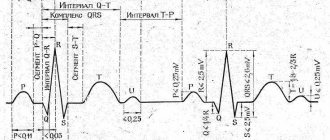
How stenting saves in case of myocardial infarction
Myocardial infarction is a lesion of the heart muscle caused by a sudden interruption of its blood supply due to thrombosis (blockage) of one of the heart arteries. The consequences of a heart attack can be minimized if blood flow in the affected vessel is quickly restored. The most effective method for this is stenting.
What causes and how does myocardial infarction develop?
Atherosclerotic plaques form in the walls of the heart arteries with age. The plaque reduces the lumen of the vessel, but may not manifest itself in any way until it ruptures. Within a few minutes, a blood clot forms on the damaged plaque, which blocks the lumen of the vessel. The access of oxygen and nutrients to the area of the heart muscle is cut off. The muscle cells in this area stop contracting and, if blood circulation is not restored within an hour, die. A focus of necrosis (necrosis) appears. This is myocardial infarction.
How does myocardial infarction manifest?
The main symptom of myocardial infarction is severe chest pain. It can spread to the left arm, shoulder, neck, and appear in the back. Pain is often accompanied by fear. This is how one of the patients describes his sensations: “Imagine that you swallowed half of a hard green apple at once. The piece got stuck halfway - neither here nor there. And it’s very painful because it’s bursting.” Sometimes myocardial infarction manifests itself with atypical symptoms: acute abdominal pain, an asthma attack, confusion and speech. In rare cases, mainly in patients with diabetes, myocardial infarction can occur completely without pain, accompanied only by sudden weakness and shortness of breath.
What to do if you suspect a myocardial infarction?
Call emergency medical help immediately. The most effective method of treating acute myocardial infarction, stenting, can only be performed in a hospital with a vascular department, where the patient must be transported as soon as possible.
When stenting, a mesh metal tube, a stent, is delivered to a blocked heart artery in a compressed form. At the site of thrombosis, the stent is expanded.
When expanded, the stent restores normal blood flow in the vessel and prevents its walls from closing again. Oxygen and nutrients begin to flow to the affected area of the heart muscle - the development of a heart attack stops. The entire operation is performed under local anesthesia, through a small puncture in an artery in the leg or arm. The sooner stenting is done during myocardial infarction, the less damage to the heart muscle. Doctors consider the ideal time for stenting to be 1.5-2 hours from the onset of symptoms. If there are no complications, the patient can be discharged home the very next day after stenting - he will save not only his life, but also his ability to work. Timely stenting reduces mortality from heart attack several times.
Terms that doctors often use
The common expression “myocardial infarction” is familiar to many, but now doctors more often use the term “acute coronary syndrome” (ACS) in the initial diagnosis and choice of treatment. It includes two diagnoses: unstable angina and myocardial infarction itself. Unstable angina is characterized by sudden pain in the heart and may be a warning sign of a heart attack. Myocardial infarction is said to occur when damage to the heart muscle has already occurred. ACS manifests itself in two main forms: ACS with ST-segment elevation on the electrocardiogram, which in most cases ends in myocardial infarction, and ACS without ST-segment elevation, which can manifest as both myocardial infarction and unstable angina.
Ureteral stenting for urolithiasis: problems, solutions
Streltsova O.S., Krupin V.N., Pochchin D.P., Yunusova K.E., Shcherbatyuk T.G., Yashanova M.I., Mamonov M.V.
Among urological pathologies, urolithiasis (UCD) ranks second in the world after inflammatory diseases of the kidneys and urinary tract, and occurs in at least 3-4% of the world's population. According to statistics in the Russian Federation, ICD accounts for 38-40% of all urological pathology, and in 70% of cases the disease is detected in patients of working age (20-60 years) [1]. After endoscopic removal of stones, after extensive abdominal operations on the urinary system, if there is a threat of ureteral obstruction or perforation during traumatic interventions, there is a need to install a catheter-stent in order to maintain the lumen of the ureter. [2]. Drainage with ureteral stents can be carried out for up to three months, and if necessary, longer. One of the main problems of a long stay of a stent in the lumen of the ureter is its encrustation with salts, as well as the high probability of bacterial colonization. Despite the search for various ways to eliminate this problem, the issue remains unresolved.
The purpose of the work was to study the effect of the drug "Rovatinex" on the condition of urine and stents in patients with urolithiasis.
MATERIALS AND METHODS
The results of surgical treatment of 1579 patients with urolithiasis were studied, of which 368 patients (23.3%) had catheters - stents installed in the ureter. The purpose of stenting was to relieve the inflammatory process in the kidney both during preparation for a planned operation and in the postoperative period. Obstruction of the stent by salt deposits and the development of bacterial infection were the leading indications for restenation.
As a drug that prevents lithogenic encrustation of stents, the drug Rovatinex (Rova Pharmaceuticals Limited, Ireland), which has sixty years of positive experience, was used in complex treatment.
An analysis of urine laboratory parameters (urinalysis, urine culture for flora) and the condition of stents after their removal was carried out in 40 patients: 20 - traditional management (group K), 20 - who received Rovatinex from the moment of stent installation to six weeks, two capsules three once a day (group P). At the same time, 10 patients had a usual drinking regime (P1), 10 consumed more than 2 liters of fluid per day (P2). The study used thermoplastic stents from the same company. The average time for kidney drainage with a stent was 32+10 days. After removal, all stents were assessed visually and tactilely for the presence of salt deposits. Their transverse section was examined using light microscopy with an eyepiece magnification of 10x10.
To determine the ability of urine to crystallize in patients receiving Rowatinex, we were the first to use the wedge-shaped dehydration method.
The wedge-shaped dehydration method described by V.N. Shabalin and S.N. Shatokhin, based on the analysis of the crystal structure of a biological fluid, allows you to visualize the systemic structural organization of a biological fluid when it is transferred to the solid phase by drying a drop on a glass slide [3, 4].
63 solid urine samples from 14 patients were studied: from 7 patients with urolithiasis, urine was collected before taking the drug Rovatinex and after 5 days while taking it. For comparison, dried drops of urine from 7 healthy respondents were studied. The structural features of urine crystals from each patient were assessed using three solid (dried) samples. Dried urine samples (facies) were examined using a transmitted light microscope. Photographing of facies was carried out using a MikMed 1 microscope and a CanonPowerShort A480 digital camera, followed by the formation of a computer database of images. The facies analysis included determining the presence and characteristics of zones and a detailed description of the structural features of each zone. The process of crystal formation of the facies was assessed on a 4-point scale according to the degree of severity of the indicator: 0 - absence of crystals, 1 - weak degree of expression, 2 - moderate degree of expression, 3 - high degree of expression. If necessary, intermediate values were introduced (1.5 and 2.5). Figure 1 shows variations of urine samples from healthy patients.
RESULTS
In urine cultures for flora before stent installation in both groups, flora was isolated in 22.5% (9/40) at a titer of more than 103 CFU/ml. E. coli, Enterococcus spp., Klebsiella, Staphylococcus spp. predominated. Before stent removal, flora was detected in three cases in group K; in group P, all urine cultures were negative. Initially, the number of leukocytes in the urine of more than 7 per field of view in the groups was detected in 12 and 10 patients, respectively. Before removal of the stent, the inflammatory process according to laboratory parameters in group P was recorded in three cases, in the control group twice as often - in six. The number of leukocytes in the urine analysis according to Nechiporenko in group K was 3650 per 1 ml, in group P it did not exceed the reference values. The amount of urine excreted per day differed. In group P on average 1750+250 ml (P1-1500 – 1750 ml, P21800 – 2100 ml), in group K – 1150+ 250 ml (from 900 to 1400 ml). The relative density of urine in group P was 1009-1018, in group K1014-1030.
Visual analysis of removed stents from patients in group K: in 45% (n=9) they changed color to darker or acquired a dull, rough surface - evidence of the aggressive action of urine. Of these, in 25% of cases (n=5) there were salt deposits. In group P, there were no visually lithogenic layers.
By light microscopy, 90% of the stents (n=18) of patients in group K had lithogenic deposits (Fig. 2, K a, b, c). In those receiving Rovatinex, less intense encrustation was detected in 45% (n=9), and against the background of a normal drinking regimen in 7 cases out of 10 (Fig. 2, P 1 a1, b 1, c1), against the background of increased water load - in 2 cases out of 10 (Fig. 2, P2 a2, b2, c2).
Fig.1. Examination of urine using the wedge-shaped dehydration method in healthy patients: a, b – normal variants. Intensity of crystal formation in facies in points: median 3; average 2.6
Fig.2. Study of ureteral stents using light microscopy in groups: K a, b, c examples of walls from patients in the control group; P1, P2 examples of stents from patients treated with Rowatinex; a1, b1, c1 with normal drinking regimen; a2, b2, c2 against the background of increased water load
Wedge dehydration method. The characteristics of the structure-forming elements of a dehydrated drop of urine while taking Rowatinex differed from the initial one: after administration of the drug, multidirectional changes in the structure of the facies were observed. After taking the herbal medicine (9 facies - 42.9% of three patients), an increase in the number of crystals was observed (median before treatment - 1, after treatment - 2), while the appearance of crystals of “cross-shaped” and “dendritic” shapes, characteristic of healthy volunteers (Fig. 3 a1, b1). In one patient, after treatment, a change in the structure of the “substrate” facies was observed, namely an increase in its “porosity”. The analysis showed that initially these patients had leukocyturia - more than 70 leukocytes in the field of view.
In three patients, the number of leukocytes in the urine analysis was normal, after five days of taking Rowatinex, a decrease in crystal formation was observed (median before treatment 1.5, after - 1) - 9 phases, which amounted to 42.9%. Moreover, in two cases, a decrease in the size of the crystals and a change in their shape to “uncertain” was observed in comparison with the indicators of the crystals of the facies of the patients before treatment (Fig. 3: c1, d1); and one patient had a complete absence of crystals.
Rice. 3. Urine examination using the wedge-shaped dehydration method before and 5 days after starting to take the herbal medicine (description in the text).
The use of the drug had no effect on the crystalline structure of urine facia in one patient (median-1). Patient G. (68 years old) with bilateral nephrolithiasis after percutaneous puncture nephrolithotripsy on one side was kept with a stent for three weeks. Hospitalized for stent replacement due to obstruction by salts, exacerbation of pyelonephritis: febrility, general urine analysis showed leukocytes up to 100 in the field of view. Empirical treatment with a third-generation cephalosporin antibiotic was performed in the hospital. 5 days before stent replacement, Rovatinex was introduced into treatment. The stent has been replaced. In the control analysis of urine, there were 150 leukocytes in the field of view, single crystals of uric acid against the background of an acidic urine reaction (pH 5.0). When taking Rowatinex with severe leukocyturia, no increase in crystal formation was recorded (Fig. 3e, e1).
The genesis of lithogenic deposits is multifactorial, and therefore compliance with the known general principles of metaphylaxis of urolithiasis, as well as the individual management scheme for a patient with urolithiasis with a stent, must be strictly observed by the patient and the doctor. The result of incorrect management of the patient is presented in Figure 4. A stent was installed in patient S., 51 years old, due to traumatic trypsy of a ureteral stone, conversion to ureterolithotomy. Due to dysuria, the patient limited fluid intake to 600–700 ml per day; on the recommendation of a doctor at the Central Regional Hospital, he received Furamag 100 mg 3 times a day, which is irrational for calculous pyelonephritis. It was found that after discharge from the hospital, Rovatinex was received in an incomplete course, only 10 days. On ultrasound, the pelvic end of the catheter is encrusted with salts. In urine analysis: beat. weight – 1008, leukocytes – 60 per field of view, erythrocytes – 3-5 per field of view. The stent was removed on the 28th day from the moment of installation in the operating room after mechanical destruction of the saline structures through the ureteroscope with forceps (Fig. 4).
Rice. 4. Stent of patient C. Standing time 28 days: a) visual characteristics: dark color, covered with salts, cystic and pelvic ends with stones. b) with light microscopy, the cross section is narrowed by salt layers
DISCUSSION
Kidney hypoxia is considered one of the main reasons for the development of pre-stone nephrolithiasis [5]. At the same time, it has been proven that terpenes, substances from unsaturated hydrocarbons, affect microcirculatory processes in the kidneys and are pathogenetically justified in complex treatment [6]. Rowatinex contains six terpene components (active ingredients: pinene [α+β] 31.0 mg, camphene 15.0 mg, cineole 3.0 mg, fenchone 4.0 mg, borneol 10.0 mg, anethole 4.0 mg) produced from essential oils of coniferous plants [7]. Terpenes of natural origin, which are part of Rowatinex, have antispasmodic, diuretic and anti-inflammatory effects. They are fat-soluble and quickly absorbed, undergo metabolic changes in the body, turning into glucuronides, which are excreted in the urine and are stabilizers that prevent stone formation.
Any drainages, stents in particular, as foreign bodies in the urinary tract, are themselves risk factors for recurrent stone formation. Aseptic inflammation develops around the stents with all the molecular and cellular reactions inherent in this process [8-10]. In patients with stents, even a small bacterial number of pathogenic microorganisms in the urine can lead to infectious and inflammatory changes with the formation of biofilms on the surface of the implant, which, in turn, are insensitive to the antibacterial drugs used [11]. Analysis of laboratory urine parameters in the groups indicates the anti-inflammatory effect of Rowatinex and preventing the progression of pyelonephritis. Our results are consistent with the data of other authors, indicating that patients taking Rovatinex are significantly less likely to develop clinically significant bacteriuria. In the works of I.V. Kazanskaya [12] showed that Rovatinex prevents the manifestation of infectious complications and even potentiates antibacterial therapy by affecting the microorganism through a wider range of biochemical mechanisms. The drug can be used both in the active stage of inflammation in combination with antibacterial drugs, and as maintenance anti-relapse therapy [6]. Minimal complaints from patients about the presence of a stent in the bladder, clinical and biochemical parameters of patients taking the herbal medicine indicate its antispasmodic and antiseptic effects.
In turn, structural pathological changes in the supramembrane system of the epithelium of the renal tubules, no matter what they are caused by - bacterial damage, ischemia, etc., are the main reasons for the increased release of organic substrate in the lithogenicity of urine [13]. Any change in the composition of urine is reflected in its “mechanical” characteristics (viscosity, structure, surface tension), which determine the shape of the resulting structures of the drying drop of biological fluid. The inclusion of a herbal medicine in the management regimen of patients with stents not only prevented the manifestation of infectious complications, but also influenced the biochemical parameters of urine, urine colloids, and, accordingly, its lithogenic properties, which was confirmed by the wedge-shaped dehydration method in our study. Analysis of the structure of urine in patients with urolithiasis receiving Rovatinex indicates its ability to modulate the process of formation of crystals and uroliths.
A component of comprehensive prevention of stent encrustation with salts should be the water load, which affects the saturation factor for all possible precipitating salts.
In an experiment on animals V.M. Bryukhanov et al. demonstrated a direct dependence of lithogenic processes in kidney tissue on changes in the concentration of ions in the urine, and not on the excretion of these ions [14]. Thus, an important parameter that maintains the performance of stents is the volume of daily urine. The results of our study indicate that Rowatinex stimulates renal function by increasing diuresis (group P1).
According to various authors, bacterial contamination of drainages, including urinary stents, occurs within a period of several hours to several days [11,13]. Using the wedge-shaped dehydration method, it was determined that the administration of the drug Rowatinex already after 5 days influenced the formation of the crystalline structure of dried urine.
CONCLUSION
The prescription of the herbal medicine Rovatinex, as a component in the prevention of stent encrustation with salts, is pathogenetically justified in many respects. Rowatinex is able to have a combined effect on key renal functions and potentiate the inhibitory effect of stone formation. A regimen for the prevention of lithogenic deposits on the stent should be introduced several days before planned stenting. An obligatory component of prevention should be an increased volume of fluid intake.
LITERATURE
1. Interdisciplinary problems in urology. Guide for doctors [ed. P.V. Glybochko, Yu.G. Alyaeva]. M.: Medforum, 2015.580 p.
2. Lukenda J, Biocina-Lukenda D. Stent, endovascular prosthesis, net or strut? What would British dentist Charles Stent (1807-1885) have to say on all this? Lijec Vjesn 2009;131(1-2):30-33.
3. Shabalin V.N. Shatokhina S.N. Principles of autowave self-organization of biological fluids. Bulletin of the Russian Academy of Medical Sciences 2000;(3):45-49.
4. Shabalin V.N., Shatokhina S.N. Morphology of human biological fluids. M.: Chrysostom, 2001. 304 p.
5. Tatevosyan A.S., Osipov A.A., Opolsky A.B., Tatevosyan T.S. A method of conservative treatment of urolithiasis and prevention of recurrent formation of kidney stones. RF patent publication of the patent: 06/10/2005. URL: https://www.freepatent.ru/patents/2253366
6. Gudenko Yu.A., Kazanskaya I.V., Lobzhanidze Z.D. Use of the drug Rovtinex in pediatric urology. Experimental and Clinical Urology 2013;(3):61-65.
7. Instructions for medical use of the drug Rowatinex. URL: https://grls.rosminzdrav.ru/Grls_View_v2.aspx?idReg=85572&t=
8. Seregin A.V., Mulabaev N.S., Tolordava E.R. Modern aspects of the etiopathogenesis of urolithiasis. General Medicine 2012;(4):4-10
9. Venkatesan N, Shroff S, Jeyachandran K, Doble M. Effect of uropathogens on in vitro encrustation of polyurethane double J ureteral stents. Urol Res 2011;39(1):29-37.
10. Rosman BM, Barbosa JA, Passerotti CP, Cendron M, Nguyen HT. Evaluation of a novel gel-based ureteral stent with biofilmresistant characteristics. Int Urol Nephrol 2014; 46(6):1053-1058.
11. Kogan M.I., Shvodkin S.V., Lyubushkin A.V., Miroshnichenko O.V. Directions and prospects in the development of urological stents (literature review). Experimental and Clinical Urology 2014;(4):64-71
12. Kazanskaya I.V., Babanin I.L., Rostovskaya V.V., Matyushina K.M., Vorontsov A.L. The influence of the herbal medicine "Rovatinex" on the urodynamics of the upper urinary tract and dysmetabolic processes in children with hydronephorosis and obstructive megaureter. Experimental and Clinical Urology 2015;(4):117-122.
13. Gazymov M.M., Gazymova D.M., Filippov D.S. Urolithiasis: Etiotropic and pathogenetic treatment, metaphylaxis. Cheboksary: Publishing House Chuvash. Univ., 2010.174 p.
14. Bryukhanov V.M., Zverev Ya.F., Lampatov V.V., Zharikov A.Yu., Kudinov A.V., Motina N.V. The influence of drinking regimes on the driving forces of crystallization in experimental nephrolithiasis. Urology 2011;(1):6-11.
Journal "Experimental and Clinical Urology" Issue No. 3 for 2016
Topics and tags
Urolithiasis disease
Magazine
Journal "Experimental and Clinical Urology" Issue No. 3 for 2016
Comments
To post comments you must log in or register