Causes of pathology
The following causes of cardiomyopathy are distinguished:
Primary – usually not installed. They are probably: congenital (genetically determined), acquired or mixed.- Secondary - are a complication of any underlying disease - blood pathology, infectious, endocrine, systemic, metabolic disorders, neuromuscular system, malignant tumors.
Today it is believed that the main role belongs to genetic factors that cause immune system disorders and disorders:
- Myocardial function in dilated cardiomyopathy.
- Differentiation of contractile elements of the myocardium, which leads to hypertrophy of cardiomyocytes in hypertrophic cardiopathy.
- Accumulation of eosinophils in the myocardium and their cardiotoxic effect during restrictive.
Anatomy of the heart
The heart is a hollow muscular organ located in the human chest. Most of the organ is shifted to the left, while the smaller part is located in the middle of the chest.
The inside of the heart is lined with endocardium. It is a shell of epithelial cells. When cardiomyopathy occurs, this layer often thickens, hypertrophies, and in some cases it may become inflamed.
The walls of the heart are represented by the myocardium. The myocardium is the muscle layer that ensures normal contraction. In cardiomyopathy, this is the main area of damage.
Outside, the heart is placed in a membrane called the pericardium. It is presented in two sheets. The first layer is fused directly to the heart, while the second plays the role of a “heart bag”.
The human heart is represented by four chambers. The first two chambers are the right ventricle and atrium, the second are the left atrium and left ventricle.
In order for the heart to be saturated with blood, valves are needed. These formations are needed for blood circulation.
Blood circulation in the body is ensured by vessels. The heart contains the coronary arteries, which carry nutrients and oxygen to the heart. With the development of cardiomyopathy, damage to the coronary arteries often occurs.
Clinical course of the disease and classification
| Morphofunctional variant of cardiopathy | Clinic |
| Dilatational | Significant cardiomegaly with pronounced dilatation of the ventricle (usually the left) with unchanged or thin walls. Myocardial contractility drops sharply. Heart failure is progressing. The ejection fraction is reduced. End-diastolic pressure is increased. Everyone gets sick, even infants. The disease develops gradually. Difficult to treat. There are no pathognomonic symptoms. The clinical picture is due to circulatory disorders and rhythm and conduction disorders. Frequent complaints: pain in the heart that cannot be relieved by nitroglycerin, shortness of breath, cyanosis of the nasolabial triangle and lips. |
| Hypertrophic | It is characterized by severe myocardial hypertrophy, mainly of the left ventricle, and obstruction of the outflow tract. As a rule, the cavities are not changed, cardiomegaly is insignificant. The clinical picture is different: asymptomatic or with minor symptoms: fatigue, shortness of breath during exercise, palpitations, syncope, heart pain, dizziness. |
| Restrictive | Infiltrative or fibrous damage to the myocardium, which is characterized by rigid ventricular walls and decreased diastolic volume. Normal or slightly altered systolic function and wall thickness. The onset of the disease is slow and gradual. Main complaint: shortness of breath, weakness even with minimal physical exertion, rhythm disturbances. |
Publications in the media
Hypertrophic cardiomyopathy (HCM) is a primary lesion of the heart, characterized by thickening of the walls of the left ventricle and the development of heart failure, predominantly diastolic • Hypertrophy of the left ventricular wall of more than 15 mm of unknown origin is considered a diagnostic criterion for HCM • The following options are distinguished •• Symmetrical HCM (enlargement involving all walls left ventricle) •• Asymmetric HCM (hypertrophy involving one of the walls): ••• apical HCM (hypertrophy covers only the apex of the heart in isolation) ••• obstructive HCM (interventricular septum or idiopathic hypertrophic subaortic stenosis ••• HCM of the LV free wall.
Important general features of HCM (both with and without obstruction) are a high frequency of cardiac arrhythmias, primarily ventricular extrasystole and paroxysmal tachycardia, and impaired diastolic filling of the left ventricle, which can lead to heart failure. Arrhythmias are associated with sudden death, which occurs in 50% of patients with HCM.
Statistical data. HCM is observed in 0.2% of the population, most often it is non-obstructive HCM (70-80%), less often - obstructive (20-30%, in the form of idiopathic hypertrophic muscular subaortic stenosis). Men get sick more often than women. The incidence is 3 cases per 100,000 people per year.
Etiology • Many HCM are hereditary diseases resulting from mutations in genes encoding myocardial contractile proteins. Familial hypertrophic cardiomyopathy: • type 1: 192600, MYH7, CMH1, 160760 (cardiac myosin, heavy chain b7), 14q12; • type 2: 115195, TNNT2, CMH2, 191045 (cardiac troponin 2), 1q32; • type 3: 115196, TPM1, CMH3, 191010 (tropomyosin cardiac 1), 15q22; •; type 4: 115197, MYBPC, CMH4, 600958 (myosin binding protein C), 11p11.2; • type 7: TNNI3, 191044 (cardiac troponin I), 19p13.2 q13.2; • with Wolff Parkinson-White syndrome: CMH6, 600858, 7q3
Pathogenesis. As a result of gene mutations, left ventricular hypertrophy and areas of cardiomyocyte disorganization occur.
• An increase in the content of calcium ions in cardiomyocytes and pathological stimulation of the sympathetic nervous system are important.
• Abnormally thickened intramural arteries do not have the ability to adequately dilate, which leads to ischemia, myocardial fibrosis and pathological hypertrophy.
• With asymmetric hypertrophy of the interventricular septum, according to the latest data, the obstruction is associated mainly with the abnormal forward movement of the anterior mitral valve leaflet into systole and, to a lesser extent, with septal hypertrophy (obstruction of the outflow tract of the left ventricle - muscular subaortic stenosis: the left ventricle is “divided” into two parts: a relatively small subaortic and a large apical; during the period of expulsion, a pressure difference occurs between them).
• Due to the presence of obstructions to normal blood flow, the pressure gradient between the left ventricle and the aorta increases, which leads to an increase in end-diastolic pressure in the left ventricle. Most patients have abnormal left ventricular systolic function.
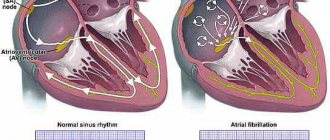
• Regardless of the pressure gradient between the left ventricle and the aorta, patients with HCM have impaired left ventricular diastolic function, leading to increased end-diastolic pressure, increased pulmonary capillary wedge pressure, pulmonary congestion, left atrial dilatation, and atrial fibrillation. The development of diastolic dysfunction is associated with a decrease in compliance and impaired relaxation of the left ventricle •• Decreased compliance occurs due to increased muscle mass, a decrease in the left ventricular cavity and a decrease in myocardial compliance due to its fibrosis •• Deterioration of relaxation is the result of systolic (incomplete emptying of the left ventricle due to outflow tract obstruction) and diastolic (decreased ventricular filling) disorders.
• HCM in some cases is accompanied by myocardial ischemia, which is associated with the following reasons •• Reduced vasodilator reserve of the coronary arteries •• Abnormal structure of the intramural arteries of the heart •• Increased myocardial oxygen demand (increased muscle mass) •• Compression during systole of the arteries passing through the thicker myocardium •• Increased diastolic filling pressure •• In addition to the above reasons, 15–20% of patients experience concomitant atherosclerosis of the coronary arteries.
Pathomorphology • Macroscopic examination •• The main morphological manifestation of HCM is thickening of the walls of the left ventricle over 30 mm (sometimes up to 60 mm) in combination with normal or reduced dimensions of its cavity •• Dilatation of the left atrium (occurs due to increased end-diastolic pressure in the left ventricle ) •• In most patients, the interventricular septum and most of the lateral wall of the left ventricle are hypertrophied, while the posterior wall is less often involved in the process. In other patients, only the interventricular septum hypertrophies. In 30% of patients there may be local hypertrophy of the wall of the left ventricle of small sizes: the apex of the left ventricle (apical), only the posterior wall, the anterolateral wall. In 30% of patients, the right ventricle, papillary muscles or apex of the heart are involved in the hypertrophic process • Microscopic examination •• Disorderly arrangement of cardiomyocytes, replacement of muscle tissue with fibrous tissue, abnormal intramural coronary arteries •• The presence of disordered hypertrophy, characterized by multidirectional arrangement of myofibrils and unusual connections between neighboring myocardial cells •• Foci of fibrosis are represented by randomly intertwined bundles of coarse collagen fibers.
Clinical manifestations are caused by obstruction of the left ventricular outflow tract, its diastolic dysfunction, myocardial ischemia and cardiac arrhythmias
• Sudden cardiac death is possible, most cases (80%) resulting from ventricular fibrillation. Other causes of sudden cardiac death include atrial fibrillation with a high ventricular rate, supraventricular tachycardia, and a sharp decrease in cardiac output with the development of shock. Risk factors for sudden cardiac death in HCM include the following •• History of cardiac arrest •• Persistent ventricular tachycardia •• Severe left ventricular hypertrophy •• Genotype features (see Etiology) or family history of sudden cardiac death •• Frequent paroxysms of ventricular tachycardia, detected by daily ECG monitoring •• Early appearance of symptoms of HCM (in childhood) •• Frequent fainting •• Abnormal response of blood pressure to physical activity (decrease).
• Complaints •• The disease can be asymptomatic for a long time, and it is accidentally detected during examination for another reason •• Shortness of breath as a result of an increase in diastolic filling pressure of the left ventricle and a passive retrograde increase in pressure in the pulmonary veins, which leads to impaired gas exchange. An increase in left ventricular filling pressure is due to worsening diastolic relaxation due to severe hypertrophy •• Dizziness and fainting during exercise as a result of worsening cerebral circulation due to worsening obstruction of the left ventricular outflow tract. Also, episodes of loss of consciousness can be caused by arrhythmias •• Chest pain due to worsening diastolic relaxation and increased myocardial oxygen demand as a result of hypertrophy. Typical attacks of angina pectoris may occur, the causes of which are a discrepancy between the coronary blood flow and the increased oxygen demand of the hypertrophied myocardium, compression of the intramural branches of the coronary arteries by subendocardial ischemia as a result of impaired diastolic relaxation •• Palpitations can be a manifestation of supraventricular or ventricular tachycardia, atrial fibrillation.
• Upon examination, there may be no external manifestations of the disease. In the presence of severe heart failure, cyanosis is detected. HCM can be combined with arterial hypertension.
• Palpation can reveal a double apical impulse (contraction of the left atrium and left ventricle) and systolic tremor at the left edge of the sternum.
• Auscultation of the heart •• Heart sounds are usually not changed, although there may be a paradoxical splitting of the second tone with a significant pressure gradient between the left ventricle and the aorta •• The main auscultatory manifestation of HCM with obstruction of the outflow tract of the left ventricle is systolic murmur ••• The occurrence of systolic murmur is associated with the presence of an intraventricular pressure gradient between the left ventricle and the aorta and mitral regurgitation (reflux of blood into the left atrium as a result of prolapse of one of the mitral valve leaflets due to excess pressure in the left ventricle) ••• The murmur has a waxing-waning character and is better heard between the apex of the heart and the left edge of the sternum. It may radiate to the axillary region ••• The murmur decreases (due to decreased obstruction of the left ventricular outflow tract) with decreased myocardial contractility (for example, due to the use of beta-blockers), an increase in left ventricular volume, or an increase in blood pressure (for example, in the squatting position, taking vasoconstrictors) ••• The murmur increases (due to increased obstruction) as a result of increased contractility (for example, during physical activity), a decrease in left ventricular volume, a decrease in blood pressure (for example, with the Valsalva maneuver, taking antihypertensive drugs, nitrates).
Instrumental data
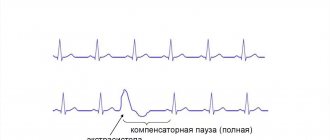
• ECG changes in HCM are detected in 90% of patients •• Main ECG signs: left ventricular hypertrophy, changes in the ST segment and T wave, the presence of pathological Q waves (in leads II, III, aVF, chest leads), atrial fibrillation and flutter , ventricular extrasystole, shortening of the P–R (P–Q) interval, incomplete blockade of the His bundle branches. The causes of pathological Q waves are unknown. They are associated with myocardial ischemia, abnormal activation of the interventricular septum, imbalance of the resulting electrical vectors of the interventricular septum and the wall of the right ventricle •• Less commonly, ventricular tachycardia and atrial fibrillation are recorded on the ECG in patients with HCM •• With apical cardiomyopathy, “giant” negative T waves often occur ( depth more than 10 mm) in the chest leads.
• Daily ECG monitoring: supraventricular arrhythmias are detected in 25–50% of patients with HCM, ventricular tachycardia is detected in 25% of patients.
• EchoCG is the main method for diagnosing this disease •• Determine the localization of hypertrophied areas of the myocardium, the severity of hypertrophy, and the presence of obstruction of the outflow tract of the left ventricle. Asymmetric hypertrophy is detected in 60%, symmetric in 30%, apical in 10% •• In Doppler mode, the severity of mitral regurgitation and the degree of pressure gradient between the left ventricle and the aorta are determined (a pressure gradient of more than 50 mm Hg is considered pronounced). In addition, Doppler can also detect concomitant mild or moderate aortic regurgitation in 30% of patients with HCM •• In 80% of patients, signs of left ventricular diastolic dysfunction can be detected •• Left ventricular ejection fraction may be increased •• Signs of HCM also include • •• small size of the cavity of the left ventricle ••• dilatation of the left atrium ••• reduced amplitude of movement of the interventricular septum with normal or increased movement of the posterior wall of the left ventricle ••• mid-systolic closure of the aortic valve leaflets •• The following are considered signs of obstructive HCM ••• Asymmetric hypertrophy of the interventricular septum with a ratio of its thickness to the thickness of the posterior wall of the left ventricle more than 1.3:1 (and the thickness of the interventricular septum should be 4–6 mm greater than normal for this age group) ••• Systolic movement of the anterior leaflet of the mitral valve forward.
• X-ray examination: the contours of the heart may be normal. With a significant increase in pressure in the pulmonary artery, bulging of its trunk and expansion of its branches are noted.
Diagnostics. The main diagnostic method is echocardiography, which allows to detect myocardial thickening and assess the presence of obstruction of the left ventricular outflow tract. Before diagnosing HCM, it is necessary to exclude causes of secondary hypertrophy, incl. acquired and congenital heart defects, arterial hypertension, coronary artery disease, etc.
Differential diagnosis • Other forms of cardiomyopathy • Aortic stenosis • Mitral valve insufficiency • IHD.
TREATMENT
General recommendations. In case of HCM (especially in the obstructive form), it is recommended to avoid significant physical activity, since this may increase the pressure gradient between the left ventricle and the aorta, causing cardiac arrhythmias and fainting.
Drug therapy • For asymptomatic HCM, it is possible to prescribe b-blockers (from 40 to 240 mg/day propranolol, 100–200 mg/day atenolol or metoprolol) or slow calcium channel blockers (verapamil at a dose of 120–360 mg/day), although this issue still remains controversial • In the presence of moderately severe symptoms, either b-blockers are prescribed (propranolol in a dose of 40 to 240 mg/day, atenolol or metoprolol in a dose of 100–200 mg/day) or slow calcium channel blockers (verapamil in dose 120–360 mg/day). They decrease heart rate and prolong diastole, increase passive left ventricular filling, and decrease filling pressure. Similar therapy is also indicated for atrial fibrillation. In addition, due to the high risk of thromboembolism in atrial fibrillation, patients should be prescribed anticoagulants • For severe symptoms of HCM, in addition to b-blockers or verapamil, diuretics are prescribed (for example, hydrochlorothiazide at a dose of 25–50 mg/day) • For obstructive HCM the use of cardiac glycosides, nitrates, and adrenergic agonists should be avoided • In obstructive HCM, it is necessary to prevent infective endocarditis, since vegetations may appear on the anterior leaflet of the mitral valve as a result of its constant trauma.
Surgery. Surgical treatment is carried out for the obstructive form of HCM with a pressure gradient between the left ventricle and the aorta of more than 50 mm Hg. In this case, septal myotomy-myectomy (Morrow operation) is performed. In the presence of frequent paroxysms of ventricular tachycardia, they resort to implantation of a cardioverter-defibrillator.
The course is variable. In most patients, the disease is relatively stable or even tends to improve (in 5–10% over 5–20 years). Women with HCM usually tolerate pregnancy well. With a long course of the disease, the development of heart failure is increasingly observed.
Prognosis • Without treatment, the mortality rate of patients with HCM is 2–4% per year • Patients with more than one risk factor for sudden cardiac death are classified as a high-risk group • In 5–10% of patients, spontaneous reverse development of hypertrophy is possible • In 10%, the transition to hypertrophic cardiomyopathy into dilated cardiomyopathy • In 5–10% of patients, a complication develops in the form of infective endocarditis.
Concomitant pathology • Arrhythmias • Systemic arterial hypertension • Aortic stenosis • IHD.
Reduction. HCM is hypertrophic cardiomyopathy.
ICD-10 • I42.1 Obstructive hypertrophic cardiomyopathy • I42.2 Other hypertrophic cardiomyopathy.
Methods for diagnosing functional cardiopathy
Clinical and instrumental methods are used for diagnosis
ECG: signs of myocardial hypertrophy, rhythm and conduction disturbances, ST changes.
X-ray of the lungs: you can see hypertrophy, dilatation of the myocardium, congestion in the lungs.
EchoGK: allows you to evaluate the size of the heart cavities, the condition of the valves, the thickness of the walls and interventricular septum, and evaluate systolic and diastolic functions.
Sometimes they use: MRI, radioisotope ventriculography, angiocardiography, cardiac catheterization, endomyocardial biopsy is taken.
Tests and diagnostics
To assess the state of the cardiovascular system, the following examinations are carried out:
- Electrocardiogram , which shows the electrical activity of the heart, the frequency and rhythm of contractions.
- Echocardiography or ultrasound of the heart makes it possible to see all the structural features and movements of structures.
- Scintigraphy allows you to assess the level of blood supply to the myocardium, detect necrotic areas, and understand the risk of complications.
- Load tests – as a result of a bicycle ergometer test , you can evaluate the functionality of the heart under conditions of increasing load.
- Magnetic resonance imaging, as the most modern method, allows you to obtain images of the heart in various planes and in high definition, as well as assess the speed of blood flow and see the features of its functioning.
Treatment options
There is no specific treatment.
For dilated cardiomyopathy, treatment for heart failure is carried out:
- Digoxin in low doses.
- ACE inhibitors: captopril (adolescents - enalapril).
- Diuretics: furosemide.
- For severe heart failure in the intensive care unit, dopamine and dobutamine, anti-inflammatory steroids, and oxygen therapy are used as indicated. Treatment of arrhythmias according to protocols.
- In case of impaired microcirculation and a tendency to thrombus formation: heparin subcutaneously or intravenously, indirect anticoagulants (warfarin, rivaroxaban, dabigatran).
- Cardioprotectors: panangin, mildronate, cardonate.
For hypertrophic cardiopathy:
Cardiac glycosides and other cardiotonics are contraindicated.- Physical activity is limited (especially if it is a teenager).
- Beta blockers are used: propranolol. Sometimes calcium antagonists: verapamil.
- Prevention of infective endocarditis: antibiotics.
- For heart failure: ACE inhibitors, diuretics.
- Antiarrhythmics if necessary.
- If conservative therapy is ineffective, cardiac surgery is recommended.
For restrictive cardiomyopathy:
- Cardiac glycosides and other cardiotonics are contraindicated.
- Sports are prohibited. Physical activity is limited (especially for children).
- Calcium antagonists: verapamil, diltiazem.
- Antiarrhythmics: amiodarone.
- Treatment of heart failure.
Prevention
To prevent the development of cardiopathy it is necessary:
- quit smoking, alcohol and drugs;
- regularly undergo a complete health examination;
- do not take medications randomly and do not self-medicate;
- control weight;
- avoid psychological stress;
- adhere to proper nutrition;
- normalize physical activity and rest - at least an hour of walking and eight hours of sleep.
conclusions
The prognosis, unfortunately, is unfavorable. Heart failure progresses very quickly, life-threatening arrhythmias and thromboembolism often occur, which leads to sudden death.
For dilated cardiomyopathy, the 5-year survival rate is 30%. Functional cardiopathy leads to disability in children. Therefore, people with this pathology must take adequate, ongoing treatment in order to prolong their stable condition. Patients with cardiomyopathy are also potential candidates for heart transplantation. After this procedure, the duration and quality of life improves significantly.
Pathogenesis
Damage to the heart muscle is primarily associated with its mechanical (blood pumping) and electrical functions (conductivity). The basis of pathological processes is:
- the presence of infectious, viral and inflammatory aspects;
- autoimmune disorders - heart proteins can acquire antigenic characteristics, which provokes the synthesis of antibodies to them and leads to stretching of the heart chambers and systolic dysfunction;
- poisoning with various chemicals, including heavy metals, medications, alcohol, drugs, etc.;
- environmental factors, including radiation damage;
- decompensation of conditions;
- injuries;
- ischemia – lack of blood supply to the heart muscle itself;
- birth defects – heart defects and genetic predisposition;
- cancer formation.
Functional cardiopathy
Functional pathology is associated with impaired ability to perform the main task of the heart - pumping blood. Exposure to negative factors can lead to a decrease in the number of normally and fully functioning heart cells - cardiomyocytes. This leads to a decrease in cardiac output, which can lead to deterioration of coronary perfusion, decreased tissue oxygenation, fluid retention, activation of the sympathetic nervous and renin-angiotensin systems, peripheral vasoconstriction, tachycardia and arrhythmias .
It happens that cardiac disorders are not part of a generalized disease, but are isolated. They can lead not only to disability, but also to death.
Structure of the heart
Complications
Cardiomyopathy carries a high risk of complications. There are disturbances in the functioning of the heart and lungs. Common complications of cardiomyopathy:
- myocardial infarction;
- acute or chronic heart failure;
- pulmonary embolism (PE);
- pulmonary edema;
- arrhythmia;
- endocarditis;
- sudden cardiac arrest;
- stroke.
Chronic heart failure develops in the vast majority. As the disease progresses, irreversible changes occur that lead to the death of the patient.
The risk of developing complications can be reduced if cardiomyopathy is treated promptly and its main complications are prevented. Therefore, relatives of patients suffering from various forms of cardiomyopathy need to know the main symptoms of these conditions.
| Complications | Symptoms |
| Myocardial infarction | It manifests itself as a sharp pain in the sternum (“dagger” pain), radiating to the left arm, under the shoulder blade and into the lower jaw. The pain syndrome is accompanied by a feeling of fear, sticky cold sweat, and changes in pulse. |
| Heart failure | With the development of cardiomyopathy, acute and chronic heart failure occurs. Acute failure manifests itself in the form of cardiogenic shock, with severe hemodynamic disturbances. Atrial fibrillation develops, the person loses consciousness, and cardiac arrest occurs. Chronic heart failure lasts a long time, symptoms increase gradually. It is manifested by shortness of breath at rest, edema of the lower extremities, cyanosis of the skin, breathing problems and cough. |
| Endocarditis | It is an inflammation of the inner surface of the heart. Manifested by sharp pain in the chest, increased body temperature, fatigue, tachycardia, edema, changes in heart rhythm, cardiac murmurs on auscultation. |
| Pulmonary edema | There is noisy breathing with moist rales (“gurgling”), and the release of copious foamy sputum. This is an emergency and requires immediate medical attention. |
| Stroke | As a complication of cardiomyopathy, stroke occurs quite often and is accompanied by severe headache, loss of orientation in space, paralysis of the limbs (usually on one side), disturbances of speech and consciousness. |
| Arrhythmia | There is a change in heart rate, a feeling of “interruptions” in the work of the heart, shortness of breath, dizziness, and possible loss of consciousness. |
| Sudden cardiac arrest | Sudden cardiac arrest is more common in hypertrophic cardiomyopathy. It develops more often against the background of loss of consciousness in young patients. The person loses consciousness, cardiac activity and breathing stop. Urgent resuscitation measures are required (indirect cardiac massage at the first aid stage). |
| Pulmonary embolism | Accompanied by cough, chest pain, and breathing discomfort. Erased symptoms of cardiomyopathy in the form of a rare dry cough and increased body temperature are possible. |
Cardiomyopathy according to ICD-10
In accordance with the International Classification of Diseases, 10th revision (ICD-10), cardiomyopathy is assigned the following codes:
Cardiomyopathy (code I42)
Excluded:
- cardiomyopathy, complicating: pregnancy (O99.4)
- postpartum period (O90.3)
Types of cardiomyopathy according to ICD-10
:
- Dilated cardiomyopathy – I42.0 Congestive cardiomyopathy
- Obstructive hypertrophic cardiomyopathy – I42.1 Hypertrophic subaortic stenosis
- Other hypertrophic cardiomyopathy – I42.2 Non-obstructive hypertrophic cardiomyopathy
- Endomyocardial (eosinophilic) disease I42.3 Endomyocardial (tropical) fibrosis Loeffler's endocarditis
- Endocardial fibroelastosis – I42.4 Congenital cardiomyopathy
- Other restrictive cardiomyopathy – I42.5 Constrictive cardiomyopathy NOS
- Alcoholic cardiomyopathy – I42.6
- Cardiomyopathy due to exposure to drugs and other external factors – I42.7
- Other cardiomyopathies – I42.8
- Cardiomyopathy, unspecified – I42.9 Cardiomyopathy (primary) (secondary) NOS
Degrees and stages
Cardiomyopathies are classified into degrees and stages of dilated cardiomyopathy and other types.
They have 3 degrees of severity:
- Moderate severity. The disease was diagnosed at an early stage, there are no pronounced pathological changes, the symptoms are not pronounced, and there are no signs of heart failure.
- Moderate severity. There are pronounced changes in the functioning of the heart muscle, myocardial decompensation, and the initial stage of heart failure.
- Severe degree. Heart failure develops, symptoms are severe, and the pathological process decompensates.
Cardiomyopathy goes through 3 stages in its development:
- Stage 1 – initial. The first symptoms of cardiomyopathy are observed; they are of an unexpressed nature.
- Stage 2 – height. There is a gradual increase in clinical symptoms, and there may be a sharp progression.
- Stage 3 – terminal. At this stage, irreversible heart damage occurs. Severe clinical symptoms with symptoms of heart failure are observed. At this stage, treatment is no longer effective and therefore therapy is symptomatic.
Cardiomyopathy in pregnant women
Cardiomyopathy can develop during pregnancy. This is due to increased load on the heart muscle, hormonal changes, and stress to the body during childbirth.
Complications during this period play an important role in the occurrence of cardiomyopathy during pregnancy. For example, gestosis in the second half of pregnancy. When it occurs, disturbances occur in all vital organs and not only in the kidneys and nervous system, but also in the heart.
In addition, gestosis increases blood pressure and increases the load on the heart.
Cardiomyopathy during pregnancy occurs in the third trimester. And it can persist into the postpartum period. Or this pathological condition occurs after childbirth.
In pregnant women, the most common are hypertrophic and dilated cardiomyopathies. They are accompanied by the following symptoms:
- increased shortness of breath;
- increased shortness of breath at rest;
- change in color of the nasolabial triangle (it becomes pale with the addition of cyanosis);
- increased blood pressure;
- increased swelling;
- change in fetal activity.
If cardiomyopathy was diagnosed during pregnancy, then, as prescribed by the doctor, the disorders are corrected with the help of medications in individual dosages.
Delivery of women with cardiomyopathy is carried out by cesarean section. As a maternity hospital, it is necessary to choose a specialized center for women in labor with cardiac pathology. If cardiomyopathy is detected in the postpartum period, then standard therapy with medications is carried out. The prognosis is more favorable than with other variants of the disease.
Is it possible to plan a pregnancy with cardiomyopathy?
Cardiomyopathy in women is associated with a high risk of complications during pregnancy. Therefore, the feasibility of planning depends on many factors. It is necessary to take into account the severity of cardiac dysfunction, the age of the mother, and whether there is a risk of heart failure.
For women with severe forms of cardiomyopathy, pregnancy is not recommended, because there is a high probability of severe consequences during pregnancy and directly during childbirth. Moreover, the degree of risk is high for both the health and life of the mother and the child. Preparing for pregnancy in patients with heart disease should be carried out not only with a gynecologist, but also with a cardiologist.
Main causes and risk factors
Some of the common causes of cardiomyopathy are genetic mutations, connective tissue diseases and storage diseases. The latter are pathological deposits of metabolic products.
Often the causes of the disease remain unknown. In this case, they speak of an idiopathic form of cardiomyopathy.
Causes of cardiomyopathies:
- heredity (caused by gene mutation);
- connective tissue diseases (systemic lupus erythematosus, rheumatoid arthritis);
- frequent attacks of tachycardia;
- hypertonic disease;
- inflammatory heart diseases (myocarditis);
- tumor formations (tumors of the adrenal cortex and thyroid gland);
- excessive physical activity (“athlete’s heart”);
- heart defects;
- metabolic disorders in the body (for example, diabetes mellitus, acromegaly, pheochromocytoma);
- congenital pathologies of the nervous system;
- muscular dystrophy (Duchenne muscular dystrophy);
- infectious diseases;
- cardiac ischemia;
- helminthiases;
- and some medications;
- heavy metal poisoning (mercury, lead);
- exposure to ionizing radiation;
- severe allergic reactions;
- hormonal disorders in the body;
- severe diseases of the urinary system (acute and chronic renal failure;
- granulomatous diseases (sarcoidosis);
- storage diseases (hemochromatosis, Fabry syndrome);
- severe disruption of electrolyte balance (decreased calcium, magnesium and sodium in the body);
- Loeffler's syndrome (respiratory disease).
There are also some risk factors, the appearance of which increases the likelihood of pathology. These include:
- radiation or chemical exposure;
- pregnancy;
- postpartum period;
- excess body weight;
- alcohol abuse;
- smoking;
- increased blood cholesterol levels;
- drug use;
- previous viral infections during pregnancy.
Cardiomyopathy in children
The development of cardiomyopathy in children is often associated with genetic predisposition, muscular dystrophic diseases, neuroendocrine pathologies and infectious lesions of the myocardium.
The onset of the disease occurs both in early childhood and later as the child grows. At an early age, the symptoms of cardiomyopathy are subtle and can easily be confused with other diseases or with the child’s character traits.
The following symptoms of cardiomyopathy occur:
- lethargy;
- moodiness;
- restless night's sleep with frequent awakenings;
- lack of daytime sleep;
- prolonged crying for no apparent reason;
- drowsiness or insomnia;
- lack of appetite;
- decreased sucking reflex;
- sweating;
- fainting;
- bulging fontanel;
- convulsions;
- delayed psychomotor development;
- pale skin;
- cyanosis (in children with congenital heart defects).
In older children, the symptoms of cardiomyopathy are more pronounced. The following clinical picture is observed:
- dyspnea;
- dizziness;
- fatigue;
- chest pain;
- pale skin;
- cough;
- nausea and vomiting;
- sudden changes in mood (nervousness, tearfulness);
- fainting.
Treatment methods for cardiomyopathy depend on the form of the disease and the age of the child. They are selected strictly individually by a pediatric cardiologist.
Depending on the cause of the disease, medications or, in some cases, surgery may be chosen.