Why do stenting of the celiac trunk and mesenteric artery?
Inside the abdominal cavity there are organs that are responsible for digestive processes.
They are abundantly supplied with blood by large vessels - the celiac trunk, the superior and inferior mesenteric and renal arteries. Impaired blood flow in these arteries complicates the work of internal organs and impairs their functionality. This disorder can occur suddenly, for example, when a vessel is blocked by a blood clot, or develop in a chronic form, when the flow of blood to the organ is maintained, but noticeably decreases. Acute circulatory disorders require immediate surgical intervention, as they can be fatal. Chronic forms worsen a person’s quality of life, cause other diseases of internal organs, and also require surgery.
Celiac stenosis (Dunbar syndrome) - symptoms and treatment
Treatment of stenosis can be conservative or surgical. The first option is possible at the stage of compensation and subcompensation. It is aimed at relieving symptoms. If conservative therapy is ineffective, the patient is prescribed surgical treatment.
Conservative therapy
Conservative treatment does not affect the underlying cause of the disease. It is purely symptomatic or aimed at concomitant diseases: hiatal hernia or cholelithiasis.
Various medications are used as symptomatic treatment :
- gastroprotectors - protect and restore the gastric mucosa;
- enveloping drugs - used for the same purpose immediately before meals;
- prokinetics - have a positive effect on gastric motility, prescribed for nausea while eating or gastroesophageal reflux (GERD);
- antispasmodics - affect smooth muscles, reducing pain;
- non-steroidal anti-inflammatory drugs (NSAIDs) - used for pain relief.
The next important point of conservative treatment is a gentle diet . It is important to exclude spicy, fatty and fried foods from your diet. To reduce the load on the stomach, it is recommended to eat in small portions. It is also important to avoid stress and excessive physical activity. All this helps reduce the intensity of pain after eating.
Some clinics include psychotherapy and acupuncture in complex treatment [16]. The latter treatment method does not have an unconditional evidence base, but many practitioners note its effectiveness in complex therapy.
If conservative treatment does not help, the question of surgery arises.
Surgical treatment
To find out whether a patient needs surgery, the doctor must determine the clinical stage of the disease, the severity of symptoms, the frequency of their occurrence and evaluate the results of the examination: ultrasound or CT angiography.
Then you need to choose a method of surgical treatment:
- open or laparoscopic decompression of the celiac trunk [7];
- reconstructive surgery;
- endovascular surgery;
- hybrid surgical treatment.
Open surgery is the most common method of surgical treatment. To get to the celiac trunk, the doctor dissects the abdominal wall, and then the structures pressing on it: the median arcuate ligament of the diaphragm and the tissue of the celiac plexus.
This operation is effective, but quite traumatic. After the intervention, the body recovers in about three months. Ventral hernias can form, in which internal organs are displaced outward. Sometimes stenosis developed again [17].
Laparoscopic decompression of the celiac trunk in terms of the technique of execution is not fundamentally different from open surgery. The difference is a less traumatic (minimally invasive) access and convenient display of the intervention area, which is visible to the entire operating team.
The pain in the wound after such an operation is less pronounced, the patient recovers faster: on average, he is in the hospital for 4–5 days under the supervision of a doctor, and after 3–4 weeks he returns to normal life and usual activities.
The American surgeon S. Roayaie was the first to report successful laparoscopic decompression of the celiac trunk in 2000 [19]. Since then, many surgeons have mastered this technique, but so far it has not been able to “displace” open decompression. This is due to technical difficulties during the operation and the adherence of older surgeons to the traditional, more familiar technique.
Reconstructive surgeries are recommended for severe changes that do not allow the celiac trunk to be freed from compressive tissue. They are performed by vascular surgeons in specialized centers. Doctors perform plastic surgery of the celiac trunk or replace it with a graft - artificial or natural, that is, another vessel. These operations are performed in an open manner and are tolerated by the patient in the same way as open decompression of the celiac trunk.
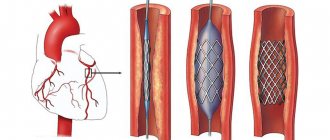
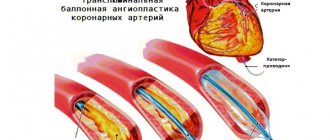
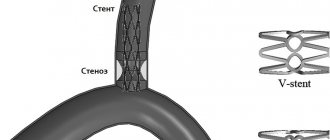
Endovascular surgery is recommended for patients over 60 years of age whose arteries are narrowed by atherosclerotic plaques. The celiac trunk is expanded using a balloon dilator or stent [18]. Both options can be performed only after preliminary surgical decompression [12].
Although endovascular operations are the most minimally invasive method of treatment, they are accompanied by certain risks: deformation, fracture, migration of stents or thrombosis of the stented artery.
Hybrid (combined) treatment is carried out if the celiac trunk is narrowed both from the inside and from the outside. It consists of the simultaneous or step-by-step implementation of two treatment methods: decompression of the celiac trunk and installation of a stent [18].
After endovascular and reconstructive operations, you need to be observed by a vascular surgeon and constantly take blood thinning medications. Dosage regimens depend on the operation performed. These mandatory measures will help avoid re-stenosis.
Preparation for stenting of the celiac trunk and mesenteric artery
The doctor may prescribe a set of studies to the patient, including:
- general analysis and biochemical parameters of urine and blood, assessment of coagulation parameters;
- video gastroscopy;
- X-rays of light;
- electrocardiogram;
- Ultrasound of the heart;
- Ultrasound of the abdominal cavity;
- multislice CT of the aorta.
Immediately before the operation you need to refrain from eating (8 hours before the intervention). Two hours before surgery you should not even take liquids.
Celiac trunk compression syndrome (CTCS) is a rare disease caused by extravasal compression of the celiac trunk by the median arcuate ligament of the diaphragm. This disease is one of the causes of chronic abdominal ischemia [1, 2]. The basis of SKChS is a violation of the relationships of anatomical structures. In 1965, the American physician J. Dunbar [3] first described this syndrome, which subsequently began to be called after him [3]. Normally, the celiac trunk extends from the aorta below the lumbar part of the diaphragm, which eliminates its compression. In the case of a high origin of this vessel from the abdominal aorta or a low location of the lumbar part of the diaphragm, or a combination of these anatomical deviations in the process of ontogenesis, compression of the celiac trunk is formed. Based on clinical and genealogical research, a group of scientists led by L.V. Potashova established that the anatomical variant of the structure, in which the development of this syndrome is possible, is congenital in nature and is transmitted according to an autosomal dominant type of inheritance [4]. According to A.V. Pokrovsky, with extravasal compression of the celiac trunk, “permanent injury to the artery leads to its cicatricial stenosis,” which causes a steadily progressive course of the process. The mechanism of origin of abdominal pain is similar to that of angina pectoris and is associated with a lack of blood flow to actively functioning abdominal organs due to impaired blood flow along the celiac trunk [1].
In case of SCS, there are difficulties both for diagnosis and for treatment. The difficulty lies in the fact that the presence of extravasal compression, identified by instrumental research methods, often does not correspond to the clinical picture and patient complaints. According to the literature, 10-24% of the population, to one degree or another, have compression of the celiac trunk by the median arcuate ligament of the diaphragm [5, 6]. Most of these people do not have any clinical manifestations of this condition, since due to the long-term congenital nature of such changes, the circulation is compensated by collateral blood flow. Only 1% of patients experience clinical symptoms [5]. The reasons why in some patients the blood circulation in the celiac trunk is completely compensated by collateral blood flow, while in others ischemia develops, are not fully understood. It is possible that in young patients, clinical symptoms manifest themselves due to the completion of the formation of the muscle-tendon framework of the diaphragm, which occurs by the age of 20-25 [7]. In elderly patients, a block of collateral blood flow is highly likely to be a consequence of atherosclerotic lesions in the superior mesenteric artery [1].
The celiac trunk, superior and inferior mesenteric arteries are connected to each other by congenital collateral anastomoses, forming a single vascular path of blood flow, along which, depending on the stage of digestion and the blood supply needs of a particular organ, blood can move both in the caudal and cranial directions [1, 8 ]. Due to this, the depletion of blood flow in SCS often reaches a peak after a meal and is provoked by a decrease in blood flow and subsequent ischemia [1]. During ischemia, the tissues of the mucous membrane of the gastrointestinal tract are primarily affected, which leads to organic and morphological changes in organs in the form of dystrophy, atrophy, erosion and ulceration [9].
According to some authors, the nature of abdominal pain in SCS may be neurogenic in nature. Thus, back in 1963, P. Harjola [10] reported a violation of the patency of the celiac trunk, caused by its compression by the neuroganglionic tissue of the celiac plexus and accompanied by the clinical picture of angina abdominalis. Proponents of this theory believe that mechanical irritation of the celiac plexus tissue due to its compression between the celiac trunk and the median arcuate ligament of the diaphragm, which increases during digestion due to increased arterial pulsation (the so-called “water hammer”), is the cause of epigastric pain in patients [4] . However, this theory does not have a solid evidence base. There is also an opinion that ischemic pain in this disease is associated with neurogenic spasm of peripheral arteries [1, 4].
Thus, the pathophysiological aspects of this disease still raise many questions. In this regard, and on the technical aspects of surgical intervention, different authors have disagreements. Thus, some authors consider it necessary to excise the nerve ganglia during decompression of the celiac trunk. S. Thoolen et al. [11] note that the neurogenic factor plays a major role in the development of clinical manifestations characteristic of Dunbar syndrome. The authors draw attention to the fact that relief of the manifestations of SCS can be achieved by excision of the celiac ganglia rather than by restoring blood flow through the main vessel. Until now, unanimity has not been achieved on this issue. At the moment, there is no clear idea of how much nerve ganglia need to be removed or even whether they need to be removed at all [4, 11].
The key issue in the presence of SCS is determining the indications for surgical intervention. Identifying patients in whom it is compression of the celiac trunk that causes the clinical signs of the disease is not an easy task. Thus, to make a diagnosis, it is necessary to have an appropriate clinical picture in combination with characteristic changes according to instrumental research methods (ultrasound scanning, CT angiography). According to the guidelines for the treatment and diagnosis of peripheral vascular disease of the European Society of Cardiology, the initial diagnostic test should be ultrasound of the abdominal aorta, celiac trunk and superior mesenteric artery. The most important method for diagnosing SCS is CT angiography. Catheter X-ray contrast angiography is used exclusively for endovascular angioplasty and stenting [12].
Symptoms can be varied: chronic abdominal pain that occurs or intensifies after eating, in some cases accompanied by dyspeptic symptoms (nausea, vomiting, flatulence, diarrhea, weight loss), neurovegetative disorders (palpitations, sweating), and depressive asthenohypochondriacal syndrome. Very often, with extravasal compression of the celiac trunk, clinical symptoms are not associated with chronic abdominal ischemia, but are caused by other, previously undiagnosed diseases (chronic pancreatitis, ulcers, gastritis, tumors of the gastrointestinal tract, etc.).
An important step in the differential diagnosis of SCS is the exclusion of psychiatric diseases in patients who, according to instrumental research methods, have extravasal compression of the celiac trunk. Thus, at the Oryol State Medical University, during preoperative preparation, 74 patients with the presence of SCS were examined, of which 47 (63.5 ± 4.9%) were diagnosed with various mental disorders (asthenodepressive, asthenic, hypochondriacal, anxiety-depressive syndromes, epilepsy ) [13].
Thus, based on all of the above, we can formulate the clinical definition of SCS as follows: extravasal compression of the celiac trunk, confirmed by instrumental research methods, accompanied by clinical symptoms of abdominal ischemia, which cannot be associated with any other disease.
There are ultrasound criteria for extravasal compression of the celiac trunk: its angular deformation in the cranial direction when examined in B-mode with an aliasing effect in the color Doppler mapping mode, an increase in the peak systolic velocity of blood flow in the celiac trunk in the deep expiratory phase by at least 80% compared with the deep inspiration phase, as well as a decrease in peak systolic blood flow velocity, peripheral resistance indices and “extension of acceleration” in the splenic artery [14, 15]. In arterial stenosis, as a rule, a decrease in the lumen of the vessel by less than 50% is considered hemodynamically insignificant. A stenosis of more than 50% in diameter is regarded as hemodynamically significant [14]. However, with an increase in systemic arterial pressure, especially pulse pressure, signs of local hemodynamic significance of vessel damage may also appear with a lesser degree of stenosis.
According to A.I. Kanaev [16], compression of the celiac trunk can be considered hemodynamically significant when the degree of narrowing of the vessel lumen is more than 50%, the peak systolic blood flow velocity is more than 2 m/s and the blood pressure gradient in the celiac trunk is more than 15 mm Hg. at maximum exhalation. To detect SCS, it is recommended to perform ultrasound scanning on exhalation, inhalation, as well as in a vertical position of the body, and at the same time take into account the results of the study as diagnostically significant in assessing stenosis and regional hemodynamic disorders in the celiac trunk [4].
The issue of correlation between the hemodynamic significance of extravasal compression of the celiac trunk according to instrumental research methods and the clinical manifestations of SCS is controversial. Often, clinical symptoms in the presence of hemodynamically significant compression of the celiac trunk are absent, while in the presence of hemodynamically insignificant changes in blood flow, the clinical picture of CSCS can be observed.
When performing CT angiography, a “fish hook” symptom is characteristic of extravasal compression of the celiac trunk, which allows differential diagnosis with another cause of celiac trunk stenosis - atherosclerotic lesions [6].
The issue of assessing the results of patient treatment remains extremely important. There is an opinion that, given the congenital nature of SSCS, it is not advisable to operate on such patients [17]. However, the available treatment experience demonstrates that the effectiveness of surgical intervention in patients with a clinical picture of KSCS in combination with hemodynamically significant stenosis is quite high. Thus, according to a retrospective analysis conducted in one of the Orel clinics, out of 261 patients who underwent open decompression of the celiac trunk, excellent results of surgical treatment were observed in 2/3, good (significant improvement) in another 1/5. These results remained stable over a long follow-up period [13]. Currently, the prevailing opinion in the scientific community is that the indication for surgical treatment of such patients is hemodynamically significant stenosis of the celiac trunk according to instrumental research methods in combination with the clinical picture of chronic abdominal ischemia [18].
In turn, surgical tactics are supported by a study by a group of scientists from the Catholic University of Lille under the leadership of E. Ducasse, which indicates that in 80% of patients with compression of the celiac trunk, aneurysmal transformation of the arteries of the abdominal aorta is detected. In this case, the arteries of the pancreatoduodenal arcade system, the gastroepiploic artery and the celiac trunk were predominantly affected; the development of complications associated with the presence of aneurysms was noted in 3-18% of cases. The danger is that this condition is asymptomatic until the aneurysm ruptures, with a corresponding risk of death [19].
For many years, when identifying disturbances in blood flow along the celiac trunk, the method of choice was to correct this condition by dissecting the median arcuate ligament of the diaphragm using upper midline laparotomy or even thoracophrenolumbotomy, which was accompanied by a difficult course of the postoperative period due to the traumatic approach. According to some authors, decompression of the celiac trunk using upper midline laparotomy is the most reliable and relatively less traumatic method [4]. Postoperative mortality is less than 1% [1]. Also, open access allows for a thorough examination of the abdominal organs and, if necessary, performing simultaneous surgery [20].
With the development of laparoscopic surgery, the first reports of laparoscopic interventions for decompression of the celiac trunk appeared. Thus, such an operation was first reported in 2000 by S. Roayale et al. from Mount Sinai-New York University Medical Center [21]. There are also several similar messages -A. Carbonell et al. [22] and L. Dordoni et al. [23], which noted excellent results within 3 to 7 months after surgery. J. Jimenez et al. [18] in their work collected 7 clinical series, which included 121 patients after laparoscopic decompression of the celiac trunk, including clinical improvement in 116. The laparoscopic approach seems preferable compared to the open one due to the low incidence of postoperative complications and speed recovery, however, there is a high risk of conversion (9.1%) with serious technical difficulties, such as bleeding (7.4%) and pneumothorax (2.5%) [18]. Despite many reports describing mostly isolated observations with positive reviews about the results of laparoscopic decompression of the celiac axis, at the moment, much experience has not yet been accumulated in using this technique.
In Russia, the first such operation was performed in 2005 at the Institute of Surgery named after. A.V. Vishnevsky, 40 laparoscopic decompressions of the celiac trunk have been performed to date.
N. Jaik et al. [24] described the first experience with robot-assisted decompression of the celiac trunk; since then, 9 papers have been published. The results report one case of conversion to open access due to aortic injury. According to S. Thoolen et al. [11], the advantages of using robotic technologies for celiac trunk compression syndrome are an increase in the “dexterity” of the operation and the presence of a three-dimensional image, which, as the authors believe, significantly improves working conditions in the area of the celiac trunk, especially with existing fibrous adhesions and difficult to distinguish structures located on the anterior wall of the great vessels.
After 20 years of errors and failures in isolated endovascular interventions, the results of the successful use of a hybrid approach using surgical and endovascular methods for the treatment of patients with chronic abdominal ischemia syndrome were first published in 1980 [25]. According to L. Reiley et al. [26], patients who underwent only decompression of the celiac trunk were more likely to experience pain recurrence (44%) than patients who additionally underwent an endovascular stage of revascularization using stenting.
Balloon dilatation and stenting as the only method of treatment are not recommended for SCS, since the median arcuate ligament, being an extremely dense anatomical structure, prevents adequate dilatation of the celiac trunk [20]. According to the literature, the isolated use of endovascular treatment methods for SCS, even with the use of stenting, is associated with a high failure rate [27]. The use of endovascular stents for the purpose of dilatation of the celiac trunk, according to some surgeons, is not justified, since in some cases the stents break during breathing. Despite this, endovascular interventions can be very effective in some situations, such as visceral artery aneurysms. This complication most often develops in the gastroduodenal arcade system (“lesser arch of Riolan”), and can also appear in the celiac trunk in the form of poststenotic dilatation and in the gastroepiploic artery; the reason for its development is an increase in collateral blood flow against the background of SCS [27].
One of the issues discussed is the need to perform intraoperative ultrasound control. Thus, a number of surgeons do not perform this stage of the operation due to the fact that they do not have the appropriate equipment in their arsenal and do not know this technique. Surgeons proficient in this technique consider intraoperative ultrasound to be necessary to ensure a favorable technical and clinical result of decompression [1, 21]. Intraoperative ultrasound control allows faster access to the celiac trunk, which is especially important in the situation of anatomical anomaly in the location of the vessels of the coelomenteric basin, which occurs in 45% of cases [28, 29], as well as in cases of severe abdominal obesity. To determine the anatomy of the operated area, one can rely on CT angiography data, which must be performed on all patients in the preoperative period. However, the fastest way to determine the vascular anatomy and the orifice of the celiac trunk is with the help of intraoperative ultrasound. It is believed that intra-abdominal ultrasound of the celiac trunk and superior mesenteric artery during surgery is less prone to errors due to technical difficulties, allows you to more accurately measure the diameter of the vessel, the speed of blood flow in it and assess the adequacy of decompression of the celiac trunk [30]. Assessing the degree of restoration of the lumen of the celiac trunk during decompression during surgery allows us to give a convincing prognosis. It is known that the final restoration of the lumen of the celiac trunk after decompression occurs gradually in the postoperative period. Complete restoration of the lumen, as a rule, does not occur at all, which is due to the congenital nature of the anomaly and changes in the wall of the celiac trunk itself during the formation of the body throughout a person’s life. Considering that the formation of the diaphragm frame and the development of blood vessels in children have not yet been completed, we can expect more convincing results of celiac trunk decompression in pediatric patients.
Thus, at the moment, every patient with celiac trunk compression syndrome remains difficult for the clinician. The most important issue in the examination and treatment of such patients remains the determination of indications for surgical intervention. Until now, surgical decompression of the celiac trunk raises many tactical questions, although with the development of this technique, many of them have already been answered. Today it is obvious that the optimal approach for performing decompression of the celiac trunk is laparoscopic.
The authors declare no conflict of interest.
How is stenting of the celiac trunk and mesenteric artery performed?
Before the operation, a sedative can be administered intravenously or intramuscularly, after which the patient is accompanied to the operating room, where his thigh or elbow is prepared for surgical access: the skin is treated with a septic tank, and the surgical field is covered with sterile sheets.
The surgeon numbs the puncture site, punctures the artery and installs a special tube. Through a tube (introducer), the doctor inserts a guidewire into the aorta, and through it a catheter for contrast examination of the arteries. Filling the bloodstream, a radiopaque substance marks the areas of narrowing. Right during the examination, the doctor can place a guidewire below the site of narrowing, deliver a balloon with a stent there and open it to increase the lumen of the vessel. The stent supports the vascular wall, and the artery begins to allow blood flow to the required extent.
After the intervention, it is necessary to conduct a control angiography to exclude complications. The catheter and introducer are removed from the vessel, and the access site is subjected to compression to stop bleeding. Special stitching devices are also used to close the incision.
Material and methods
Since 2005 at the Institute of Surgery named after. A.V. Vishnevsky we performed 21 laparoscopic interventions for extravasal compression of the celiac trunk. The results of treatment were monitored in the long term. Among the patients there were 8 women and 13 men. The age of the patients ranged from 28 to 72 years and averaged 44 years. The duration of the disease until diagnosis and surgical treatment varied from 3 to 20 years and averaged 6 years. The results of surgical treatment in the long-term postoperative period were studied in 17 of 21 patients. The characteristic clinical picture of the disease in the form of severe abdominal pain in the epigastric region, neurovegetative disorders, such as attacks of tachycardia, panic attacks, was observed to one degree or another in all patients. Dyspeptic symptoms in the form of nausea, vomiting, flatulence, and weight loss were observed in 9 patients. Astheno-hypochondriacal syndrome was present in 6 patients. It should be noted that the long course of any disease accompanied by chronic pain syndrome can lead to mental and behavioral disorders. Before making a verified diagnosis, a long history of the disease is accompanied by a diagnosis of irritable bowel syndrome, functional abdominal pain, refractory gastroparesis, aggravation due to drug addiction, and somatoform disorders of the autonomic nervous system in patients. The emergence of psychogenic factors is based on the patient’s prolonged suffering, the “helplessness” of doctors and the modern “diagnostic machine”. The result of this is the patient’s refusal to eat in order to exclude the development of possible abdominal pain, and a decrease in quality of life. Thus, when deciding whether to carry out surgical treatment for decompression of the celiac trunk, it is necessary to involve a psychiatrist both at the preoperative stage and after the operation in order to eliminate the possibility of developing a false relapse of the disease.
In all cases, the diagnosis was established using ultrasound duplex scanning data with measurement of blood flow velocity during respiratory tests, and was also confirmed by CT angiography data (Fig. 1, 2,
Rice. 1. Ultrasonic duplex scanning. Reduction of peak systolic blood flow velocity during inspiration to 150 cm/s.
Rice. 2. Ultrasonic duplex scanning. Increase in peak systolic blood flow velocity to 350 cm/s during exhalation. rice. 3, 4).
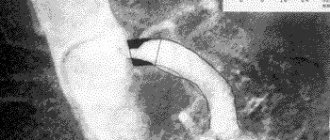
Rice. 4. Extravasal compression of the celiac trunk (CT angiography, 3D reconstruction).
Rice. 3. CT angiography. Extravasal compression of the celiac trunk. a — in the sagittal plane; b - in the axial plane.
Ultrasound criteria for extravasal compression of the celiac trunk are: angular deformation of the celiac trunk in the cranial direction in B-mode, acceleration of the peak systolic velocity of blood flow in the celiac trunk in the deep exhalation phase by more than 80% compared to the deep inspiration phase, as well as a decrease in peak systolic velocity blood flow For arterial stenoses, it is generally accepted that stenoses that reduce the lumen of the vessel by more than 50% in diameter are, as a rule, hemodynamically significant [6]. According to some authors, compression of the celiac trunk can be considered hemodynamically significant when the degree of narrowing of the vessel lumen is more than 50%, with a peak systolic blood flow velocity of more than 2 m/s and a blood pressure gradient in the celiac trunk of more than 15 mm Hg. at maximum inspiration [7].
In all patients, according to instrumental research methods, the indicators of blood flow along the celiac trunk were within the range of hemodynamic significance. According to duplex scanning, in the preoperative period the average degree of narrowing of the celiac trunk was 72.5%. Peak systolic blood flow velocity in the celiac trunk (Vs) was 312 cm/s, and the acceleration of peak systolic blood flow velocity in the deep expiratory phase compared to the deep inspiration phase (Vs exp - Vs inhalation) was 144.5 cm/s. Also, before the operation, all patients were comprehensively examined at the Institute of Surgery named after. A.V. Vishnevsky in the scope of ultrasound of the abdominal organs, esophagogastroduodenoscopy, consultation with a therapist and psychiatrist. Thus, the connection between the clinical symptoms of abdominal ischemia and other diseases was excluded.
All patients underwent laparoscopic decompression of the celiac trunk.
Operation technique
Patients were positioned on the table with their legs apart in the Fowler position. 5 trocars (5 and 10 mm) were installed: 10 mm (optical) - in the middle of the distance between the xiphoid process and the umbilicus along the midline, 10 mm - in the right and left hypochondrium along the midclavicular lines for the hepatic retractor and working instrument, respectively, 5 mm - under the xiphoid process in the midline and 5 mm along the anterior axillary line on the left for the clamp and working instrument, respectively.
At the first stage, all patients underwent topical diagnosis of the celiac trunk using laparoscopic ultrasound, which also determined the extent, degree of narrowing and deformation of the celiac trunk, and characteristic poststenotic dilatation.
Intraoperative ultrasound navigation allows you to quickly find and isolate the celiac trunk, which is especially important in the situation of an anatomical anomaly in the location of the vessels of the coelomesenteric region, which occurs in 45% of cases, as well as in conditions of severe abdominal obesity.
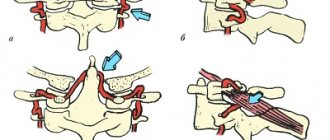
Two types of access to the median arcuate ligament of the diaphragm were used: antegrade or retrograde, and the decision on the choice of access was made intraoperatively depending on the specific anatomical situation and the location of the anatomical structures relative to each other. Antegrade dissection consisted of dissection of the hepatogastric ligament in the avascular zone to create access to the right crus of the diaphragm, from which dissection continued in a caudal direction. To identify the muscle fibers of the chiasm of the diaphragm, dissection was performed in the retroesophageal space. The muscle fibers of the chiasm of the diaphragmatic crura were cut with a hook using monopolar coagulation to expose the anterior surface of the aorta. An important point when using both antegrade and retrograde approaches was the extensive dissection of the muscular chiasm of the diaphragmatic crura above the aorta up to 3-4 cm in order to prevent the occurrence of restenosis in the postoperative period due to scarring of the dissected structures.
In order to better visualize the median arcuate ligament, traction of the stomach downwards and to the left was performed with an atraumatic clamp. The intersection of the ligament fibers was performed with a hook using monopolar coagulation. Pulling the ligament fibers from the vascular wall using a hook made it possible to avoid electrical trauma to the aorta and celiac trunk. Particular importance was given to the complete dissection and dissection of all lymphatic and nerve fibers, as well as the nerve ganglia surrounding the mouth of the celiac trunk to achieve the maximum effect from the decompression performed. The procedure was considered complete when the celiac trunk was freed from any external stenotic structures up to its mouth, which was necessarily confirmed by intraoperative laparoscopic ultrasound data.
In most cases, a retrograde dissection was performed, which differed from the previous approach in that the arcuate ligament was accessed by dissecting the main branches of the celiac trunk in a retrograde direction to the orifice of the celiac trunk, with the left gastric or common hepatic artery most often identified and isolated. Delicate traction of the isolated trunks using vascular holds made it possible to obtain adequate exposure of the mouth of the celiac trunk and subsequent decompression.