Blood osmolarity (BOS) implies plasma osmolarity, since it is in it that osmotically active substances are dissolved . The osmolarity of blood plasma is the totality of all kinetically active particles (anions, cations, organic compounds) dissolved in one liter of it.
What are they - osmotically active substances that determine the indicator called blood osmolarity? First of all, these are sodium cations (Na+), which, together with chlorine anions (Cl-), determine the osmotic activity of the plasma, as well as the bicarbonate anion (HCO3-). Osmotically active ions freely pass through the capillary wall, enter the vessel, where they pick up water molecules (H2O) and carry it into the intercellular (interstitial) space. For example, just one sodium ion can capture up to 300 H2O molecules.
Blood plasma osmolarity is a significant laboratory indicator used in clinical laboratory diagnostics to identify acute renal failure (acute renal failure) in the early stages of its development, when other biochemical tests (creat - creatinine, urea - urea) are still “silent”.
Osmolarity of blood and urine
Definition
- Osmosis is the one-way movement of a solvent (water) through a semi-permeable membrane, separating two solutions with different concentrations of solutes (osmotically active substances), towards a solution with a high concentration.
- Osmotically active substances are sodium ions (Na+), chloride (CL-) and bicarbonate (HCO3-), as well as glucose, urea, and proteins.
- Sodium, potassium and glucose cannot diffuse (pass) through the cell membrane, therefore, with pathological changes in their concentrations, a significant change in blood osmolarity and associated complications occur.
- Substances such as urea and ethanol diffuse freely through the cell membrane and therefore do not have a significant effect on blood osmolarity.
- Osmolarity – osmotically active substances dissolved in 1 liter of solution (water). The unit of measurement is milliosmol per liter (mOsm/L).
- Osmolality is the concentration of the same particles dissolved in a kilogram of water. The unit of measurement is milliosmole per kilogram of solution (mOsm/kg).
- Blood and urine osmolarity can be measured using instruments or can be calculated using a mathematical formula (theoretical osmolarity).
- Osmotic window is the difference between actual (measured) and theoretical osmolarity (see below). To calculate theoretical osmolarity, it is necessary to take tests for sodium, potassium, glucose and urea in the blood.
Indication
- Diagnosis of hyponatremia (low sodium) or hypernatremia (high sodium).
- Diagnosis of diabetes insipidus or primary polyuria (large volume of urine).
- Determination of the osmotic window is used to assess the presence of osmotically active substances that are not taken into account in the formula for calculating theoretical osmolarity (see below), for example, in toxicology. Osmotically active substances are also: ethanol, methanol, ethylene glycol, isopropanol, dichloromethane, lactate, ketone bodies, etc.
- Urine osmolarity is also used to diagnose hypo- or hypernatremia.
Methods (osmometers of various modifications)
- Freezing point depression method (the higher the osmolarity, the lower the freezing point of the solution).
- Method of increasing the boiling point (the higher the osmolarity, the higher the boiling point).
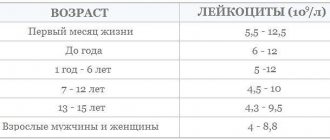
Reference values (normal limits)
- Units of measurement are mOsm/l = mOsm/kg.
- Reference values taken from Thomas L. Labor und Diagnose 2012.
| Age | mOsm/kg |
| 275-300 | |
| 7 days to | 276-305 |
| 28 days to | 274-305 |
| children >1 year old and all others | 280-300 |
| Urine | 50-1200 |
| Osmotic window |
The indicated limits of the norm may differ from those of your laboratory. Therefore, be guided by the norms indicated on the analysis form.
d = days after birth; m. = month; l. = years
Determination of the osmotic window
- Serum osmolarity (OSM) can be calculated using the following formula. Theoretical TMR in blood = 1.86 x sodium (mmol/l) + glucose (mmol/l) + urea (mmol/l) + 9.
- Blood osmotic window (mOsm/kg) = actual OSM (measured using instruments) minus theoretical OSM. For example, osmotic window = 285-282 = 3 mOsm/kg.
Interpretation of blood osmolarity (BOS) results
- TSC changes in parallel with the concentration of sodium in the blood. The interpretation depends on hyponatremia or hypernatremia.
- An increase in blood osmolarity >290 mOsm/kg activates the feeling of thirst due to the secretion of antidiuretic hormone.
- An increased TSC of 40-60 mOsm/kg due to loss of water or due to an increase in sodium or glucose can lead to cerebral edema and death.
- Decreased blood osmolarity
- Osmotic window >6-
- >10 mOsm/kg - poisoning with ethanol, methanol or other substances.
- In severe bleeding, the osmotic window is >15 mOsm/kg without detecting any osmotically active substances.
Other indicators related to USC
Thus, osmolarity of blood (plasma or serum) is an important parameter indicating the preservation or disorder of the dynamic balance of water in the body. It is measured using special laboratory equipment or calculated using a formula after carrying out the necessary biochemical tests (sodium, urea, glucose).
In addition to the described object of study (osmolarity), the table above shows other laboratory tests: free water clearance (SWR - a rather sensitive and important indicator of the concentrating ability of the kidneys) and osmolarity index (IO - the ratio of urine osmolarity to blood plasma). They are directly related to determining the functional abilities of the kidneys during the development of acute renal failure (ARF) and are also calculated using formulas.
True, and that’s not all: there is another indicator related to osmolarity, which is called the osmotic window. Its norm is less than 6 mOsm/l. The osmotic window is measured in mOsm/l or mOsm/kg, calculated based on the values of the osmotic window obtained by osmometry - actual, and the osmotic value derived from the formula - theoretical:
Osmotic window = OSK fact. – USC theory.
For example, 287 mOsm/kg – 284 mOsm/kg = 3 mOsm/kg (corresponds to the norm). If the osmotic window is more than 6 but less than 10 mOsm/L, then doctors suspect the development of keto-, lactate- or renal acidosis. If the level of this indicator crosses 10 mOsm/l and tends to increase, then there is reason to think about severe poisoning (ethyl or methyl alcohol, as well as other organic substances that can affect OSC).
Results and transcript
Transferrin: what is it, functions, definition and norms in tests, deviations.
The results obtained allow the doctor to determine the nature of the pathology and the severity of the disease. An osmolarity value ranging from 800 to 1200 mOsm/L is considered normal.
If the result shows hypoosmolarity, this may mean the following pathologies:
- Pyelonephritis.
- Kidney failure.
- Diabetes insipidus.
- Necrosis of renal tubules.
- Violation of water-salt metabolism.
- Fluid retention in the body.
The severity of violations can be determined by the degree of deviation of the indicator from the norm:
- 400-600 mOsm/l indicates a moderate decrease in the filtering function of the kidneys.
- 600-800 mOsm/l reflects primary changes in the functioning of the renal system.
- Below 400 mOsm/l indicates significant disturbances in the genitourinary area.
The hyperosmolar state (in which the value exceeds 1200 mOsm/l) is characterized by the formation of edema, hypertension, and problems with the heart.
Such a deviation from the norm is provoked by:
- Congestive heart failure.
- Renal artery stenosis.
- Dehydration.
- Glucosuria.
- Dehydration.
- Pyelonephritis.
- Shock.
A strong excess of osmolarity concentration leads to serious disturbances in the functioning of organs, coma.
Only a doctor can accurately interpret the examination. At the same time, the doctor takes into account other tests taken by the patient. Studying the clinical picture and research results provides the basis for making the correct diagnosis and selecting an effective treatment regimen.
source
Application
Diastolic pressure - what is it, normal indicators, reasons for increased or decreased values
Diagnosis of non-ketotic hyperglycemic coma. Control of water-electrolyte balance
- Identify abnormal serum water levels to evaluate for hyponatremia
- Measurements of plasma and/or urine osmolality are more valuable in assessing hydration status than changes in hematocrit, urea, or plasma proteins, as they depend on many other factors.
Promotion
Hyperglycemia.
Diabetic ketoacidosis (osmolality should be determined constantly in decompensated diabetes mellitus).
Non-ketotic hyperglycemic coma.
Hypernatremia with dehydration
- Diarrhea, vomiting, fever, hyperventilation, inadequate water intake.
- Diabetes insipidus is central.
- Nephrogenic diabetes insipidus - congenital or acquired (hypercalcemia, hypokalemia, chronic kidney disease, sickle cell anemia, effects of certain medications).
- Osmotic diuresis - hyperlycemia, administration of mannitol.
Hypernatremia with normal hydration - occurs when the hypothalamus is damaged.
- Impaired sensitivity of osmoreceptors (primary hypernatremia) - water load does not lead to normalization of osmolarity; Chlorpropamide may lower sodium levels to near normal levels.
- Impaired thirst (hypodipsia) - drinking water quickly returns sodium levels to normal.
Hypernatremia with overhydration is iatrogenic and accidental (eg, children being fed high-sodium diets and water, or CPR with soda). Drinking alcohol; alcoholic coma with hyperosmolar status. Decreased (equivalent to hyponatremia) Hyponatremia with hypovolemia (urine sodium typically >20 mEq/L)
- Adrenal insufficiency (for example, congenital forms of adrenal hyper- and hypoplasia with sodium loss, adrenal hemorrhage, inadequate corticosteroid therapy).
- Renal losses (osmotic diuresis, proximal renal tubular acidosis, sodium-losing nephropathies, pyelonephritis, diseases of the renal medulla, polycystic kidney disease).
- Losses through the gastrointestinal tract (diarrhea, vomiting).
- Other losses (burns, peritonitis, pancreatitis).
Hyponatremia with normal volume or hypervolemia (dilution syndrome)
- Congestive heart failure, liver cirrhosis, nephrotic syndrome.
- SNS ADH.
Formulas for calculating and predicting serum osmolality (do not compete with direct osmolality measurement):
mOsmol/L = (1.86 x serum Na) + (serum glucose: 18) + (BUN: 28) + 9 (in mg/dL)
or
in SI units: = (1.86 x serum Na) + serum glucose (mmol/l) + BUN (mmol/l) + 9
easier:
Na+ + K+ 4- (BUN: 28) + (glucose: 18). But since there is relatively little potassium in the serum, and the level of urea has little effect on the distribution of water, the formula is further simplified: 2Na + + (glucose / 18).
Sources
- https://nefrol.ru/diagnostika/osmolyarnost.html
- https://medintercom.ru/articles/osmolyarnost_krovi
- https://2pochki.com/diagnostika/chto-takoe-osmolyarnost-mochi
- https://1pokrovi.ru/analizy-krovi/osmolyarnost-krovi.html
- https://med-slovar.ru/diagnostika-i-issledovaniya/analizy/12-spetsificheskie-laboratornye-issledovaniya/34-osmolyalnost
- https://www.medmoon.ru/med/osmoljalnost_krovi_norma_tablica.html
Methods for determining the indicator
Concept, composition and properties of blood
There are a number of methods based on physical laws that allow you to measure the osmolarity of urine. These methods have found implementation in the designs of osmometers designed to study the water-salt balance in human biological fluids. Among the most well-known methods for determining osmolarity are:
- a method based on taking measurements when passing a biological fluid through a membrane made of natural or artificial materials;
- osmometers based on the principle of reducing the vapor pressure of a substance above a liquid;
- devices whose operating principle is based on an increase in boiling point, which occurs with an increase in the osmolarity of the mixture;
- devices based on lowering the freezing point with increasing osmolarity of the solution are the most common design for medical research.
In addition, there are known methods for determining the indicator by the electrical conductivity of the solution and by measuring the surface tension of the liquid.
Specifics of the examination and accuracy of results
To obtain a reliable value, it is necessary to follow a number of rules, ignoring which can seriously distort the desired indicator:
- In order to prevent bacteria from entering the urine, women and men must wash their genitals and release the first few drops into the toilet before taking the test. The remaining urine is collected in prepared sterile containers.
- Individual recommendations from physicians may include advice to refrain from drinking liquids 12 hours before sample collection.
- 24 hours before the test, you must adjust your diet according to the requirements of your doctor.
The accuracy of the results may be affected by the use of medications containing sucrose and dextran. Before the test, you must inform your doctor about the medications you are taking. In addition, an unreliable result may occur if an X-ray examination using contrast liquid is performed several days before the test.
Preparing for the test
Plasma osmolarity testing is very complex because it can be influenced by many factors. To avoid repeated collection of material and not waste time, specialists insist that the patient undergo special preparation. Despite its importance, it is very simple.
First of all, the patient must tell the doctor about all the medications he is currently taking. He is obliged to listen carefully to the patient and determine whether it is possible to continue taking pharmaceuticals or whether it is better to temporarily stop so as not to affect the result of the analysis.
The second thing you need to point out to your doctor is taking dietary supplements, since they can also affect the interpretation of the study.
Read also: What is a urea test, why is it needed and what diseases are detected?
Important! Blood for plasma osmolarity is donated only on an empty stomach, so the patient is prohibited from taking any food or drinks 9 hours before collecting the material.
Prohibited the day before the study:
- Smoking.
- Drink alcohol.
- There are flour products.
- Abuse sweet foods.
Experts advise eating “light” steamed food a few days before collecting whey.
If a patient regularly participates in donor programs, he can take an osmolarity test only 15-18 days after the last donor collection or blood transfusion. This pause is needed for the body to recover and correctly show its condition.
The collection of biomaterial occurs quickly and does not cause discomfort or pain to the patient. Over many years of medical practice, no complications were recorded after the test. Only in some patients did a small bruise or swelling form at the site of needle penetration. As a rule, everything went away within 2-3 days.
Indications for analysis
Urine osmolarity must be determined if the patient has the following pathologies:
- excessive urination;
- infections of the genitourinary system that cause complications;
- indirect signs indicating an increase or decrease in sodium concentration in the body;
- assessing the results of therapy for hyperosmolar comas;
- diabetes mellitus;
- diagnosis of renal dysfunction.
Normal values and deviations
Normal osmolarity values are considered to be fluctuations in the range of 800–1200 mOsm/L. Deviations from these values manifest themselves in a decrease in osmolarity, called hypoosmolarity, or an increase in it, called hyperosmolarity. Hypoosmolarity can be caused by a violation of the water-salt balance due to overhydration, the manifestation of renal dysfunction, severe pyelonephritis and necrotization of the kidney canals. Hyperosmolarity can be associated with dehydration, stenosis of the artery in the kidney area, disruption of the heart muscle, and shock. Possible fluctuations in the indicator can be represented by the following results:
- When the value decreases to 600 mOsm/l, it indicates moderate impairment of kidney function.
- When a reading is less than 400 mOsm/L, serious kidney problems can be diagnosed.
- In the case of a change in osmolarity towards values exceeding 1200 mOsm/l, it leads to the appearance of edema of varying severity, an increase in blood pressure and disturbances in the functioning of the cardiovascular system, including severe conditions and coma.
It should be noted that in some cases, to make a diagnosis, the attending physician recommends that the patient undergo a blood omolarity test. The relationship between the values of the indicator for urine and blood is an important criterion for judging the presence of a disorder in the kidneys and the timely initiation of its therapy. The results obtained during the analysis allow the doctor to determine the nature of the pathology in case of violation of the concentration of electrolytes and the amount of fluid in the body
The importance of determining osmolarity is that a violation of water-mineral metabolism leads to a change in the overall metabolism of the body, which entails many different diseases
Osmotic activity of blood plasma
KAZAKH NATIONAL MEDICAL UNIVERSITY NAMED AFTER S.D.ASFENDIYAROV
Specialty: General medicine
Direction of training: “Surgery” 7th year
Name of discipline: Anesthesiology, resuscitation and intensive care in oncology with mammology
SYLLABUS
Topic: Features of intensive care of cancer patients. Infusion therapy. Parenteral nutrition. Pain syndrome.
Almaty 2021
The syllabus was compiled by: Abdymoldaeva Zh. A., according to instruction letter No. 10 on the basis of the TUP internship (2011), developed in accordance with the State Standard of Education of the Republic of Kazakhstan 2006 and the Work Program for the specialty "Surgery"
Discussed and approved at a meeting of the department, protocol No. dated “___” _________2016.
Head Department, Doctor of Medical Sciences, Professor _____________________ Israilova V.K.
| Information about teachers: list, degrees and positions, priority scientific interests |
| №№ | Full name | Degree | Job title | Priority scientific interests, achievements |
| 1 | 2 | 3 | 4 | 5 |
| 1. | Israilova V.K. | head department, professor | Doctor of Medical Sciences | Specialist in anesthesiology and resuscitation |
| 2. | Dzholdybekov T. S. | assistant professor | Ph.D. | Specialist in anesthesiology and resuscitation |
| 3. | Berdalina G.S. | assistant professor | Ph.D. | Specialist in anesthesiology and resuscitation |
| 4. | Utegenova Zh.A. | assistant professor | Ph.D. | Specialist in anesthesiology and resuscitation |
| 5. | Mukhamadiev B.T. | assistant | Ph.D. | Specialist in anesthesiology and resuscitation |
| 6. | Suleimenov B.K. | assistant | Doctor of Medical Sciences | Specialist in anesthesiology and resuscitation |
| 7. | Abdymoldaeva Zh.A. | assistant | Specialist in anesthesiology and resuscitation | |
| 8. | Yuldashev A.A. | assistant | Specialist in anesthesiology and resuscitation | |
| 9. | Zryachev V.M. | assistant | Specialist in anesthesiology and resuscitation | |
| 10. | Jarkenbekova D. S. | assistant | Specialist in anesthesiology and resuscitation | |
| 11. | Kamalova G.T. | assistant | Specialist in anesthesiology and resuscitation | |
| 12. | Shmygaleva A.A. | assistant | Specialist in anesthesiology and resuscitation |
| 2. | Contact information: location of the department (address, building, auditorium), telephone numbers, email address |
| Name of the department | Address | Audience | Phones Email address |
| Department of Anesthesiology and Reanimatology | Almaty, Kazybek bi, No. 96 GKP at RV GBSNP | — | — |
| 3. | Topics of classes and volume of training hours |
| № | Subject | Form of conduct | Cont. in hours |
| 1 | 2 | 3 | 4 |
| 1 | Features of the body of a cancer patient. Assessment of the functional state of patients. Tumor and body. | Practical lesson. Patient supervision, interpretation of clinical and laboratory data, clinical analysis, medical management. documentation | 6 |
| 2 | Features of anesthesiological support for cancer patients. Preoperative preparation of the patient. Operational and anesthetic risk. | Practical lesson. Patient supervision, interpretation of clinical and laboratory data, clinical analysis, medical management. documentation | 6 |
| 3 | Features of intensive care of cancer patients. Infusion therapy. Parenteral nutrition. Pain syndrome. | Practical lesson. Patient supervision, interpretation of clinical and laboratory data, clinical analysis, medical management. documentation | 6 |
| 4 | Resuscitation and intensive care in the early postoperative period. Prevention of complications. | Practical lesson. Patient supervision, interpretation of clinical and laboratory data, clinical analysis, medical management. documentation | 6 |
| 5 | Features of the body of patients with breast cancer pathology. Functional state of the patient. Compensation for somatic disorders. | Practical lesson. Patient supervision, interpretation of clinical and laboratory data, clinical analysis, medical management. documentation | 6 |
| TOTAL: | 30 |
DISORDERS OF METABOLISM, WATER AND ELECTROLYTE
BALANCE AND ACID-BASE STATE
Water sectors of the body
DEFINITION Water sectors are various structures of the body, separated by biological semi-permeable membranes (take part in the redistribution of water between these structures).
CLASSIFICATION
All water in the human body is distributed between two water compartments.
• Intracellular fluid makes up approximately 40% of the total body weight (25 L).
• Extracellular fluid - 20% of body weight (15 l).
Components of extracellular fluid.
• Interstitial fluid - 15% of total body weight (or 12 l).
• Intravascular fluid - plasma - makes up approximately 5% of body weight
(or 3 l).
• Transcellular fluid.
• Lymph.
The total water content in the body depends on the age and constitution of the person.
The total water content in the body of newborns is 80% of body weight, in an adult man or middle-aged woman - 60% and 50% of body weight, respectively. In old age, the total water content decreases: in men to 51%, in women - up to 45% of body weight.
Water balance
The liquid enters the body of a healthy person with the food or water he eats. As a result of metabolic processes occurring in the body, water is also formed. The amount of liquid received when drinking is 1500 ml (approximately 60% of all liquid entering the body during the day), with food - 750 ml (30%). The amount of metabolic water formed in the human body, as a rule, does not exceed 250 ml (10%). Normal fluid loss is 2500 ml per day. The excretion of water from the body occurs through urine - 1500 ml (60%), through the skin (imperceptible losses) and through perspiration through the lungs - 700 ml (28%), with sweat - 200 ml (8%) and feces - 100 ml (4 %) (Fig. 2-7).
Osmotic activity of blood plasma
The osmotic activity of a biological fluid is determined by the concentration of biologically active substances - dissociating electrolytes (they have a relatively high molecular concentration and low molecular weight) and non-dissociating compounds. This activity (corresponding to 1 liter of solution) is expressed in milliosmoles (mine); one moe corresponds to one milliequivalent (meq) of monovalent ions, with the ratios meq/L and mmol/L being equal. The total plasma concentration is 285-295 mOsm/L. Osmotic concentration is denoted by the terms “osmolarity” and “osmolality”.
• Osmolarity is the number of osmoles of solute contained in 1 liter of solution. The units of osmolarity are mOsm/L.
• Osmolality is the number of osmoles of solute contained in 1 kg of solvent. Osmolality is measured in mOsm/kg water. Approximately half the value (50%) of the osmotic pressure of blood plasma is created by the sodium ions (Na) present in it. The contribution of chlorine ions (C1) is almost two times less (30% of the osmotic pressure of blood plasma).
| The equation for calculating the osmolality of blood plasma Opl = 2 x [Na1 + [C6H12061 + [urea], where Opl is the osmolality of blood plasma (mOsm/kg); [Na^]—concentration of sodium ions in blood plasma (mOsm/l); [C6HI2OJ—plasma glucose concentration (mosm/l); [urea] - concentration of urea in blood plasma (mOsm/l). In toxemia, plasma osmolality increases as a result of the possible presence of substances such as ethanol and ethylene glycol, complex carbohydrates, and undifferentiated “unmeasured osmoles.” Colloidal osmotic pressure of plasma Colloidal osmotic pressure of plasma (COP) is the osmotic pressure created by blood plasma proteins: albumin, globulins and fibrinogen. Normally, the COD value is 25 mm Hg. (3.4 kPa). The CODE depends on the molecular weight of the solute and its concentration. Albumins create 80% of the plasma CODE, globulins - up to 16-18%, and proteins of the blood coagulation system - no more than 2%. The ratio of colloid-osmotic and hydrostatic pressure determines the processes of filtration and reabsorption occurring in the body (Starling’s law, see Chapter 2.6). A decrease in plasma albumin (below 30 g/l) and a decrease in COP (less than 14-16 mm Hg) are independent factors of unfavorable outcome. Regulation of water-electrolyte metabolism A decrease in fluid volumes and an increase in plasma osmolarity causes thirst and increased secretion of antidiuretic hormone (ADH) by the hypothalamic-pituitary system. ADH acts on the renal medulla, stimulating water resorption. As a result, interacting with the renin-angiotensin-aldosterone (RAAS) system in response to a decrease in fluid circulating volume, ADH restores it. The combined action of these two systems results in balanced sodium and water retention. As plasma osmolarity decreases, ADH secretion decreases. In this case, excess water is released and plasma osmolarity increases to normal values. Inappropriate increased secretion of ADH leads to water retention in the body and a decrease in sodium concentration in the extracellular fluid. Adrenal insufficiency is considered one of the possible causes of water and electrolyte imbalance. INFUSION-TRANSFUSION THERAPY INTRODUCTION Infusion therapy is one of the main tools of an anesthesiologist-resuscitator and can give an optimal therapeutic effect only if two essential conditions are met: the doctor must clearly understand the purpose of using the drug and have an idea of the mechanism of its action. The main tasks of ITT: • restoration and maintenance of the volume and composition of all water sectors of the body (vascular, interstitial, cellular); • optimization of parameters of central, regional hemodynamics and microcirculation; • correction of homeostasis parameters: ionic and acid-base balance; <osmolarity and oncotic pressure; • ensuring adequate oxygen transport to organs and tissues (the main condition for adequate ITT); • prevention of reperfusion injuries. An obligatory component of intensive care for critical conditions is the use of transfusion methods to correct the deficiency of cellular and plasma components of blood acquired by the patient as a result of the disease, in the process of cytostatic therapy, in case of blood loss due to injury or surgery. INTENSIVE POSTOPERATIVE THERAPY FOR PATIENTS WHO HAVE SUFFERED MASSIVE INTRAOPERATIVE BLOOD LOSS The specific treatment of patients who have suffered massive and supermassive intraoperative blood loss has become relevant in connection with the development of aggressive surgery and the expansion of indications for surgical treatment of cancer, especially its locally advanced forms. Among ICU patients, 20% are patients after oncosurgical interventions with blood loss of 60% or higher; maximum 700% bcc. Massive and supermassive blood losses are mainly accompanied by surgical interventions performed for: • kidney cancer with a tumor thrombus spreading through the inferior vena cava (17%); • retroperitoneal tumors (16%); • tumors of bones and soft tissues (15%); • colon cancer (8%); • stomach cancer (6%); • cardioesophageal cancer (5%); • pancreatic cancer (5%). Depending on the volume of intraoperative blood loss, ITT varies. • With blood loss up to 100% of the blood volume, systemic hemodynamics are at an acceptable level; for recovery, as a rule, the use of adequate volumes of ITT is sufficient. • In case of blood loss of 100-200%, ITT is performed with crystalloid solutions (130-150% of the blood loss volume) and colloids (50-70%), which consist of 30-35% FFP, 20-50% of synthetic colloids, mainly , hydroxyethyl starches. • Blood loss over 100-200% of the blood volume requires, in addition to massive ITT, the use of cardiovasotonics to create centralization of blood circulation and maintain perfusion of vital organs. The need to use cardiovasotonics in the early postoperative period often persists in patients who have suffered a blood loss of more than 200% of their blood volume. • To stabilize the hemostatic system in case of massive intraoperative blood loss of 600-700% of the bcc, FFP is required in a volume of at least 30% of the blood volume in patients with developed acute DIC syndrome. A sufficient volume of injected red blood cells (donor + autotransfused) is 20-30% of the blood volume. Early complications of massive blood loss and the use of cardiovasotonics. • Lactic acidosis. • Disturbances in the hemostatic system caused by hemodilution coagulopathy and acute disseminated intravascular coagulation syndrome in patients who have suffered a blood loss of more than 100% of their volume. The tactics of using blood components in the early postoperative period, in particular FFP or red blood cells, depends on the need for anti-shock, hemostatic therapy for ongoing bleeding or DIC syndrome. Cancer patients who have suffered severe surgical trauma, massive intraoperative blood loss, hemorrhagic shock, acute disseminated intravascular coagulation syndrome and massive ITT constitute a special risk group for the development of organ failure and MODS, including ALI/ARDS. It is very difficult to build an ITT program and maintain an adequate fluid balance in the body in the early postoperative period in patients with massive intraoperative blood loss. The doctor must take into account the contradiction between the need to adequately replenish blood loss and prevent fluid overload, which leads to the development of non-cardiogenic OA and AHF; on the first day of the postoperative period, no more than 2-2.5 liters of fluid are prescribed to patients admitted to the ICU with stable circulatory parameters. Prevention of overhydration is based on an accurate calculation of fluid balance, the excess of which should not exceed 80 ml/kg, which is achieved by using diuretics (furosemide 10-15 mg). It is necessary to maintain the COP of blood plasma at the level of 20-25 mm Hg. Sufficient hydration during diuretic therapy is indicated by normonatremia, the degree of hemodilution of 28-30%, coagulogram data, as well as stable hemodynamic parameters that do not require the administration of cardiovasotonics. In patients admitted to the ICU with hemorrhagic shock, the infusion program should be structured in such a way as to help maintain the COP within normal values, since by the end of the operation not only low COP values are detected, but also significant fluid retention in the body. The volume of administered colloid solutions should exceed the crystalloid infusion by an average of 1.5 times. This situation, along with the use of diuretic therapy and achieving a negative fluid balance during the observation of patients in the ICU, are preventive measures against ALI/ARDS. POSTOPERATIVE BLEEDING Diagnosis of postoperative bleeding in the early stages does not cause difficulties only if there is an active excessive release of blood through the drainage (more than 200 mm/h) or the presence of clots in hemorrhagic fluid with a large admixture of hemoglobin. However, sometimes even large-diameter drainage tubes become clogged with blood clots during intense bleeding. In this case, hypovolemia, decreased blood pressure and central venous pressure, tachycardia, oliguria, severe pallor of the skin, despite adequate ITT, should be regarded as signs of bleeding. It is necessary to carry out ITT aimed at correcting systemic hemostasis and hemodynamic disorders, simultaneously with active step-by-step observation of the surgeon and examination of the hemostatic system. Even moderate changes in the coagulogram in patients with postoperative bleeding with a high degree of certainty indicate the need for additional surgical hemostasis. An unfavorable sign is hypofibrinogenemia (less than 1 g/l) against the background of a high level of D-dimer, which is observed in patients with severe hemorrhagic shock. Causes of postoperative ongoing bleeding requiring conservative therapy. • Hemodilutional coagulopathy is characterized by moderate hypocoagulation, as well as the absence or low level of DIC markers. The patient is prescribed conservative therapy with replenishment of coagulation factors. • Acute DIC after surgery, complicated by massive blood loss and hypovolemic shock, is characterized by: severe hypocoagulation (aPTT 120-180 s), caused by a deficiency of blood coagulation factors both due to consumption and due to hemodilution; o thrombocytopenia (50 thousand in 1 μl and below) with a complete lack of platelet aggregation ability, high levels of intravascular coagulation markers. Gastrointestinal bleeding from acute ulcers, so-called stress bleeding, is observed in later postoperative periods. The genesis of stress bleeding is multifactorial; a key role is assigned to disturbances of microcirculation in the gastrointestinal tract. Activation or neutralization of potentially damaging factors (hydrogen ions, bile acids, pepsin) by increasing bicarbonate secretion and increasing mucus formation are associated with adequate blood circulation in the mucous membrane of the stomach and duodenum. Oxygenation disorders (a combination of tissue ischemia and acidosis) are the starting point for a decrease in the activity of protective factors, which results in an increase in the number of oxygen and hydrogen radicals, damage to the cells of the gastrointestinal mucosa and the development of erosive and ulcerative bleeding. The main risk group consists of patients who have suffered shock of various origins, sepsis, patients with respiratory, acute liver and kidney failure. Diagnosis is based on a decrease in hemoglobin during dynamic observation and identification of gastric contents stained with blood or the color of coffee grounds, and the appearance of melena. If these symptoms persist for more than 12 hours or hemodynamic changes develop (decreased pressure, tachycardia, decreased hemoglobin), gastroscopy should be performed. The main objectives of prevention: • ensuring sufficient oxygenation and restoration of local blood circulation; • adequate treatment of respiratory failure; • sufficient volume replacement and timely elimination of circulatory disorders of internal organs with drugs with positive inotropic effects and other vasoactive agents; • combating infectious complications; • preventing stressful situations with the help of analgesics and sedatives, which reduces the incidence of bleeding. Among the means of drug prevention of stress ulcers, omeprazole, which inhibits the proton pump and has a maximum inhibitory effect on acid secretion, is of particular interest. In case of widespread damage, stopping diffuse bleeding is carried out by irrigating the surface with hemostatic solutions. If a damaged vessel is visible, clipping it is most effective, as well as injections of solutions to compress the bleeding area and vasoconstriction (saline solution of sodium chloride with the addition of adrenaline). The advent of the drug eptacog alfa (Novoseven*) (recombinant activated coagulation factor VII) has opened up new opportunities for the treatment of uncontrolled bleeding after various surgical interventions. The mechanism of action of eptacog alfa is based on the local initiation of hemostasis at the site of injury; the drug forms complexes with tissue factor of damaged tissues. Features of the mechanism of action of the drug, characterized by a local effect, explain the safety of its use; there are no absolute contraindications for use. Pain management and monitoring techniques used in oncology |
·
End of form
| Anesthesiologists have to proceed from the fact that the indications for surgery in a cancer patient are always absolute, therefore, even if he has severe disorders associated with the underlying or concomitant diseases, one should strive to perform surgical treatment. This is consistent with the current modern trend towards expanding indications for surgical treatment due to the improvement of anesthetic and surgical techniques. Patients with reduced functional reserves of respiration and circulation, other organs and systems are shown anesthesia methods that provide an adequate level of anesthesia and areflexia for the surgical injury, prevent pathological circulatory reactions in response to surgical trauma and do not have undesirable side effects. Recently, general anesthesia is based on the principle of multicomponentity, when the necessary components of anesthesia (switching off consciousness, anesthesia, areflexia, autonomic stabilization, muscle relaxation) are achieved by a combination of components with selective properties (central analgesic, tranquilizer, hypnotic, antipsychotic, muscle relaxant). Regional anesthesia makes a significant contribution to the provision of anesthesia and areflexia during oncological interventions, but for large intracavitary operations it can only be used as part of a combined general anesthesia with artificial ventilation. A significant proportion of patients in an oncology hospital are elderly and senile people who, in addition to cancer, have involuting organic and functional changes, and often severe concomitant diseases with varying degrees of insufficiency of the functions of organs and systems. Disorders that have developed as a result of preoperative radiation and chemotherapy should also be taken into account. All this dictates the need for special measures for preoperative correction of disorders in the optimal time frame. The modern level of surgical treatment, therapeutic and diagnostic procedures poses a number of challenges for the anesthesiology service. First of all, these include ensuring the safety and psychological comfort of the patient, reliable pain relief, preventing pathological circulatory reactions, reliable airway patency, reducing side effects and, of course, creating optimal conditions for the surgeon’s work. The choice of anesthetic management method is determined by several circumstances: the need to agree with the patient on the method of anesthesia in case of any, even small, danger to him (for example, consent to regional anesthesia); the ability to reduce the harmful effects of anesthetic agents on the anesthesiologist and operating room staff; creating optimal conditions for the surgeon’s work. The choice of anesthesia method is also influenced by the following factors: duration of the operation; · area of operation; · need for relaxation; “full” stomach; · initial condition of the patient and concomitant pathology; · age; · postoperative phase: inpatient or outpatient. Criterion “duration of operation”. If the operation lasts up to 30 minutes, mask or intravenous anesthesia can be performed; the danger is aspiration of gastric contents. An increase in the duration of the operation beyond this period is an indication for endotracheal anesthesia. Alternative: laryngeal mask and regional anesthesia (when possible). Criterion "area of operation". Operations on the thoracic and upper abdominal organs are performed under endotracheal anesthesia or in combination with regional anesthesia. When the patient is positioned on his stomach, tracheal intubation is indicated; an alternative is regional anesthesia. Operations on the lower floor of the abdominal cavity, pelvic organs and extremities can be performed under regional, mask, laryngeal-mask, endotracheal and combined anesthesia. The criterion is “the need for relaxation.” Sufficient relaxation is necessary in: · abdominal surgery; · thoracic surgery; · gynecology with abdominal access; · urology for abdominal and retroperitoneal interventions. Particularly reliable relaxation is necessary in: neurosurgery during intracranial interventions; · eye surgery. “Full stomach” criterion. All patients with a “full stomach” should be intubated under conditions of protection against aspiration according to the rule of anesthesia for intestinal obstruction. Patients with a “full stomach” include patients: · in whom less than 6 hours have passed since the last meal; · with intestinal obstruction; · with stenoses of the upper gastrointestinal tract (esophagus, stomach, duodenum); · with large abdominal tumors (high position of the diaphragm, impaired passage of food); · in an unconscious state. Criterion “initial condition and concomitant pathology”. Key points: · Lung disease: inhalational or regional anesthesia is preferred, and all respiratory depressants (opioids, fentanyl) are administered with caution. · Cardiac disease: care must be taken when inducing anesthesia, tracheal intubation, when dosing inhalational anesthetics, and take into account the cardiodepressive effect of anesthesia drugs. · Muscle diseases: surgery can be performed without the use of muscle relaxants or with minimal doses of depolarizing relaxants, but caution must be exercised when using opioids. · Liver disease: the use of inhalational anesthetics should be avoided if possible; alternatives include the use of neuroleptanalgesia, ataralgesia and regional anesthesia. · Kidney disease: Since almost all muscle relaxants are eliminated by the kidneys, their doses should be reduced, and preference is given to non-depolarizing relaxants with an intermediate duration of action. Age criterion. In elderly patients, the possibility of combining regional and general anesthesia should be kept in mind. The criterion is “postoperative phase—inpatient or outpatient.” During outpatient operations, general anesthesia with the use of facial and laryngeal masks, with the use of short-acting intravenous anesthetics, analgesics and hypnotics, dominates. The use of regional anesthesia in outpatient practice is controversial due to the duration of action and its possible side effects and complications. It must be remembered that in oncoanesthesiology, drug doses should be reduced by 15-20%. Operations in oncology, as a rule, are highly traumatic and lengthy, and the more extensive and lengthy the surgical intervention, the greater the likelihood of complications and unfavorable outcomes. Against the background of limited functional capabilities of the cancer patient’s body, surgical intervention creates extreme conditions for the activity of all organs and systems, and especially the cardiovascular, respiratory, and excretory systems. One of the main tasks of an anesthesiologist is to ensure the safety of a patient under anesthesia. This requires a system for effectively monitoring vital body functions during anesthesia and surgery. The American Society of Anesthesiologists has adopted standards for intraoperative monitoring, which can be supplemented as necessary by each anesthesiologist. When using complex technical monitoring methods, indications, contraindications, complications that may arise during their use, as well as economic feasibility should be taken into account. Due to the fact that the patient's condition changes rapidly during anesthesia, the presence of an anesthesiologist is necessary throughout the entire duration of anesthesia, despite the presence of monitoring. It is imperative to monitor: blood circulation; breathing. According to indications, monitoring is used: central nervous system; body temperature; diuresis. Blood circulation monitoring. Non-invasive blood pressure monitoring. Absolute indications are general or regional anesthesia. The measurement technique and frequency depend on the type of surgery and the patient’s condition. Invasive blood pressure monitoring is indicated for controlled hypotension, possible significant fluctuations in blood pressure, and the need to monitor arterial blood gases. Electrocardiography is of great diagnostic value in all operated patients. Modern monitors make it possible to automatically analyze the ECG, including the ST segment, which makes it possible to diagnose early signs of myocardial ischemia. Based on the sound signal, the anesthesiologist can recognize changes in heart rate and heart rhythm, but the operation of electrosurgical equipment limits the possibilities of intraoperative analysis of arrhythmias. In order to monitor central venous pressure and provide infusion-transfusion therapy, central venous catheterization is performed. Catheterization of the pulmonary artery with a floating Swan-Ganz catheter allows you to determine left ventricular preload, diagnose air embolism, myocardial ischemia, and also obtain mixed venous blood samples. There are catheters with built-in electrodes for recording intracavitary ECG and cardiac pacing, as well as with thermistors for measuring cardiac output. When using this study, one should keep in mind the possibility of such serious complications as rupture of the pulmonary artery and cardiac arrhythmias. Breath monitoring. Pulse oximetry is one of the standards for mandatory intraoperative monitoring, and even more so in elderly cancer patients with limited functionality. Pulse oximetry is designed to non-invasively measure peripheral blood oxygen saturation, assess tissue perfusion (by pulse amplitude) and measure heart rate. This method allows you to quickly recognize developing hypoxia and assess the adequacy of oxygen delivery to vital organs, and in the postoperative period, monitor the development of hypoventilation, atelectasis and bronchospasm. Capnography makes it possible to monitor breathing, blood circulation and the tightness of the respiratory circuit. The method allows you to quickly and accurately diagnose erroneous intubation of the esophagus, kinking of the endotracheal tube or other airway, as well as leakage of the breathing circuit. Other types of monitoring. Intraoperative monitoring of the central nervous system (electroencephalography, evoked potentials) is carried out during interventions with a risk of damage to the central nervous system (brain tumor, aortic aneurysm, carotid endarterectomy, etc.), when using controlled hypotension, a heart-lung machine to assess the adequacy of cerebral oxygenation. Monitoring body temperature is desirable during long-term operations under general anesthesia and is mandatory when conducting sessions of general hyperthermia during the complex treatment of cancer patients. Monitoring diuresis allows you to indirectly assess the condition of the kidneys, circulatory system, water balance and circulating blood volume. Have you heard about the use of lithotherapy in oncology? Skilled oriental healers know how to prepare a magical elixir for the treatment of cancer from a special “stone of dawn”. |
Stress adaptation syndrome. Painful stress In the development of the general adaptation syndrome, not only stress mechanisms (in particular, sympathetic-adrenal and corticosteroid), but also anti-stress mechanisms are included. These mechanisms at different levels limit the degree of sympathetic-adrenal excitation, reduce the release of ACTH and glucocorticoids when stress agents act on the body. In the central nervous system, anti-stress mechanisms are represented by GABAergic and serotonergic neurons, which weaken sympathetic influences and reduce the release of corticoliberin. In peripheral organs, limited release of norepinephrine from nerve endings and a decrease in the effectiveness of its action are caused by the neurotransmitter acetylcholine, some prostaglandins, possibly adenosine and other factors. The nonspecificity of the general adaptation syndrome is relative. For example, the response of the endocrine and other body systems to cooling, which includes the obligatory participation of thyroid hormones, differs from the response to hypoxia, in which the production of thyroid hormones may be weakened. Exaggerating the role of the conditions of action of a stressor agent (so-called conditional factors) in the formation of the characteristics of the body's response and even the nature of the pathological processes that arise in this case is unfounded. If we take this point of view, it turns out that the properties of the stress agent itself (especially its nature) and the properties of the organism (its reactivity) do not seem to have significant significance in the adaptation of the organism or in the occurrence of pathological processes. The characteristics of stress largely depend on the individual properties of the organism, in particular on the degree of its adaptation to a given agent; long-term adaptation to stress is an important factor in preventing its damaging effects. Pain stress is the general tension of the body associated with the occurrence of pain, which is usually formed under the action of tissue-damaging agents. Pain can act as a decisive pathogenetic component of a number of pathological conditions, in the development of which changes in brain activity under the influence of excessive pain afferentation, such as shock, are essential. Pain (Latin dolor, Greek nocens) in the narrow sense is an unpleasant subjective sensation, accompanied by interconnected emotional, vegetative and locomotor reactions. As with any other stress, pain stress activates the sympathetic-adrenal and pituitary-adrenal systems, which take part in the formation of the above reactions. Currently, pain is considered as a psychophysiological condition that most often occurs when destructive agents act on the body, in which, along with a subjective feeling of a negative nature, the nociceptive system operates, ensuring the elimination of this sensation.
Osmolality (blood)
Key words: renal failure diabetes insipidus blood
Osmole - When determining the concentration of a solution in terms of the number of particles, instead of grams, a unit called the osmole is used.
One osmole is 1 gram molecule of an osmotically active solute. So, 180 g of glucose, i.e. 1 gram molecule of glucose is equivalent to 1 osmole of glucose, since glucose does not dissociate into ions. If a solute dissociates into 2 ions, 1 gram molecule of solute will correspond to 2 osmoles, since the number of osmotically active particles in this case is twice as large as for a non-dissociating solute. When completely dissociated, 1 gram molecule of sodium chloride, or 58.5 g, is equivalent to 2 osmoles (i.e., the osmolarity of a 1-molar NaCl solution will be 2 osmol/l).
A solution containing 1 osmol of solute in every kilogram of water is said to have an osmolality of 1 osmol per 1 kg. A solution containing 1/1000 osmol of solute per kg has an osmolality of 1 milliosmol (mosm) per kg. The normal osmolality of extracellular and intracellular fluids is approximately 300 mOsm per 1 kg of water.
Serum osmolality is a quantitative measure of osmotically active substances dissolved in blood serum, i.e. the sum of the concentrations of cations, anions and non-electrolytes, expressed in milliosmoles per kilogram of water (mOsm/kg H 2 O). The main anions that determine serum osmolality include sodium and other anions (potassium, chlorine, bicarbonate ions, etc.). A more accurate measurement also takes into account the glucose and urea content, finding an expression in the formula:
Serum osmolality= 2Na + + serum glucose + urea (urea nitrogen)
Plasma osmolality can be determined by knowing the concentration of the main osmotic components of the extracellular fluid - sodium, glucose and urea. For example: sodium is 140 mEq/L, glucose is 4 mmol/L, blood urea is 6 mmol/L. Plasma osmolality = 2 x Na + glucose (mmol/l) + urea (mmol/l) = 2 x (140) + 4 + 6 = 290 mOsm/kg H2O
One of the main factors regulating antidiuretic hormone (ADH) secretion and thirst is plasma osmolality. Osmoreceptors located in the hypothalamus are sensitive to fluctuations in osmolality. A change of 1% already leads to noticeable changes in ADH secretion.
When determining blood osmolality, two main conditions are distinguished: hyperosmolality and hypoosmolality.
Hyperosmolality is caused by an increase in serum osmolality, which is one of the common causes of coma in diabetes mellitus and brain dehydration. As blood osmolality increases, ADH secretion increases. When the osmolality reaches about 295 mOsm/kg, the concentration of ADH becomes sufficient to ensure the maximum antidiuretic effect (urine volume less than 2 l/day; urine osmolality more than 800 mOsm/kg). At the same time, the thirst quenching mechanism is activated, which leads to an increase in water consumption and prevents dehydration of the body.
Hypoosmolality is a decrease in blood osmolality. Hypoosmolality can lead to osmotic cerebral edema and the development of intracranial hypertension syndrome. The reason for the decrease in osmolality can be various factors, for example, an excess of free water contained in the blood plasma relative to the volume of kinetic particles dissolved in it. When blood osmolality decreases below the threshold level (about 280 mOsm/kg), ADH secretion is inhibited. This leads to the excretion of a large volume of maximally diluted urine. Increased water excretion prevents a further decrease in plasma osmolality, even with significant water intake.
The osmolal concentration of urine ranges from 50 to 1400 mosmol/l (in plasma 295 mosmol/l). When the osmolal concentration of urine is higher than that of plasma, the difference between these values indicates the amount of solutes removed from the plasma without equivalent loss of water. For example, if with a daily urine volume of 2 liters the osmolality is 100 mOsmol/L, then the clearance will be 1.43 liters of free water during this period. The lowest osmolality is observed in uncontrolled diabetes insipidus.
Osmolality exceeds standard value
A critical increase in serum osmolality is considered to be 298 mOsm/kg. This deviation is called hyperosmolarity. It can be provided by the following factors:
- Severe dehydration of the body.
- Diabetes insipidus.
- Mechanical head bruises.
- Strokes.
- Increased glucose levels.
- An increase in sodium concentration in the body.
- The inability of the kidneys to fully remove harmful toxins from the human body, which over time leads to the development of intoxication.
- Carbon monoxide poisoning and household chemicals.
Read also: Excess of total protein in the blood, causes of this condition, methods of diagnosis and treatment
Types of hyperosmolarity
There are three types of hyperosmolarity conditions.
Isotonic
It is characterized by excessive accumulation of salt and water in the body, which provokes the development of heart and kidney diseases. Treatment of the deviation involves the patient taking cardiac glycosides and minimally drinking water. Pharmacological agents are prescribed:
- Furosemide.
- Prednisolone.
- Triamterene.
Hypertensive
It is characterized by the accumulation of water and salts in blood vessels and intercellular membranes, causing low hemoglobin, protein and hematocrit. Therapeutic measures include:
- A solution of insulin and glucose.
- Albumen.
- Lasix.
- Veroshpiron.
Important! Depending on the patient’s condition, hemodialysis and peritoneal therapy are performed. It is strictly forbidden to administer crystalloids.
Hypotonic
Accumulation of fluid in blood vessels, in the cell and its membranes. Because of this, sodium, protein and hemoglobin drop sharply in the body. Therapy involves the use of mannitol solution, hypertonic mixtures and corticosteroids. To accelerate fluid removal, hemodialysis with ultrafiltration mode is performed.
Specifics of the study
Several basic values are assessed, which are studied by specialists in the resulting material. After the examination and grouping of the necessary data is completed, laboratory assistants enter the obtained indicators into a special correspondence table, with the help of which acceptable values and their violations are later displayed.
Checking the osmotic concentration is determined by the following factors:
- To obtain information about the amount of fluid in the blood.
- As a source of indicators of the chemical composition of serum.
- To monitor increases and decreases in serum fluid concentrations.
- To check the level of the hormone that is responsible for fluid retention in the body;
- To detect the underlying causes of dehydration and swelling of the extremities.
- To diagnose the body for the presence of pathological processes.
- To diagnose the presence of poisons, methanol and other dangerous substances.
The study of plasma osmolarity is characterized by the content of chemical substances in it. To carry out the event, the laboratory assistant takes venous blood from the patient.
Several basic values are assessed, which are studied by specialists in the resulting material. After the examination and grouping of the necessary data is completed, laboratory assistants enter the obtained indicators into a special correspondence table, with the help of which acceptable values and their violations are later displayed.
Links[edit]
- DJ Taylor, NPO Green, GW Stout Biological Science
- IUPAC Golden Book
- ^ abcdefg Widmaier, Eric P.; Herschel Raff; Kevin T. Strang (2008). Wander's Human Physiology, 11th ed. McGraw-Hill. pp. 108–12. ISBN 978-0-07-304962-5.
- 1947-, Costanzo, Linda S. (2017-03-15). Physiology
. Previous: Costanzo, Linda S., 1947- (Sixth ed.). Philadelphia, Pennsylvania. ISBN 9780323511896. OCLC 965761862.CS1 maint: numeric names: authors list (link) - Page 158 in: Martin, Alfred N.; Patrick J. Cinco (2006). Martin's Physical Pharmacy and Pharmaceutical Sciences: Physicochemical and Biopharmaceutical Principles in the Pharmaceutical Sciences
. Phila: Lippincott Williams and Wilkins. ISBN 0-7817-5027-X. [1] - Blood Density Collection of facts on physics
. Edited by Glenn Ehlert. Retrieved March 26, 2009. - Earley, L.E.; Sanders, C. A. (1959). "The effect of changes in serum osmolality on antidiuretic hormone release in some patients with decompensated cirrhosis and low serum osmolality". Journal of Clinical Research
.
38
(3):545–550. doi:10.1172/jci103832. PMC 293190. PMID 13641405.
Features of osmolarity
An increased blood osmolarity provokes a decreased urine osmolarity. This imbalance is the main symptom of abnormalities in the renal parenchyma. The slightest violation of this norm is provoked by the processes that are responsible for the distribution of fluid in the body.
According to basic physiology, a person needs to consume 1-2 liters of water daily for normal existence, since it enriches the body with useful substances and microelements. Most of them enter us through drinking, the rest through liquid found in food. Unnecessary or waste water is removed from the body by the epidermis, pulmonary, intestinal and renal systems. The daily rate of fluid excreted in urine and feces is 0.8 - 1 liter.
If a person’s water balance is disturbed, or fluid is not properly removed from the body, the osmolarity of blood and urine is disrupted. An excess of fluid provokes swelling and heaviness in the limbs, and a lack of it will cause severe dehydration and plasma viscosity.
More than 30 percent of serious diseases develop due to impaired water balance. For example, excess fluid and electrolyte imbalance in most cases cause:
- Kidney diseases.
- Cardiac pathologies.
- Blood diseases.
- Circulatory disorders.
Fluid deficiency provokes the following changes in the body:
- Excess glucose in the blood.
- Diseases of the adrenal glands and kidneys.
- Diabetes.
Thanks to osmolarity analysis, it is easier for a specialist to determine the state of water-salt balance and, if necessary, correct it with medications.
Interpretation of results
The study of oncotic and osmotic pressure of blood plasma is very important for the examination and treatment of patients suffering from diabetes. This is explained by the fact that this disease is characterized by an excess of the osmolarity norm, so if several tests do not show a decrease in this indicator, the specialist should prescribe the patient another treatment.
The study of serum osmolarity is carried out in order to find out the quantitative indicator of urea, glucose and sodium. Urea is the result of protein breakdown in our body. The study of osmolality allows doctors to understand the state of water-salt balance in the body of the person being studied.
In most cases, a specialist prescribes this test if the patient has:
- Dehydration.
- Lack of sodium.
- Kidney failure.
- Poisoning from chemicals or gases.
Generally accepted medical standards for serum osmolality
| Unit | Non-pregnant adults | 1st trimester | 2nd trimester | 3rd trimester |
| mOsm/kg water | 274 296 | 273 282 | 278 291 | 276 – 285 |
| mmol/kg | 274 296 | 273 282 | 278 291 | 276 285 |
Calculation of osmolality using the formula:
The formula is very simple: Osm = 1.86 Pa + G + M + 10.
PA is a quantitative indicator of sodium.
G – glucose concentration.
M – urea index.
Determination of osmolality of aqueous solutions
The following methods can be used to determine osmolality: cryoscopic, membrane and steam osmometry.
Cryoscopic method
The method is based on lowering the freezing point of solutions compared to the freezing point of a pure solvent.
1 osmol per kilogram of water lowers the freezing point by 1.86 °C. Measuring these changes is the basis of the cryoscopic method.
This dependence can be expressed by the following formula:
Where:
Sosm - osmolality of the solution (mOsm/kg)
T2 is the freezing temperature of a pure solvent (˚C);
T1 is the freezing temperature of the test solution (˚C);
K is the cryometric constant of the solvent (for water: 1.86).
Currently, the determination of the osmolality of solutions is carried out using automatic cryoscopic osmometers.
The required amount of the test solution is placed in the cell of the device. Next, the measurement is carried out according to the instructions included with the device. If necessary, the device is calibrated using standard solutions of sodium or potassium chloride that cover the detectable osmolality range (Table 1).
Table 1 - Standard reference values for the freezing point depression and the efficiency of the osmotic concentration of aqueous solutions of sodium and potassium chlorides
| Analytical concentration of salt p, g/kg H2O | Decrease in freezing temperature DTzam., K | Effective (osmotic) concentration mef, mmol/kg H2O |
| Sodium chloride solutions | ||
| 5,649 | 0,3348 | 180 |
| 6,290 | 0,3720 | 200 |
| 9,188 | 0,5394 | 290 |
| 9,511 | 0,5580 | 300 |
| 11,13 | 0,6510 | 350 |
| 12,75 | 0,7440 | 400 |
| 16,00 | 0,9300 | 500 |
| Potassium chloride solutions | ||
| 7,253 | 0,3348 | 180 |
| 8,081 | 0,3720 | 200 |
| 11,83 | 0,5394 | 290 |
| 12,25 | 0,5580 | 300 |
| 14,78 | 0,6696 | 360 |
| 20,71 | 0,9300 | 500 |
Membrane osmometry method
The method is based on the use of the property of semi-permeable membranes to selectively allow molecules of substances to pass through.
The driving force behind the process is the process of osmosis. The solvent penetrates the test solution until equilibrium is established; the additional hydrostatic pressure that arises is approximately equal to the osmotic pressure and can be calculated using the formula:
(5)
Where:
Osmolality can be calculated using the formula:
Сcm =pcmR ∙ T (6)
where R is the universal gas constant (8.314 J/molK)
T – absolute temperature (˚K).
Note. This method is applicable only for solutions of high molecular weight substances (104 – 106 g/mol). When analyzing solutions containing electrolytes and other low molecular weight substances, only the osmotic pressure created by high molecular weight components of the solution will be determined.
Determination of the osmolality of the test solution is carried out using a membrane osmometer. Preliminary calibration of the device and measurements are carried out in accordance with the instructions for the device.
Steam osmometry method
1 osmol per kilogram of water lowers the vapor pressure by 0.3 mmHg. Art. at a temperature of 25 °C. Measuring these changes is the basis of vapor osmometry.
The method is based on measuring the temperature difference that occurs across thermistors placed in a measuring cell saturated with solvent vapor if a drop of pure solvent is applied to one of them and a drop of the test solution is applied to the other. The temperature difference occurs due to the condensation of solvent vapor on a drop of solution, since the vapor pressure of the solvent above this surface is less. In this case, the temperature of the solution drop increases due to the exothermic condensation process until the vapor pressure above the solution drop and the pressure of the pure solvent in the cell are equal. When pure solvent is applied to both thermistors, the temperature difference is zero. The temperature difference is practically proportional to the molal concentration of the solution.
The osmolality of the test solution is determined using a steam osmometer. Preliminary calibration of the device and measurements are carried out in accordance with the instructions for the device.
Download in PDF GPM.1.2.1.0003.15 Osmolarity
SALT.
Norm: none.
Pathology options:
Interpretation of results: hypovolemia (diarrhea, vomiting, excessive sweating), severe pneumonia, leukemia when taking cystostatic drugs.
Interpretation of results: hypovolemia (diarrhea, vomiting, excessive sweating), severe pneumonia, leukemia when taking cystostatic drugs.
Interpretation of results: inflammation of the urinary tract (pyelonephritis, cystitis, etc.).
Interpretation of results: rheumatism, anemia.
Interpretation of results: has no diagnostic value, may occur when using sulfur mineral waters.
Interpretation of the results: diabetes mellitus, consumption of lingonberries, blueberries, intake of salicylic and benzoic acids.
Interpretation of results: intake of plant foods, cystitis.
Interpretation of the results: repeated vomiting, frequent gastric lavage.
Interpretation of results: no diagnostic value.
Interpretation of the results: eating large amounts of tomatoes, spinach, sorrel, apples, grapes, oranges.
Interpretation of results: no diagnostic value.
Interpretation of results: hereditary cystinosis.
Interpretation of results: protein decomposition products, liver diseases, B-12 deficiency anemia, leukemia.
Interpretation of the results: products of the cleavage of purine bases, promotes stone formation.
Typical results: amyloidosis, renal tuberculosis, cystitis.
Interpretation of results: hyperbilirubinemia.
Interpretation of results: bleeding from the urinary tract.
Interpretation of results: intravascular hemolysis.
Interpretation of results: fatty degeneration of organs.
Interpretation of results: treatment with sulfonamide drugs.
What does it consist of??
The process by which urine is concentrated or diluted is a very complex one, requiring the proper integration of two independent renal systems: the creation of a solute gradient and antidiuretic hormone activity.
Urine concentration and dilution
The creation of an osmolar gradient of solutes occurs in the loop of Henle and in the renal medulla. There, urine osmolarity increases from values similar to those of plasma (300 mOsm/kg) to levels close to 1200 mOsm/kg, all due to the reabsorption of sodium and chloride in the thick portion of the ascending loop of Henle.
Urine then passes through the cortical and medullary collecting tubules, where water and urea are reabsorbed, helping to create osmotic gradients.
Likewise, the thin portion of the ascending loop of Henle helps reduce urine osmolarity due to its permeability to chlorine, sodium and, to a lesser extent, urea.
As the name suggests, antidiuretic hormone prevents or reduces the release of urine to normally conserve water.
This hormone, also known as vasopressin, is then activated in situations of high plasma osmolarity (>300 mOsm/kg) to absorb water, which ultimately dilutes the plasma but concentrates the urine.