Pressure on the vertebral bodies and posterior parts of the ribs leads to their deformation, to the point that the aneurysmal sac can protrude between the inner edge of the left scapula and the spinal column. These patients may develop lower paraplegia (complete loss of voluntary movements of both lower extremities). Compression of the left lung by an aneurysm leads to its atelectasis and creates favorable conditions for the occurrence of pneumonia. Compression of the esophagus in some cases can lead to difficulty passing food through it (dysphagia). When the wall of the esophagus is destroyed due to prolonged pressure on it from the aneurysm, small bleeding from the esophagus occurs, after which, as a rule, the aneurysm ruptures into its lumen with the development of massive bleeding. When a descending aortic aneurysm ruptures into the pleural cavity, rapidly increasing anemia (anemia) and large hemothorax (accumulation of blood in the pleural cavity) develop.
Aneurysm of the thoracoabdominal (thoracoabdominal aorta) aorta is rare and is usually caused by syphilis. The aneurysm compresses the esophagus and upper stomach, which leads to pressing pain in the epigastric region, which can be associated with food intake, sometimes belching, vomiting, and disruption of the passage of food through the esophagus. An aneurysm of the thoracoabdominal aorta can cause narrowing or complete occlusion of the lumen of the superior mesenteric artery and the celiac trunk, which supply blood to the abdominal organs, which is manifested by attacks of painful abdominal pain (the so-called abdominal pain). Due to the reasons described above, an aneurysm of this location leads to weight loss for the patient.
An abdominal aortic aneurysm over time manifests itself as pain caused by the pressure of the aneurysm on the nerve plexuses and nerve roots located directly next to it. Pain may be in the lumbar or epigastric region. A large aneurysm located below the origin of the renal arteries can compress the ureters, causing the development of hydronephrosis and anuria. If compression of the renal arteries occurs, symptomatic hypertension occurs. When the duodenum is compressed by an aneurysm, the passage of food through it is disrupted, which is manifested by vomiting and weight loss. Most often, an abdominal aortic aneurysm is manifested by the presence of a pulsating tumor-like formation in the abdominal cavity at the level of the navel or just below and slightly to the left of it. A thrombosed aneurysm does not pulsate, and therefore can be mistaken for a tumor. Sometimes there is a rise in body temperature. An aneurysm ruptures into the abdominal cavity quickly and, as a rule, painlessly, and into the retroperitoneal tissue - with severe pain in the abdomen and lower back, with the development of shock. After some time, the patient may die due to increasing blood loss.
Dissecting aortic aneurysm is manifested by sudden onset of acute chest pain that is not relieved by painkillers and collapse. Sometimes a complete loss of the ability to voluntarily move both lower extremities develops, which can be temporary or permanent. Due to the localization and nature of the pain that occurs, the clinical manifestations of dissecting aortic aneurysm can be mistaken for acute myocardial infarction.
WHEN TO CONTACT THE DOCTOR
When the above symptoms appear.
You can make an appointment with a cardiologist at Medical by calling 8 (49244) 9-32-49.
Aortic stenosis
Causes:
- Rheumatic valve stenosis (fusion and calcification usually occurs at the edges of the valves).
- Fibrocalcific aortic stenosis (degenerative changes in the valve are associated with the destruction of collagen and the accumulation of calcium deposits in the leaflets: this process begins in the area of the sinuses of Valsalva and spreads to the leaflets).
- Congenital valvular stenosis (cm).
The end result of all of the above forms of valvular aortic stenosis is fusion of the commissures and narrowing of the aortic opening. When valve degeneration and calcification develops, it is practically impossible to differentiate between these conditions.
EchoCG criteria
One-dimensional echocardiography:
- The opening amplitude of the aortic valve is less than 15 mm (since in the presence of multiple echoes from the leaflets it is difficult to identify the amount of separation, this criterion is used only with two-dimensional echocardiography).
- Thickening of the aortic valves.
- Multiple linear echoes from the leaflets in systole and diastole.
- Dense echoes from the walls of the aorta.
- Left ventricular hypertrophy and/or left ventricular dilatation.
- Decreased EF tilt of the anterior mitral valve leaflet (indirectly characterizes a decrease in left ventricular compliance).
- Diastolic flutter of the anterior mitral valve leaflet.
- Reducing aortic excursion.
- Dilatation of the aortic root.
- Dense echo signal from the left atrioventricular ring.
Two-dimensional echocardiography:
- Systolic separation between the anterior (right coronary) leaflet and the posterior (non-coronary) leaflet is less than 1.5 cm.
- Thickened aortic cusps in the parasternal long-axis view of the left ventricle or cross-section at the level of the great vessels (This phenomenon is more clearly visualized in diastole, with the right coronary cusp being damaged much more often than the left).
Fig. 131
Valvular aortic stenosis, protrusion of the aortic leaflets into the ventricular outflow tract.
- Bending of the aortic valves beyond the closure line (prolapse of the aortic valves into the outflow tract of the left ventricle) (Fig. 131).
Doppler EchoCG:
- The presence of turbulent blood flow behind the aortic valve leaflets (flow speed exceeds 1.5 m/s).
- Estimation of the obstruction gradient using Bernoulli's equation
- Calculation of the cross section of the aorta using the Kevin formula. W.
- Allows to identify concomitant aortic valve insufficiency.
- Assessment of left ventricular systolic function.
Aortic stenosis severity assessment:
- The degree of restriction of the aortic leaflets: leaflet separation of less than 8 mm indicates severe stenosis, more than 12 mm indicates mild or moderate stenosis.
- Left ventricular hypertrophy.
- Systolic gradient: moderate stenosis, obstruction gradient does not exceed 50 mmHg. Art., severe stenosis - 50-80 mm Hg, severe stenosis - more than 80 mm Hg.
- If the speed of the maximum systolic flow behind the aortic valves is more than 4 m/s, aortic valve replacement is indicated; from 3 to 4 m/s, observation and intensive treatment for several months with repeated monitoring; less than 3 m/s, conservative treatment.
- If there is an increase in the obstruction gradient of 8 mm Hg. per year and a narrowing of the valve area by 0.1 cm2 - progression of stenosis occurs.
Differential diagnosis:
- Sclerosis of the aortic leaflets: In this condition, the leaflets are thickened, but do not have restriction of movement.
- Fibrosis and calcification of the aortic root: In this condition, it is difficult to visualize the structure of the aortic leaflets.
- Low cardiac output: In this condition, cardiac output is reduced and if the leaflets are sclerotic, it is almost impossible to distinguish from aortic stenosis.
Causes of aortic aneurysm
Most often, an aortic aneurysm develops as a result of an atherosclerotic process or is of syphilitic origin. Recently, atherosclerosis has taken first place among the causes of aortic aneurysm development, which is due to advances in the treatment of syphilis and an increase in average life expectancy. In addition, syphilis is more often the cause of the development of a thoracic aortic aneurysm, while atherosclerosis more often leads to the formation of an abdominal aneurysm.
Other causes of aortic aneurysm development are medianecrosis and nonspecific aortoarteritis. Traumatic aneurysms are also possible (for example, after a closed abdominal injury) and false anastomotic aneurysms after operations on the aorta. Aneurysms of mycotic (fungal) origin are also described in the scientific medical literature.
The most common cause of the development of dissecting aortic aneurysm is long-standing arterial hypertension against the background of atherosclerosis. In this case, on the inner lining (intima) of the aortic wall, as a rule, there are already various pre-existing small defects. Less commonly, the causes of dissecting aortic aneurysm can be hypertension due to coarctation of the aorta (a congenital defect manifested by segmental narrowing of the aortic lumen); arterial hypertension caused by other factors; Marfan syndrome (hereditary pathology of connective tissue), which is accompanied by severe weakness of the aortic wall. It is possible to form an acute dissecting aneurysm of the ascending aorta due to its rupture caused by a closed injury (for example, a car injury). Sometimes dissecting aortic aneurysm can occur as a result of iatrogenicity: as a complication of cannulation of the arteries and aorta for the purpose of perfusion during cardiopulmonary bypass.
How is MSCT of the aorta performed?
The duration of the study itself is approximately 15-20 minutes. The examination is usually performed in the supine position. You cannot move during the examination. You need to breathe calmly and evenly. You may be asked to hold your breath for a short time. Holding your breath is very important for obtaining high-quality images. The contrast agent is administered intravenously in a volume of up to 50-150 ml using a special injector device synchronized with the operation of the computed tomograph.
Sometimes, when a contrast agent is administered, nausea, a feeling of heat, anxiety, and skin reactions at the injection site may occur. These reactions usually disappear without a trace after the study and do not require any treatment.
Complications of aortic aneurysm
1. Aortic valve defects and heart failure . With an aneurysm of the ascending aorta of syphilitic origin, cardiac decompensation may develop due to a defect of the aortic valve or blockage of the mouth of the coronary arteries.
2. Rupture of an aneurysm with bleeding . Bleeding can occur into the respiratory organs (bronchi, trachea), pleural cavity, cardiac sac, into the esophagus, large blood vessels located in the chest cavity, and sometimes even out through the skin when the sternum is destroyed. In case of bleeding into the pericardial cavity, cardiac tamponade occurs. Bleeding results in rapidly increasing blood loss.
3. Acute and subacute thrombosis of the aortic aneurysm. Most often it develops in the abdominal aorta and leads to the closure of its branches located here.
The listed complications quickly lead to the death of the patient if appropriate measures are not taken in time.
Marfan syndrome
Hereditary determined connective tissue disease of an autosomal dominant type. Most often with Marfan syndrome there are various changes in the aorta and mitral valve (Fig. 132).
Fig. 132
Aneurysmal dilatation of the ascending aorta in Marfan syndrome (scheme).
EchoCG criteria:
- Dilatation of the ascending aorta.
- Dilatation of the sinuses of Valsalva (the right coronary sinus is most often damaged - 69%, non-coronary - in 26%, left coronary - in 5%).
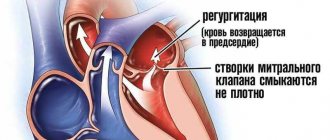
- Aortic valve insufficiency (relative, associated with large dilatation of the aortic root) (see sinus of Valsalva aneurysm).
- Mitral valve prolapse.
- Mitral regurgitation (caused by myxomatous valve degeneration and/or dilatation of the left atrioventricular orifice).
Doppler EchoCG:
Identification of valvular (mitral, aortic) regurgitation and its severity.
Instrumental examination
X-ray examination. For aneurysms of the thoracic aorta, radiography is performed in three projections with mandatory contrasting of the lumen of the esophagus. The expansion of the shadow of the vascular bundle is characteristic. Aneurysms of the descending aorta bulge into the left pulmonary field. Most patients experience displacement of the contrast-enhanced esophagus. Sometimes calcification (calcification) of the aneurysmal sac is determined. In case of aneurysms of the abdominal aorta, plain radiography of the abdominal cavity in two projections allows us to identify calcification of the aortic wall and urination of the lumbar vertebral bodies.
Ultrasound examination (ultrasound) of the aorta and heart. Ultrasound allows us to identify the presence and size in diameter and along the length of aneurysms of the ascending, descending aorta, aortic arch, abdominal aorta, the condition of the vessels extending from the aorta, as well as the presence of aortic valve defect, the nature of changes in the aortic wall.
Computed tomography (CT). When the lumen of the aorta is more than 4 cm, its expansion is considered to be aneurysmal. When performing computed tomography, it is possible to determine the involvement of large arteries in the process and identify signs of dissection (dissection) of the walls (in case of dissecting aortic aneurysm). Angiographic examination (aortography). It is used, as a rule, before surgery when planning its nature and volume.
Aneurysm of the sinuses of Valsalva
Causes:
- Marfan's disease.
- Aortoarteritis.
- Supravalvular aortic stenosis.
The right coronary sinus is damaged in 69% and usually ruptures into the right atrium or right ventricle. The non-coronary sinus is damaged in 26% and usually ruptures into the right atrium. The left coronary sinus is damaged in 5% of cases.
EchoCG criteria
One-dimensional echocardiography:
- Dilatation of the aorta at the level of the sinuses of Valsalva (more than 40 mm).
- Signs of compression of the left atrium.
- Increasing the distance from the aortic valves to the underlying wall during systole.
- Systolic bulging of the aortic wall.
- Hemodynamic manifestations depending on which sinus of Valsalva is damaged and whether it has a breakthrough. The presence of volume overload of the right atrium and ventricle, premature opening of the pulmonary valve is characteristic of a breakthrough of the sinus of Valsalva into the right atrium, volume overload of the left parts is observed when the sinus breaks into the left ventricle or atrium.
Two-dimensional echocardiography:
- Protrusion of one or more sinuses in the parasternal short-axis view at the level of the aortic valve.
- Direct visualization of the sinus aneurysm and the site of its rupture.
Doppler EchoCG:
- Allows you to determine the presence of a breakthrough of the sinus of Valsalva into the corresponding chamber of the heart and the amount of regurgitant discharge.
- Assessment of left ventricular systolic function.
Treatment of a patient with an aortic aneurysm
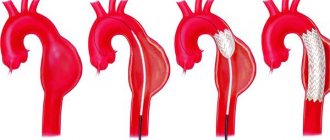
A thoracic aortic aneurysm with a diameter of more than 5 cm is subject to surgical treatment due to the high risk of its rupture and the development of thromboembolic complications. The surgical intervention is performed under conditions of artificial circulation and hypothermia (low temperature) and is reduced to resection (removal) of the aneurysm with simultaneous replacement of the removed area with a prosthesis.
In case of an asymptomatic course of an abdominal aortic aneurysm, the indication for planned surgical intervention is its diameter of more than 4 cm. In cases of increasing pain and the threat of rupture, emergency surgical intervention is indicated. The operation is reduced to resection of the aneurysm with direct replacement of the abdominal aorta or bifurcation aortofemoral replacement.
Prognosis for aortic aneurysm
In the absence of timely treatment and the occurrence of severe complications of aortic aneurysm, the prognosis is unfavorable. Death can occur as a result of decompensation of cardiac activity caused by the development of aortic valve defects with an aneurysm of the ascending aorta, cardiac tamponade due to the breakthrough of an aneurysm into the pericardial cavity, massive blood loss as a result of a breakthrough of the aneurysm into the hollow organs and the pleural or abdominal cavity.
However, the successes currently achieved in the surgical treatment of aortic aneurysms make it possible, in the case of timely and adequate surgical intervention, to save the lives of the majority of patients. With a planned operation, the mortality rate is 0-5%, and in the case of aneurysm rupture, even with emergency surgery, it is 50-80%. The five-year survival rate among operated patients is 80%, and among non-operated patients it is 5-10%.
Idiopathic dilatation of the aortic bulb (anuloaortic ectasia)
EchoCG criteria
One-dimensional echocardiography:
- Premature opening of the aortic valve.
- Dilatation of the aortic root.
- Partial early-mid-systolic closure of the aortic valve.
- Paradoxical systolic movement of the posterior wall of the aorta.
Two-dimensional echocardiography:
- Dilatation of the aortic ring in cross section at the level of the great vessels.
- Prolapse of the aortic valve into the left ventricular outflow tract.
Doppler EchoCG:
- The presence or absence of aortic regurgitation is determined.
- Assessment of left ventricular systolic function.