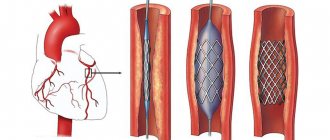
Bypass grafting of the arteries of the lower extremities below the inguinal ligament are:
- femoral-popliteal bypass surgery,
- femoral-tibial bypass,
- femoral-distal popliteal bypass.
Above the inguinal ligament the following is performed:
- aortofemoral bypass surgery,
- aorto-bifemoral bypass (aorto-femoral bifurcation bypass),
- aorto-iliac bypass surgery,
- iliofemoral bypass,
- femoro-femoral cross bypass,
- aorto-mesenteric bypass, depending on which vessel needs bypass.
Read more about types of aortic bypass surgery
Aortofemoral bypass surgery, femoral-popliteal bypass surgery, femoro-tibial bypass surgery and other types of bypass surgery create new pathways for delivering blood to tissues, bypassing diseased arteries. Arterial bypass surgery does not cure artery disease. This type of treatment (surgery) is used when drug therapy and minimally invasive procedures (balloon angioplasty of arteries, arterial stenting) do not relieve the existing symptoms of the disease.
Aortofemoral bypass surgery (aortobifemoral bypass surgery)
This is the implementation of a shunt connecting the aorta to the femoral arteries, which bypasses the diseased arteries and increases blood flow to the patient's legs.
Access to the aorta is achieved either by a midline laparotomy or by a Robe oblique transverse incision. The femoral arteries are accessed through a vertical incision in both groin areas. Using thin threads, the shunt is sutured above and below the blocked arteries. Then the tissue is sutured layer by layer over the shunt.
Subclavian-femoral / axillary-femoral / axillary-bifemoral bypass
This type of bypass is used in some difficult situations.
Instead of the aorta as the source of blood, the subclavian or axillary artery is used. An incision is made below the collarbone and in one or two groin areas. The shunt is sutured with thin threads to the subclavian or axillary artery and femoral artery(s). Pain in the area of postoperative sutures can be observed for several days.
The operation time varies widely, depending on the person’s weight, scar tissue changes, and the severity of the disease.
Color duplex scanning after bypass surgery on the arteries of the lower extremities
Ultrasound machine RS85
Revolutionary changes in expert diagnostics.
Impeccable image quality, lightning-fast operating speed, a new generation of visualization technologies and quantitative analysis of ultrasound scanning data.
Introduction
Modern clinical guidelines for the diagnosis and treatment of steno-occlusive diseases of the aorta and arteries of the lower extremities consider color duplex scanning as an effective method in assessing the nature, localization, extent and extent of damage in each segment of the arterial bed (class of indications I, level of evidence B) [1-4 ]. During the dynamic monitoring of patients who have undergone bypass surgery on the arteries of the lower extremities, ultrasound examination of blood vessels is the method of choice for diagnosing bypass stenosis and complications of reconstructive operations [5]. Numerous studies have shown that without preventive surgery, the risk of thrombosis of a stenotic bypass is about 25% [6-8], and regular monitoring using duplex scanning allows you to clarify or determine the indications for preventive interventions, including angioplasty or replacement of a fragment of the bypass vessel [4].
Types of bypass operations
Bypass operations, depending on the level of proximal (central) and distal anastomoses, are divided into aorto-femoral (aorto-bifemoral) bypass, iliofemoral, femoro-popliteal (above or below the knee joint gap), femoral-posterior tibial bypass, etc. As a bypass vessel (transplant), an autovenous vein and prostheses (synthetic or biological) can be used, which are anastomosed with the arteries according to the “end to end” (Fig. 1, 2) or “end to side” type (Fig. 3-5 ) [9,10].
Rice. 1.
Femoro-popliteal bypass with a combined prosthesis, end-to-end anastomosis.
Longitudinal section, arrow 1—the junction of the synthetic Dacron prosthesis (arrow 2) and the biological prosthesis (bovine internal mammary artery, arrow 3). The location of the intermediate anastomosis is above the level of the knee joint gap (the level of the upper edge of the patella).
Rice. 2.
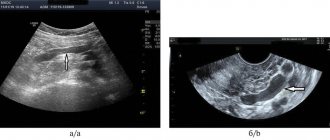
Iliofemoral bypass with a synthetic Dacron prosthesis, proximal end-to-end anastomosis.
A)
Angiogram. Longitudinal section, arrow 1 - the junction of the common iliac artery (arrow 2) with the prosthesis (arrow 3).
b)
Echogram. Longitudinal section, arrow 1 - the junction of the common iliac artery (arrow 2) with the prosthesis (arrow 3).
Rice. 3.
Aorto-femoral bypass with a synthetic Dacron prosthesis, proximal anastomosis with the aorta in an “end-to-side” manner.
Longitudinal section, arrow 1 - aorta, arrow 2 - prosthesis, arrow 3 - lumen between the aorta and the prosthesis.
Rice. 4.
Iliofemoral bypass with a synthetic Dacron prosthesis, distal end-to-side anastomosis with the common femoral artery.
A)
Longitudinal section at the level of the distal anastomosis. Arrow 1 - wall of the synthetic prosthesis, arrow 2 - segment of the superficial femoral artery, arrow 3 - segment of the deep femoral artery, arrow 4 - posterior wall of the common femoral artery.
b)
The cross section is 3-5 mm above the level of the distal anastomosis (the level of the cross section is shown by a dot in figure “a”). Arrow 1 - prosthesis, arrow 2 - common femoral artery.
V)
Cross section at the level of the distal anastomosis. Arrow 1 - anterior wall of the prosthesis, arrow 2 - posterior wall of the common femoral artery.
Rice. 5.
Femoro-popliteal bypass with an autovenous vein, distal anastomosis with the popliteal artery according to the “end to side” type.
Longitudinal section. Arrow 1 - autovenous, arrow 2 - popliteal artery.
During reconstructive operations on the arteries of the femoral-popliteal and tibial segments, autovenous bypass (in the in situ position or reversed great saphenous vein) is characterized by a lower incidence of bypass thrombosis in the long-term period compared to bypass with a prosthesis [4, 11-12] (Fig. 5-7 ).
Rice. 6.
Stenosis of the femoral-popliteal autovenous shunt is more than 70%, the highest risk group. Stenosis outside the anastomotic zone - upper 1/3 of the bypass (middle 1/3 of the thigh).
A)
Dopplerogram of blood flow from the shunt 3-5 cm above the stenosis zone (Vps - 83 cm/s).
b)
Dopplerogram of blood flow from the area of stenosis (Vps - 430 cm/s).
V)
Dopplerogram of blood flow 8-10 cm distal to the zone of stenosis (Vps - 35 cm/s).
G)
Dopplerogram of blood flow from the shunt 2-3 cm above the level of the distal anastomosis (Vps - 25 cm/s).
Rice. 7.
Femoropopliteal autovenous shunt without signs of stenosis, low risk of shunt thrombosis.
A)
The area of the proximal anastomosis, longitudinal section, there is no filling defect in the CD mode.
b)
Dopplerogram of blood flow from the proximal anastomosis area (Vps - 125 cm/s).
V)
Dopplerogram of blood flow from the middle 1/3 of the shunt (Vps - 76 cm/s).
G)
Dopplerogram of blood flow from the shunt 2-3 cm above the level of the distal anastomosis (Vps - 80 cm/s).
Aortofemoral shunts have the longest effect [13-14]. For aortofemoral bypass surgery, synthetic prostheses are used, the material of which can be fluorolavsan or dacron with a ribbed structure (see Fig. 2-4), polytetrafluoroethylene or polyurethane [4, 10] with a smooth inner surface (Fig. 8).
Rice. 8.
Aortofemoral bypass with a synthetic prosthesis made of polytetrafluoroethylene, proximal anastomosis.
Arrow 1 - aorta, arrow 2 - prosthesis.
A number of studies have shown the high efficiency of operations using biological prostheses [15-16], which are specially treated umbilical veins or vessels of animals (cattle, pigs). Biological prostheses do not differ in structure from the native artery or vein (Fig. 9). However, biological prostheses, compared to autovenous and synthetic ones, can be more susceptible to aneurysmal expansion and subsequent thrombus formation (Fig. 10).
Rice. 9.
Femoro-popliteal bypass with a biological prosthesis (internal thoracic artery of a bull), middle 1/3 of the prosthesis.
Arrow 1 - biological prosthesis, arrow 2 - anechoic liquid formation (exudate) in the paraprosthetic space, an indirect sign of inflammation.
Rice. 10.
Aneurysmal dilatation and parietal thrombosis of a biological prosthesis.
The middle 1/3 of the femoral-popliteal bypass is shown, the prosthesis is unevenly expanded. Arrow 1—functioning lumen of the prosthesis, arrow 2—thrombomass at the level of maximum aneurysmal dilatation.
In some cases, a combination of prostheses is used as a bypass vessel (a synthetic prosthesis is connected to an autovenous vein or synthetic and biological prostheses are connected to each other) (see Fig. 1). If reconstruction of the femoropopliteal segment is performed after correction of a lesion in the aortoiliac segment, then the best results are observed when an anastomosis is formed with the branches of the prosthesis in the area of the distal anastomosis [4].
Duplex scanning frequency
Patients who have undergone autovenous bypass grafting should be periodically (at least during the first 2 years after surgery) examined by duplex scanning with measurement of peak systolic blood flow velocity (Vps) and calculation of the blood flow velocity ratio throughout the entire length of the shunt (indication class I, level evidence C) [4]. Duplex scanning examination is recommended in the first month after surgery. If autovenous graft stenosis is not detected in the first month (approximately 80% of grafts), observation is recommended at 6-month intervals during the first year, and then annually after the first year after autovenous grafting [10, 17]. Observation of prosthetic reconstructions, as after autovenous reconstructions, is recommended at 6-month intervals [17]. However, one of the key clinical recommendations, the Trans-Atlantic Inter-Society Consensus (TASC II), notes the need only for regular clinical examination and measurement of the brachial-ankle pressure index [18].
Basic Duplex Scanning Tasks
Due to the fact that shunt thrombosis is accompanied by a low probability of limb salvage, the primary objectives of dynamic ultrasound examination of vessels after bypass surgery are the detection of ultrasound signs of an increased risk of developing shunt thrombosis and early diagnosis of shunt thrombosis.
Diagnosis of shunt thrombosis is not difficult. The conclusion about thrombosis is made based on the detection of structures in the lumen of the shunt in B-mode, in the absence of blood flow in the shunt according to color and pulsed wave Doppler modes (Fig. 10, 11), as well as based on the characteristics of blood flow below the area of the distal anastomosis.
Rice. eleven.
Thrombosis (occlusion) of the shunt.
A)
Middle 1/3 of femoral-popliteal autovenous shunt. In the lumen of the shunt, heterogeneous, predominantly hyperechoic structures are visualized (arrow), blood flow is not recorded in the color flow mode.
b)
Femoro-popliteal bypass with a polytetrafluoroethylene prosthesis. Middle 1/3 of the prosthesis, anechoic structures in the prosthesis (arrow), blood flow is not recorded in the color flow mode.
Indirect signs of inflammation (exudate) in the projection of the prosthesis and infection of the prosthesis discovered during ultrasound examination (see Fig. 9) are unfavorable prognostic factors, accompanied by a high risk of shunt thrombosis in the early postoperative period.
The most significant and common precursors of shunt thrombosis are stenoses of the central and/or distal anastomoses, stenoses of shunts outside the anastomotic zones, as well as a decrease in the speed of blood flow through the shunt [10] (Fig. 6, 12, 13). The role of duplex scanning after autovenous bypass surgery has been studied in detail. It has been established that about 20% of infrainguinal autovenous shunts have residual postoperative stenosis or stenosis develops during the first year after reconstruction [6, 10]. Stenoses are localized both in the area of anastomosis and in any other segment of the shunt. For artificial or biological prostheses, stenotic damage to the prosthesis outside the anastomotic zones is less typical compared to autovenous shunts [10] (see Fig. 6).
Rice. 12.
Iliofemoral bypass with a synthetic Dacron prosthesis, stenosis in the area of the distal anastomosis 50-75%, high-risk group.
A)
Dopplerogram of blood flow from the shunt 3-5 cm above the level of the distal anastomosis (Vps - 34 cm/s).
b)
Dopplerogram of blood flow from the area of stenosis (Vps - 292 cm/s).
V)
Cross-section of the superficial femoral artery in the area of the distal anastomosis, planimetric assessment of the degree of stenosis “by diameter”, stenosis 59%.
G)
Main blood flow in the superficial femoral artery in the middle 1/3 of the thigh (Vps - 111 cm/s). Normal blood flow characteristics below the distal anastomosis exclude stenosis greater than 75% and confirm the results of the planimetric “diameter” assessment.
Rice. 13.
Iliofemoral bypass with a synthetic fluorolavsan prosthesis, low-velocity blood flow in the prosthesis.
Dopplerogram of blood flow from the middle 1/3 of the prosthesis (Vps - 30 cm/s), significant narrowing of the proximal and distal segments of the vascular bed were not detected.
In the protocol for ultrasound examination of the aorta and arteries of the lower extremities after bypass operations, special attention is paid to the description of structural and anatomical changes in the afferent vessels and the characteristics of blood flow in them, the characteristics of blood flow and assessment of the condition of the proximal, distal anastomoses and the bypass vessel along its entire length, as well as the description of the structural and anatomical changes in the efferent vessels and the characteristics of blood flow in them [10].
When studying a bypass vessel, emphasis is placed on analyzing Vps and calculating the ratio of the peak blood flow velocity in the zone of its local increase (in the area of stenosis) to the peak blood flow velocity recorded proximal to the stenosis (Vps-ratio). In addition, the ultrasound examination protocol requires the most complete description of the planimetric characteristics of the stenotic segment of the shunt, and in the case of autovenous shunting, an assessment of the cross-sectional diameter of the shunt vein above and below the stenosis, the extent of the stenosis, and a clear indication of the anatomical localization of the stenosis.
Doppler gradation of the degree of stenosis and the risk group for shunt thrombosis
There is a Doppler gradation of the degree of stenosis of autovenous shunts, which is based on the assessment of Vps in the area of stenosis and Vps-ratio [19], developed based on a comparison of duplex scanning data with angiography results (Table).
Table.
Dopplerographic criteria for the degree of narrowing of the autovenous shunt.
| Stenosis degree,% | Vps in the area of stenosis, cm/s | Vps-ratio, c.u. |
| 20-50 | 1,5-2,4 | |
| 50-75 | > 180 | 2,5-4,0 |
| > 75 | > 300 | > 4,0 |
However, the greatest predictive efficiency is in assessing the degree of risk of thrombosis of an autovenous shunt based on a combination of indicators such as Vps in the area of stenosis, Vps-ratio, average Vps in the shunt (the average value of the peak systolic blood flow velocity from 3-4 equidistant zones of the shunt outside the area of stenosis) and Ankle-brachial pressure index (ABP). Low blood flow through the shunt increases the risk of thrombosis [20]. The risk of thrombosis of an autovenous shunt is lower compared to an artificial prosthesis [10], and prophylactic administration of anticoagulant and antiplatelet therapy reduces the risk of thrombosis of low-speed shunts [6-7].
The highest risk group (I degree) with a threat of thrombosis in the near future includes shunts that are stenotic by more than 70% with Vps in the stenosis zone > 300 cm/s, Vps-ratio > 3.5 a.u., with low-velocity blood flow in shunt (average Vps
The group of high risk of thrombosis (grade II) includes shunts that are stenotic by more than 70% with Vps in the stenotic area > 300 cm/s, Vps-ratio > 3.5 a.u., with normal blood flow velocity along the shunt outside the area of stenosis (average Vps > 45 cm/s) and a decrease in LID (compared to the previous assessment) by less than 0.15 c.u.
Groups of the highest and highest risk of thrombosis (grades I and II) require revision of the shunt and surgical restoration of its patency. The probability of progression of stenosis or development of thrombosis in these groups within 3-6 months is 40-50%. For the highest risk group (grade I), immediate restoration of shunt patency is recommended, while for the high-risk group (grade II), planned restoration is possible within 1-2 weeks.
The group of moderate risk of thrombosis (III degree) includes shunts that are stenotic by 50-70% with Vps in the stenosis zone of 180-300 cm/s, Vps-ratio > 2 a.u., with normal blood flow velocity along the shunt outside the area of stenosis ( average Vps > 45 cm/s) and a decrease in LID (compared to the previous assessment) by less than 0.15 c.u.
The group of low risk of thrombosis (grade IV) includes shunts that are not stenotic or stenotic 45 cm/s) and a decrease in LID (compared to the previous assessment) by less than 0.15 cu. (see Fig. 7).
In risk groups of grades III and IV, shunt lesions detected in the first 3 months. from the moment of bypass, in 20-30% of cases they regress, in 10-20% of cases they remain stable, and in 40-50% of cases they progress to stenosis of more than 70%.
For the moderate-risk group, follow-up monitoring is recommended at intervals of 4-6 weeks to confirm or rule out progression of stenosis. During a dynamic study, the progression of stenosis is confirmed by an increase in Vps in the area of stenosis, a decrease in the average value of Vps along the shunt outside the area of stenosis, and a decrease in LID > 0.15 a.u. and, accordingly, transition to a high or highest risk group [10].
Compared with autovenous bypass, studies on the role of duplex scanning in monitoring prosthetic bypasses and determining the indications for surgery on modified prostheses are not so numerous. However, it has been shown that after prosthetic bypass surgery, the inclusion of duplex scanning in the monitoring program and the performance of reconstructive intervention on the bypass vessels based on its results is accompanied by a 5-year patency of the shunts of about 88% and exceeds those indicators without dynamic duplex monitoring [7]. In femorofemoral prosthetic bypass surgery, ultrasound precursors of shunt thrombosis are Vps in the afferent iliac artery of more than 300 cm/s, the presence of stenosis of the prosthesis with a local increase in Vps of more than 300 cm/s and the average value of Vps in the prosthesis of less than 60 cm/s [7]. There is no detailed Doppler gradation of the degree of stenosis and the degree of risk of thrombosis for synthetic and biological prostheses. In this regard, it is permissible to use ultrasound criteria for the degree of stenosis and the degree of risk of thrombosis of autovenous shunts for synthetic and biological prostheses (Fig. 12).
An average Vps value in the prosthesis of less than 45 cm/s can be used as a prognostic criterion for the risk of prosthetic shunt thrombosis [8]. However, the low blood flow velocity through the shunt (Fig. 13) first of all requires a thorough examination of the proximal and distal segments of the vascular bed in order to exclude/confirm significant narrowings. The independent meaning of low blood flow velocity through the shunt has not been fully determined, but requires the following indication in the ultrasound examination protocol: “a decrease in the speed of blood flow through the shunt.”
One of the predisposing factors to the development of thrombosis is kinking (torsion) of the shunt/prosthesis (Fig. 14), accompanied by a narrowing of the functioning lumen and local disturbances of blood flow in the form of an increase in Vps.
Rice. 14.
Aortofemoral bypass with a synthetic prosthesis made of Dacron, bending of the prosthesis.
A)
The longitudinal section of the prosthesis is 2-4 cm below the level of the proximal anastomosis, the arrow shows the bend of the prosthesis.
b)
Dopplerogram of blood flow from the inflection area, Vps locally increased to 150 cm/s.
Diagnosis of complications of bypass surgery
Duplex scanning is highly accurate in diagnosing such complications of bypass operations as vascular aneurysm in the area of proximal (central) and/or distal anastomoses (Fig. 15), prosthetic aneurysm along the length (more typical for biological prostheses, Fig. 10), diagnosis of pulsating hematoma (Fig. 16), false aneurysm in the area of proximal or distal anastomosis (Fig. 17), as well as in the diagnosis of arteriovenous fistulas (Fig. 18, 19).
Rice. 15.
Iliofemoral bypass with a synthetic Dacron prosthesis, aneurysm in the area of the distal anastomosis.
A)
Dopplerogram of blood flow from the shunt 1-2 cm above the level of the distal anastomosis (Vps - 120 cm/s).
b)
Zone of distal anastomosis, arrow 1 - prosthesis, arrow 2 - dilated common femoral artery (diameter - 17 mm).
V)
The main blood flow in the superficial femoral artery is 1-2 cm below the level of the distal anastomosis (Vps - 70 cm/s).
Rice. 16.
Pulsating hematoma in the ilioinguinal region. The study was conducted on the first day after surgery.
A)
Longitudinal section. Arrow 1 - hematoma cavity, arrow 2 - common femoral artery, arrow 3 - canal between the common femoral artery and the hematoma cavity, canal diameter - 3-3.5 mm.
b)
Dopplerogram of blood flow from the canal, Vps locally increased to 320 cm/s.
Rice. 17.
False aneurysm in the area of the distal anastomosis of the iliofemoral shunt. The study was conducted 3 months after surgery.
A)
Longitudinal section. Arrow 1 - aneurysmal sac, arrow 2 - wide base of the aneurysmal sac, arrow 3 - synthetic prosthesis, arrow 4 - superficial femoral artery.
b)
Dopplerogram from the shunt in front of the area of the aneurysmal sac, main blood flow, Vps - 84 cm/s.
V)
Dopplerogram from the middle 1/3 of the superficial femoral artery, distal to the aneurysmal sac, main blood flow, Vps - 103 cm/s.
Rice. 18.
Arteriovenous fistula between the superficial femoral artery and the superficial femoral vein, upper 1/3 of the thigh. Condition after thrombectomy from the superficial femoral artery.
A)
Dopplerogram of blood flow from the fistula area, fistula diameter is 2.5-3 mm. Arrow 1 - superficial femoral artery, arrow 2 - superficial femoral vein.
b)
Dopplerogram from the superficial femoral vein 3-5 cm distal to the fistula, the blood flow is pseudoarterial, Vps - 45 cm/s.
Rice. 19.
Arteriovenous fistula after autovenous shunting in situ in the lower 1/3 of the thigh. Study 1 day after bypass surgery.
A)
Dopplerogram of blood flow from an autovenous shunt 4-5 cm above the fistula (inflow of the autovenous vein), middle 1/3 of the thigh, the nature of the blood flow is main-altered (low-resistant), Vps - 120-130 cm/s, RI - 0.8 a.u.
b)
Dopplerogram from a fistula (inflow of the autovenous vein), the nature of the blood flow is arterial, Vps - 95 cm/s.
Conclusion
Obviously, duplex scanning is the primary diagnostic procedure for suspected shunt dysfunction at any time from the moment of reconstructive surgery. The results of a duplex scan performed by an experienced specialist can be considered as an indication for angiographic examination [9] or for preventive surgery on bypass vessels [4].
Literature
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Guidelines for the Management of Patients With Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic): A Collaborative Report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) // J Am Coll Cardiol. 2006. N 47. P. e1-e192.
- Layden J., Michaels J., Bermingham S., et al. Diagnosis and management of lower limb peripheral arterial disease: summary of NICE guidance // BMJ. 2011. N 345. P. e4947.
- Rooke TW, Hirsch AT, Misra S, et al. ACCF/ AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (Updating the 2005 Guideline) // J Am Coll Cardiol. 2011. V. 58. N 19. P. 2020-2045.
- National recommendations for the management of patients with diseases of the arteries of the lower extremities (Russian Society of Angiologists and Vascular Surgeons, Association of Cardiovascular Surgeons of Russia, Russian Scientific Society of X-ray Endovascular Surgeons and Interventional Radiologists, All-Russian Scientific Society in Cardiologists, Associate Phlebologists of Russia) // Angiology and Vascular Surgery. 2013. T. 19. Appendix 2. P. 2-67.
- Tendera M., Aboyans V., Bartelink ML, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC) // Eur Heart J. 2011. V. 32. N 22. P. 2851-2906.
- Tinder CN, Chavanpun JP, Bandyk DF, et al. Efficacy of duplex ultrasound surveillance after infrainguinal vein bypass may be enhanced by identification of characteristics predictive of graft stenosis development // J Vasc Surg. 2008. V. 48. P. 613-618.
- Stone PA, Armstrong PA, Bandyk DF, et al. Duplex ultrasound criteria for femoral-femoral bypass revision // J Vasc Surg. 2006. V. 44. P. 496-502.
- Brumberg SR, Back MR, Armstrong PA, et al. The relative importance of graft surveillance and warfarin therapy in infrainguinal prosthetic bypass failure // J Vasc Surg. 2007. V. 46. P. 1160-1166.
- Haimovici's vascular surgery. 6th edition / edited by Ascher Å., Veith FJ, Gloviczki P., et al. —Blackwell Publishing. 2012. 1317 pp.
- Rutherford's vascular surgery. 6th edition / edited by Cronenwett JL, Johnston KW - Elsevier Health Sciences. 2014. 2688 pp.
- Bradbury AW, Adam DJ, Bell J., et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: Analysis of amputation free and overall survival by treatment received // Journal of Vascular Surgery. 2010. V. 51. P. 18S-31S.
- Conte MS Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) and the (hoped for) dawn of evidence-based treatment for advanced limb ischemia // Journal of Vascular Surgery. 2010. V. 51. P. 69S-75S.
- Szilagyi DE, Elliott JP, Smith RF, et al. A thirty-year survey of the reconstructive surgical treatment of aortoiliac occlusive disease // J Vasc Surg. 1986. V. 3. N 3. P. 421-436.
- Nevelsteen A., Wouters L., Suy R., et al. Aortofemoral Dacron reconstruction for aorto-iliac occlusive disease: a 25-year survey // Eur J Vasc Surg. 1991. V. 5. P. 179-186.
- Barbarash L.S., Ivanov S.V., Zhuravleva I.Yu. and others. 12 years of experience in the use of bioprostheses for the replacement of infrainguinal arteries // Angiology and Vascular Surgery. 2006. T. 3. No. 12. P. 91-97.
- Zhuravleva I.Yu., Kudryavtseva Yu.A., Ivanov S.V. and others. Ways and prospects for improving infrainguinal arterial bioprostheses // Pathology of blood circulation and cardiac surgery. 2005. No. 1. P. 78-83.
- Mohler ER, Gornik HL, Gerhard-Herman M., et al. ACCF/ACR/AIUM/ASE/ASN/ICAVL/SCAI/ SCCT/SIR/SVM/SVS 2012 appropriate use criteria for peripheral vascular ultrasound and physiological testing, part 1: arterial ultrasound and physiological testing // J Am Coll Cardiol. 2012. V. 60. P. 242-276.
- Norgren L., Hiatt WR, Dormandy JA, et al. TASC II Working Group: Inter-Society consensus for the management of peripheral arterial disease (TASC II) // J Vasc Surg. 2007. V. 45. P. S5-S67.
- Jaff M., Mohler E., Roman M., et al. Guidelines for noninvasive vascular laboratory testing: a report from the American Society of Echocardiography and the Society for Vascular Medicine and Biology // Vasc Med. 2006. V. 11. N 3. P. 183-200.
- Bandyk D., Cato R., Towne J. A low velocity predicts failure of femoropoliteal and femorotibial bypass grafts // Surgery. 1985. V. 98. P. 799-809.
Ultrasound machine RS85
Revolutionary changes in expert diagnostics.
Impeccable image quality, lightning-fast operating speed, a new generation of visualization technologies and quantitative analysis of ultrasound scanning data.
Stenting or bypass surgery, which is better?
Stenting is a minimally invasive method for the treatment of obliterating vascular atherosclerosis. If patients apply in a timely manner and the disease is not advanced, performing such a procedure has an advantage.
If the situation does not allow stenting (blockage of vessels over a long distance, impossibility of passing a stent through a blocked vessel), then bypass surgery is performed. There are situations that require a hybrid approach in the treatment of atherosclerosis of the arteries of the lower extremities. For example, narrowing of the iliac artery and blockage of the femoral artery. In this case, stenting of the iliac artery and femoropopliteal bypass is performed.
In the postoperative period, the patient of our department remains under the supervision of the attending physician in superior wards for 5-7 days, where dressings and care of postoperative wounds are performed.
I often ask what disability group is given after bypass surgery. The issue of disability is resolved at the MSEC at the place of residence.
Carrying out bypass surgery
The essence of the surgical intervention is to create an artificial bypass between the occluded arteries, along which blood flow is allowed to flow. The large saphenous vein of the lower limb, which is not removed from its own bed, is traditionally used as a shunt. If its use is impossible, other autogenous veins of the arms and legs are used, which are subsequently compensated by the body on its own. Synthetic prostheses are rarely introduced due to the increased risk of blood clots and subsequent rejection.
Since the venous vessels serve to redirect blood back to the heart, and when it is included in the arterial system, the blood flow turns to the periphery, the doctor must first destroy the vein valve in order to remove obstacles to the flow of blood to the lower part. One end of the vein is fixed above the occluded section of the femoral artery, the opposite is placed on the tibial artery.
The planned duration of the operation is about 3 hours. General anesthesia or epidural anesthesia is used for pain relief. The latter is used more often and involves fixing a thin catheter in the area of the brain roots, which helps reduce the required dosage of the anesthetic drug.
Preparing for surgery
Since atherosclerosis is a chronic systemic disease, any patient must undergo a comprehensive examination and exclude damage to other vessels of the body. Minimizing the risk of cardiac and cerebral complications as a result of surgery requires preliminary diagnostic measures of a comprehensive nature.
The standard list of studies includes:
- Blood tests (general blood tests, biochemistry, coagulogram, other tests as personally prescribed by the doctor);
- Urinalysis (general);
- Electrocardiogram (ECG);
- Fluorography (chest x-ray);
- Vascular ultrasound.
Before the operation, it is necessary to notify the vascular surgeon about the prescribed medications. Some of them should be stopped a week before bypass surgery (anti-inflammatory drugs and anticoagulants). It is important to avoid smoking and drinking alcoholic beverages no later than 2 weeks before the procedure. The evening before the operation, a light dinner is allowed, but after midnight you should not eat or drink. The operation is performed on an empty stomach.
Indications and contraindications for the treatment method
Patients who are in good general condition are selected for bypass surgery. Therefore, a detailed assessment of comorbidities, obesity and other life-threatening factors is carried out. Only an immediate threat to life is a reason for risk in patients with severe concomitant diseases.
It is necessary to evaluate the vascular bed in detail using diagnostic ultrasound and angiography in order to formulate a clear concept for the operation.
A careful assessment of the saphenous veins is necessary, since the success of bypass surgery and the duration of the bypass operation depend on their quality. The use of artificial prostheses as shunts is an operation of despair, since such shunts close in half of the cases within 2 years.
Possible complications during treatment
In situ femoral-tibial bypass.
This method involves the use of the patient’s own great saphenous vein, which remains in its usual place, but with the help of special techniques, arterial blood flow is allowed through it into small arteries on the lower leg and foot. Femoro-tibial bypass is the mainstay of treatment for critical ischemia and impending gangrene. Success, with the correct indications for surgery, is achieved in 90% of patients with incipient arterial gangrene (necrosis of the fingers, arterial trophic ulcers). The ability to walk on your own leg is preserved. A venous bypass can be made from the veins of the legs or arms if the main saphenous vein is not preserved.
Peroneal artery bypass
The smallest artery of the leg is the least affected by the atherosclerotic process. However, its capacity is often not enough for the full operation of the autovenous shunt, which leads to thrombosis. The peculiarity of operations on the peroneal arteries is the need to clearly assess the volume of blood flow. To unload the artery, special techniques are often used - unloading fistulas with veins far from the anastomosis.
Multi-storey "jumping" shunts
Patients are often denied leg preservation due to the lack of a good length and patent artery in the lower leg, however, we often see individual sections and branches of arteries with preserved blood flow. Our leading vascular surgeon I.M. Kalitko For such situations, a technique for multi-story bypass of the lower leg arteries has been developed. Often several short bypasses are performed to individual patent segments of the arteries. An important condition for the normal operation of such a complex reconstruction is a reliable assessment of the incoming and distributed blood volume. When shunts are overloaded, unloading fistulas can be used.
Advantages of treatment at the ISC
The Innovative Vascular Center is the only clinic in our country where unique microsurgical, endovascular and plastic methods are comprehensively used to treat patients with incipient gangrene and diabetic foot. Vascular surgeons at our clinic successfully use the method of restoring blood circulation in the limb using a microscope, developed at the University Hospital of Aachen (Germany), for patients with damage to the small arteries of the leg and foot. This is a microsurgical bypass of the arteries of the leg and foot using the patient’s own saphenous vein. Microsurgery allows the successful connection of vein grafts and the smallest vessels with high quality. This allows doctors at our clinic to save limbs for patients already sentenced to amputation.
Since 2011, our clinic has been a leader in Russia in the use of bypass surgery of the arteries of the leg and foot and has the best results in preserving limbs against the background of atherosclerosis of small arteries.
How to prepare
Preparation consists of a full diagnosis, which primarily includes:
- ultrasound examination of the carotid arteries;
- functional diagnostics of the heart;
- ECHO-cardiography;
- Ultrasound examination of the vessels of the lower extremities.
In addition, to avoid the risk of bleeding after surgery, gastroscopy is performed. Computed angiography of the affected arteries, tests and diagnosis of the condition of the kidneys are also necessary. Before the main procedure, the existing imbalance of electrolyte and protein metabolism is corrected, and hemoglobin levels are normalized. Two days before surgery, the use of alcohol and tobacco is prohibited. Immediately before the intervention, bowel cleansing is performed using an enema.
Which surgery is better?
Each operation has its own indications and contraindications. The method of surgical treatment is chosen by the doctor based on many different factors, taking into account not only ultrasound and angiography data, but also the patient’s health status, the presence of concomitant diseases, age, etc. Some patients may require a combination of different techniques, such as stenting one section of the artery and replacing another. Such operations are called “hybrid”.
Surgery on the great vessels is one of the most common operations in the world, which has saved the lives and preserved the health of millions of people.
Online consultations (Skype, Viber, Whats App)
Sign up for a consultation
Diagnostics
Very often patients do not make any complaints. But if symptoms appear, they are nonspecific. The most common symptoms include pain in the abdomen, back, or chest area. Patients describe this pain in different ways: some feel moderate to severe pain or tenderness in the middle or upper abdomen or lower back, while others feel the aneurysm itself as a beating and pulsating mass in the abdominal cavity. But, as noted, many may not experience any symptoms. Sometimes abdominal aortic aneurysm
may be detected during a routine physical examination. When palpating the abdomen, the doctor may detect a bulge or pulsation in the abdominal cavity. But most often, an aneurysm is detected during medical tests such as an abdominal ultrasound (ultrasound) or computed tomography (CT) scan. The size and location of the abdominal aortic aneurysm, as well as the general condition of the patient, help to choose the right treatment method. If the aneurysm is small, your doctor may recommend surveillance (regular examinations to monitor its size). A large aneurysm or a rapidly enlarging aneurysm has a high chance of rupture and requires treatment.
After the procedure
Upon completion of the surgical intervention, the patient is transferred to the recovery room for one day. At this time, he is observed by doctors (anesthesiologist and surgeon). Prevention of intestinal paresis is prescribed. Then the operated patient is transferred to the general ward, where he remains for up to 7 days. After this period, the sutures are removed and the patient is prepared for discharge.
The reliability and lifespan of the shunt depend on compliance with medical prescriptions. First of all, you should stop smoking and see a doctor regularly and undergo periodic ultrasounds. To prevent the development of atherosclerosis, from time to time they normalize metabolic processes and reduce cholesterol levels.