Article for the "bio/mol/text" competition: The mechanism for stopping bleeding is essential for the survival of the body, however, despite a history of research spanning decades, many details of this system remain unclear. Eight years ago, Mikhail Panteleev told Biomolecule about blood clotting. Since then, a lot of new data has accumulated in this area. In this article we will tell you how a young team of scientists from Moscow State University lifted the veil of secrecy over two mysterious phenomena in the complex system of arterial thrombus formation, showing how dying cells move in it.
Competition "bio/mol/text"-2019
This work was published in the “Own Work” category of the “bio/mol/text” competition 2019.
The general sponsor of the competition and partner of the Skoltech nomination is the Skoltech Center for Life Sciences.
The nomination partner is the Russian Science Foundation.
Competition sponsor: the largest supplier of equipment, reagents and consumables for biological research and production.
The audience award was sponsored by BioVitrum.
"Book" sponsor of the competition - "Alpina Non-Fiction"
Specialists and non-specialists are well aware of the ominous word “thrombosis”. The word “thrombus” is traditionally perceived as something dangerous. However, not all blood clots are dangerous. If the vascular wall is damaged, the body must quickly form something like a plug that will prevent blood from flowing out of the artery or vein. Thus, the formation of plugs, or, in scientific terms, hemostatic blood clots , is a key task that is solved by the human hemostasis system (and, of course, not only humans). However, sometimes this system fails, and damage to the vessel leads to the formation of a massive intravascular thrombus, which almost completely blocks blood flow. If this process occurs in a large artery that supplies blood to a vital organ, such a blood clot can cause serious complications and even death. Myocardial infarction and ischemic stroke are perhaps the most well-known and common complications caused by arterial thrombosis, which are today the most common cause of death and disability in people in developed countries [1].
Why in some cases, in response to damage, the hemostasis system works excessively and forms a deadly plug in the vessel? Despite many decades of research, this question remains unanswered. The lack of understanding of the mechanisms that regulate the formation of a blood clot leads to the fact that today there is no reliable way to prevent thrombosis: taking existing antithrombotic drugs is associated with a fairly high risk of bleeding, including life-threatening ones.
Two mysteries of blood clots
For the formation of a blood clot in an artery, the harmonious occurrence of a whole set of processes is necessary: platelets must attach to the site of damage, be activated (“turn on”) and stick together (aggregate), while in the blood plasma, as a result of a cascade of biochemical coagulation reactions, a jelly-like fibrin must be formed a network capable of firmly bonding platelets to each other and attaching the entire unit to the site of vessel damage (more information about coagulation can be found in the article by Mikhail Panteleev mentioned in the annotation: “How does blood coagulation work?” [2]). The task becomes truly complex if we remember that all of the listed processes must develop under conditions of pulsating blood flow, which has a significant impact on the nature of their course [3].
The key role here is played by platelets - small, 1-2 micrometers in size, blood cells. Mostly they form a clot called a white thrombus . Despite their small size, platelets demonstrate a wide range of functional responses, including the secretion of granules, changes in the shape and properties of the outer membrane, as well as mechanical activity: due to the presence of special protein molecules, the platelet is able to contract like a muscle and develop forces that are quite large by cell standards - more 10 nN. This mechanism results in mechanical compression of the thrombus, a process that physiologists call contraction, but whose physiological role is still a matter of debate.
Another mysterious phenomenon is the formation of so-called procoagulant platelets - dying cells that expose their surface to the biochemical reactions of blood coagulation, which is thus significantly accelerated (hence the name). Procoagulant platelets weakly interact with other activated platelets, which are capable of not only aggregating, but also exhibiting mechanical activity [4]; in other words, for other platelets they become slippery and can no longer firmly cling to each other. A large number of studies have been devoted to dying platelets, but today there is no clear understanding of exactly what role they play in the hemostasis system. The appearance of procoagulant platelets occurs with strong cell activation, which can occur in the immediate vicinity of the site of vessel damage - that is, in the very “heart” of the thrombus - but these cells are observed mainly on the surface of the blood clots. How do they end up there? Until recently, there was no answer to this question.
Blood tests
In the category of qualitative transformations of platelets, there is a deficiency or blockade of membrane receptors or the absence of compact granules. Symptoms of hemorrhagic diathesis arise due to a shift in the release of spherosomes, when the production of thromboxane and prostaglandins is impaired. An anomaly and deficiency of von Willebrand factor, as well as a violation of the interchange of nucleotides and calcium movement, are important.
In modern medicine, the following types of blood tests are used:
- general clinical;
- biochemical research;
- determination of the degree of coagulation;
- Sukharev's test.
Blood testing is recommended as the first step in making a diagnosis. As a result of laboratory tests, pathology is revealed and the true state of the human body is reflected.
General clinical
Based on the results of the study, the level of hemoglobin, the number of leukocytes, lymphocytes are checked, the color coefficient, the degree of erythrocyte sedimentation (ESR) are determined, and the volume of platelets present is shown in the overall picture. Based on the research, the degree of functioning of the body is determined and deviations from the norm are identified.
A general analysis is prescribed by a doctor to confirm or refute:
- the appearance of inflammation;
- development of diseases of hematopoietic organs and systems;
- the occurrence of immune failures;
- allergic reactions.
The analysis is recommended for pregnant women, patients with varicose veins, heart and vascular diseases. The study is necessary for organ pathologies and autoimmune diseases. The analysis does not require complex preparation; the morning time before breakfast is more suitable for taking blood.
Biochemical research
The analysis informs the doctor and provides a detailed table of indicators, so a large volume of blood is required, which is taken from a vein. Biochemical indicators reflect the functioning of most organs and the degree of development of the disease.
Check shows:
- inflammatory processes;
- indicators of the state of the blood system;
- position of water-salt exchange;
- volumes of microelements important for life.
As a result, the indicator of proteins and carbohydrates is determined, the level of blood enzymes, and the concentration of bilirubin are checked. An advanced biochemical analysis shows whether the content of trace elements is normal or not. As a result, nitrogen metabolism is examined, the presence of urea and creatinine is established.
Determination of clotting
The study reveals the rate of blood clot formation and platelet aggregation. Extension of the indicator leads to excess blood loss, and low activity of flat bodies causes blockages of blood vessels. Pregnant women are tested twice, since timely clotting is very important during childbirth. The occurrence of traffic jams is dangerous with varicose veins because it causes the appearance of blood clots. A coagulogram is prescribed before surgical treatment or extensive blood transfusion.
The analysis is carried out before the morning meal or 8 hours after a meal; alcohol intake is not recommended. 1 ml of blood is taken from a vein and divided into two tubes. The samples are kept at a temperature of +37˚C, and the time from blood collection to the start of the clotting stage is determined.
Diagnostics according to Sukharev
In the process, the time of blood thickening to the time of its complete immobility is studied. The development of the procedure should be limited to a period of 35-120 seconds, and the end of the process is extended to 3.5-5.0 minutes.
Decreased indicators indicate changes:
- anemia of various origins;
- pregnancy;
- improper functioning of platelets;
- excessive use of blood thinning medications.
Accelerated clotting indicates hormonal imbalances, the development of atherosclerosis, and infectious lesions in the body. For analysis, a Panchenkov tube is used, blood is taken from a finger, the first drop is removed with cotton wool. The capillary is filled to the control level and placed horizontally. After 30 seconds, the laboratory technician turns the tube over, changing sides, and uses a stopwatch while working.
What we found and how we did it
Biophysical approach
Like any complex system, the formation of a blood clot in an artery needs to be managed. Identifying the mechanisms that regulate biological processes is one of the traditional tasks of biophysics, so the problem of regulating arterial thrombus formation has long attracted the attention of not only doctors and physiologists, but also biophysicists. For more than two decades, the Department of Biophysics of the Faculty of Physics of Moscow State University has been developing a direction related to the analysis of the principles of the structure and regulation of the hemostasis system: for example, in the classic works of Professor F.I. Ataullakhanov and his students demonstrated the autowave nature of the propagation of the blood plasma coagulation process in the absence of flow [5], [6].
Establishing the mechanisms that regulate thrombus formation in arterial blood flow is one of the main tasks of our research team, whose members are Professor of the Department of Medical Physics M.A. Panteleev, Professor of the Department of Biophysics F.I. Ataullakhanov, senior researcher Department of Biophysics D.Yu. Nechipurenko, as well as students and graduate students of the Faculty of Physics.
In vitro and in silico study
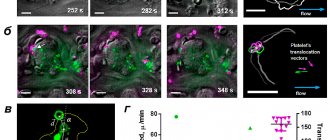
Recent studies carried out by us in collaboration with colleagues from France and the USA made it possible to link the surface distribution of dying platelets with the process of thrombus compression [7]. Using confocal microscopy in in vitro thrombus experiments, we showed that procoagulant platelets form in various parts of the growing thrombus and then move to its surface (Fig. 1).
Figure 1. Dynamics of movement of procoagulant platelets in a thrombus. a — Confocal micrographs of thrombi at different time points. Green color corresponds to the fluorescence of dying cells (a fluorescent marker of cell death is used). b — Blood clots at different points in time. Green color corresponds to the fluorescence of dying cells, purple color corresponds to the fluorescence of platelets attached to the thrombus (a fluorescently labeled antibody to the surface proteins of the platelet is used). c — The main quantities used to analyze the movement of platelets are the movement vector d , the translocation angle α between the direction of movement and the initial radius vector of the center of the dying cell, drawn from the center of the thrombus. d — Results of analysis of the modules of average movement speeds and translocation angles of dying cells (green) and “fresh” platelets attached to the surface of the thrombus (purple). Scale: 10 micrometers.
[7]
This redistribution is accompanied by the formation of fibrin on the surface of the thrombus. Since dying (procoagulant) platelets interact rather weakly with other cells and do not participate in the contraction process, it has been suggested that their redistribution is the result of mechanical displacement during the process of active compression of the thrombus. To test this hypothesis, a computer model of platelet aggregate compression was created, which demonstrated the functionality of the formulated hypothesis (Fig. 2).
Figure 2. Simulation of cell aggregate contraction. a — Platelet aggregate before and after compression. Spheres imitating procoagulant cells, which do not participate in the contraction process and interact relatively weakly with other spheres, are marked in green. Contraction is described as a decrease in the equilibrium length of the pair potential (Morse) interaction between the centers of the spheres. b — Aggregate before and after contraction, in which the “procoagulant” spheres, initially located inside the aggregate, had different radii. The spheres that remained inside the aggregate after contraction are marked in purple, and those outside the aggregate in green. c — The value of the absolute values of the movements of procoagulant platelets in experiments (ex vivo) and “procoagulant” spheres in the model (in silico). d — The fraction of spheres displaced as a result of compression of the aggregate onto its surface. Calculation results for spheres of different radii are shown.
[7]
An important evidence base for the work was experiments with the blood of unique genetically modified mice, whose platelets are deprived of the ability to exhibit mechanical activity and, therefore, ensure thrombus contraction. In accordance with the predictions of the model and the formulated hypothesis, dying cells did not move to the surface of the thrombus in the absence of contraction (Fig. 3). The lack of surface distribution of dying platelets was also accompanied by the lack of surface localization of fibrin.
Figure 3. Comparison of the distribution of procoagulant cells and fibrin for normal and genetically modified mice. a — Distribution of procoagulant platelets (green) in normal mice (top panel) and modified mice (bottom panel). The outline of the thrombus, constructed from the image in the differential interference contrast mode, is marked in yellow. b — Analysis of the ratio of the total fluorescence of the surface of dying cells located outside the dense part of the thrombus to the fluorescence of the same surfaces inside the thrombus for normal (WT) and genetically modified mice (MYH9). c — Distribution of procoagulant surfaces (green) and fibrin (purple) in thrombi from wild-type mice (top panel) and thrombi from genetically modified mice (bottom panel). Scale: 10 micrometers.
[7]
Platelets are...
A thrombus is a blood clot (translated from Greek), the basis of which is platelets.
A platelet is a small, colorless, spherical platelet of blood (Bizzocero plaque). Plates are formed from the plasma structure (megakaryocytes - plasma cells) of the bone marrow. Platelets do not have a nucleus, but are equipped with an abundant number of granules (more than 200).
The value of granulations is due to the high content of special components of platelet growth (thromboxane, thrombin, adenosine diphosphorate and other factors), which ensure the formation of amino acids and enzymes (what are they?) that destroy the membranes of bacterial cells, preventing the penetration of pathogens into the blood.
The size of platelet plates varies within their age (young, middle, mature), from 2 to 5 microns.
However, when a platelet comes into contact with a surface that does not correspond to the internal vascular endothelium or the cavity of the cardiac pericardium, the plates are activated, releasing up to 10 pseudopods (processes) tens of times larger than the size of the plate itself.
This feature allows platelets to serve as a kind of “patch” that, if necessary, covers the wound surfaces of blood vessels, preventing bleeding.
- High platelets with RA - why worry - Arthritis - 2021
It is the pseudopods that ensure the movement of the plates through the circulatory system. Platelets have the ability to adhere to foreign agents, capture them and destroy them, form a blood clot by aggregation (gluing plates together) to prevent hemorrhagic processes (bleeding).
» alt=»»>
The main role of platelets is to actively participate in the process of blood clotting (hemostasis).
They provide a transport function, delivering nutritional components to the tissues (endothelium) lining the internal cavity of the vascular walls. They remain viable for up to 10 days, after which they are destroyed in various organs (spleen, lungs, or liver).
Recent developments by Japanese scientists have proven that megakaryocytes are not the only sources of formation of Bitsocerro plaques (platelets). They were able to obtain platelets from the patient's own stem cells.
These studies increase the success of transplantation, since the presence of such platelets does not cause the body to reject donor organs.
How platelets participate in the process of hemostasis can be seen in the diagram:
Having appreciated the basic functions of platelets, you can understand what their imbalance in the body entails and what the negative consequences may be.
Any imbalance in platelet activity and number is medically called thrombocytopathy.
- A decrease in the concentration of platelet platelets is called thrombocytopenia.
- And an increased concentration is thrombocytosis.
- A dysfunction of their activity is diagnosed as thrombosthenia.
Summarizing
Our study made it possible to describe a new mechanism of cell redistribution within the thrombus: during contraction, “slippery” procoagulant platelets are mechanically squeezed onto the surface of the thrombus, forming a heterogeneous structure of its outer part.
But the point has not yet been made in determining the role of procoagulant platelets in hemostasis. The formation of a weakly adhesive, that is, unsuitable for the adhesion of new cells, layer of dying cells and fibrin on the surface of the thrombus can help stop its growth by reducing the efficiency of fixation of non-activated platelets brought by the blood stream. However, this hypothesis requires further research.
It is pleasant to note that an important contribution to this work was made by young co-authors - students of the Department of Biophysics of the Faculty of Physics of Moscow State University - Roman Kerimov and Alexandra Yakusheva, as well as a student of the Faculty of Fundamental Medicine of Moscow State University Taisya Shepelyuk (Fig. 4). The results of the work were published in one of the leading journals of the American Cardiovascular Association and reported at several international conferences, including the Gordon Conference on Hemostasis.
Figure 4. Roman Kerimov, Alexandra Yakusheva, Taisya Shepelyuk and Dmitry Nechipurenko
This version is a modification of a note that was published in the physics department newspaper “Soviet Physicist”.
The author expresses gratitude to Anastasia Masaltseva and Yuri Nechiporenko for their assistance in editing the article, as well as to all his colleagues - co-authors of the original work.