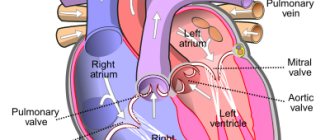
With the normal structure of the heart, the pulmonary veins flow into the left atrium, and it is through them that oxygenated blood enters the left ventricle, and then into the aorta. It is then carried by arteries throughout the systemic circulation.
Under the influence of a number of external negative factors in the prenatal period, conditions can be created for the formation of such a congenital heart defect as anomalous pulmonary venous drainage (ADPV), in which these vessels flow not into the left atrium, but into the right atrium, vena cava or coronary sinus. Such an abnormal arrangement of vessels can be partial or total, that is, part of the individual pulmonary veins or all the mouths of these vessels can communicate with the right atrium. The patient’s condition largely depends on these factors.
According to statistics, ADLV is detected in 1.5-3% of patients with congenital heart defects. More often this pathology is found among men.
Often, ADLV is combined with such anomalies of cardiac development as a patent foramen ovale and atrial septal defect. And in 20% of patients it is accompanied by other defects: the common truncus arteriosus, tetralogy of Fallot, dextrocardia, ventricular septal defect, underdevelopment of the left chambers of the heart, etc. In addition, patients with this diagnosis often have extracardiac developmental anomalies: horseshoe kidney, polycystic kidney disease, hydronephrosis, umbilical hernias, intestinal diverticula and malformations of the musculoskeletal system or endocrine system.
In this article we will introduce you to the suspected causes, types, manifestations, methods of identifying and treating abnormal drainage of the pulmonary veins. The information received will help you understand the essence of such a congenital heart defect, and you will be able to ask your doctor any questions you may have.
A little history
This rare congenital defect was first described by Wilson back in 1798, but its variants were examined in more detail only after sufficient cardiac surgical experience had been accumulated in the 50-60s of the 20th century. The first successful operation to correct this pathology was performed by Miller in 1951. Subsequently, cardiac surgical techniques were improved, and in 1956, radical correction of ADLV was carried out using superficial hypothermia. In the same year, a similar intervention was performed using artificial circulation.
On the territory of the USSR, the first successful operation to eliminate such a heart defect was performed by Nikolai Mikhailovich Amosov in 1978, and one of the leading Russian cardiac surgeons, Georgy Eduardovich Falkovsky, reported on a series of successful corrections in young children in 1984.
Causes
Heredity probably plays a certain role in the development of ADLV.
The probable causes of the development of ADLV may be the same external factors that provoke other congenital anomalies of the structure of the heart and blood vessels:
- heredity (chromosome mutations);
- taking certain teratogenic drugs;
- exposure to toxic substances on the body during pregnancy;
- bad habits of the expectant mother;
- infectious diseases suffered by the pregnant woman;
- endocrine disorders;
- toxicosis;
- unfavorable environment.
Disconnection of the pulmonary veins from the left atrium can be caused by the following factors:
- the absence of their connection with the left atrium is formed due to the fact that, under the influence of the external causes described above, the left atrial outgrowth cannot communicate with the venous plexuses of the future lung;
- early pulmonary vein atresia - occurs with the initial connection of the pulmonary vascular bed and the common pulmonary vein, the lumen of which is subsequently obliterated, and blood from the lungs begins to flow through other collateral pathways.
Congenital left atrial aneurysm
A congenital defect manifested by aneurysmal dilatation of the left atrium without diseases of the left ventricle or mitral valve (Fig. 90).
Fig.90.
Congenital aneurysm of the left atrium.
EchoCG criteria
One-dimensional echocardiography:
- Marked dilatation of the anteroposterior size of the left atrium.
- Normal echocardiogram pattern of the mitral valve (may be mitral valve prolapse).
Two-dimensional echocardiography:
- Large mass formation associated with the left atrium.
- There may be compression of the wall of the left ventricle by the aneurysmal body.
- Changes in the position of the chambers of the heart due to their displacement by a space-occupying formation.
Doppler EchoCG:
- Assessment of the obturator function of the mitral valve.
- Assessment of pulmonary hemodynamics.
Differential diagnosis:
- Congenital mitral valve insufficiency.
- Mitral stenosis.
- Restrictive cardiomyopathy.
- Hypertrophic cardiomyopathy with a small cavity of the left ventricle.
Varieties
ADLV is of two types:
- Total (or complete). With this defect, blood circulation between the greater and lesser circles completely stops, since there is no communication between the pulmonary veins and the left atrium. This form of the defect is incompatible with life, however, the child may have concomitant anomalies of heart development such as atrial or ventricular septal defects, which slightly compensate for such a significant hemodynamic disturbance. In such cases, the baby can live for some time. However, in most cases, without timely cardiac surgical correction of total ADVC, children die before the first year of life.
- Partial. With such an anomaly, one of the branches of the pulmonary veins communicates with the left atrium, and the patient’s hemodynamic state depends on the volume of blood discharge.
Depending on the level of entry of the pulmonary veins into the left atrium, four anatomical variants of the ADLV are distinguished:
- supracardial (or supracardial) - observed in almost 55% of newborns and is accompanied by the flow of the pulmonary veins into the superior vena cava system;
- cardiac (or intracardiac) - detected in 30% of patients and is accompanied by the flow of the pulmonary veins into the right atrium or coronary sinus;
- subcardiac (or subcardial, infracardial) - observed in 12% of children and is accompanied by the flow of the pulmonary veins into the inferior vena cava or portal vein (sometimes into the lymphatic duct);
- mixed - detected in 3% of newborns and may be accompanied by various variations in the confluence of the pulmonary veins described above.
Publications in the media
Anomalous pulmonary vein drainage (ADPV) is a congenital heart disease characterized by the drainage of all pulmonary veins into the right atrium or the main veins of the systemic circulation. Thus, total ADLV or partial ADLV are distinguished.
Statistical data • Total ADLV makes up 2.6% of all CHDs, partial ADLV - 1.5% • The combination of total ADLV with atrial septal defect (ASD) occurs in 0.7–9% of all CHDs, partial ADLV with ASD - in 5, 7% • The right lung is most often abnormally drained (97.2%) • The most common type of ADLV is supracardiac (75% partial and 45% total ADLV).
Etiology: factors that form congenital heart disease (see Tetralogy of Fallot).
Pathogenesis • Changes in hemodynamics with partial ADPV are similar to those with ASD (see Atrial septal defect) and almost do not depend on the anatomical type of the defect (see below) • With total ADPV, blood from all veins of the body enters the right atrium, and the child’s life is impossible without the presence of communication between the systemic and pulmonary circulation • Most often, such a shunt is an open foramen ovale or a true secondary ASD, the size of the latter determines the speed of development and severity of pulmonary hypertension and, therefore, the prognosis in the natural course.
Classification of anatomical types of partial ADLV
• Supracardial type - drainage of individual pulmonary veins or their common collector into the superior vena cava or its tributaries •• Right superior pulmonary vein ® superior vena cava •• Accessory right superior pulmonary vein ® superior vena cava •• Left superior pulmonary vein ® left brachiocephalic vein •• Left superior pulmonary vein ® left brachiocephalic vein ® right atrium (separate from the superior vena cava) •• Right superior pulmonary vein + right inferior pulmonary vein ® accessory superior vena cava
• Cardiac type - drainage of individual pulmonary veins or their common collector into the right atrium or coronary sinus •• Right superior pulmonary vein + right inferior pulmonary vein ® right atrium (isolated or in combination with ASD) •• Left superior pulmonary vein + left inferior pulmonary vein ® coronary sinus
• Subcardial type - drainage of individual pulmonary veins or their common collector into the inferior vena cava •• Right inferior pulmonary vein ® inferior vena cava •• Left inferior pulmonary vein ® inferior vena cava
• Mixed type.
Clinical picture and diagnosis. Diagnosis is almost entirely based on these instrumental methods, since the pathogenesis and clinical picture of the defect are identical to those of ASD, and also due to the frequent combination of ASD and ADLV.
Complaints: see Atrial septal defect.
Objectively: see Atrial septal defect.
Instrumental diagnostics
• ECG •• Signs of hypertrophy and overload of the right sections •• Most often, the ECG is normal.
• X-ray of the chest organs •• Strengthening the pulmonary pattern •• Bulging of the arches of the pulmonary artery and the right chambers of the heart •• In the most common variant of ADPV (drainage of the right superior pulmonary vein into the superior vena cava), the expansion of the shadow of the lower segment of the superior vena cava and the root of the right lung is determined •• With the supracardial type of total ADLV, radiological data are highly specific ••• With the most common variant of total ADLV (drainage of the common collector into the left brachiocephalic vein or accessory superior vena cava), the shadow of the heart has a configuration resembling the number 8, or the figure of a snow woman (Snellen sign - Albers) ••• Total ADLV into the superior vena cava leads to an aneurysm of its central section, the latter is visible in the form of a local protrusion along the right contour of the shadow of the vascular bundle •• Cardial and subcardial types of ADLV do not have specific signs •• Sometimes a homogeneous spotting of the pulmonary pattern resembles a picture miliary pulmonary tuberculosis •• When the right inferior pulmonary vein flows into the inferior vena cava in the anteroposterior projection, the shadow of an abnormally running vessel is visualized, resembling the shape of a “Turkish saber” (symptom of the “Turkish saber”).
• EchoCG •• Absence of the orifices of the pulmonary veins in the left atrium •• Diagnosis of the defect is practically based only on the registration of pathological flows from the pulmonary veins according to the study in the color Doppler mapping mode •• EchoCG is a highly sensitive method for diagnosing ADPV, but in most cases the anatomical variant of ADPV is diagnosed erroneously or incompletely •• All adults and children of the older age group undergo transesophageal echocardiography •• For the rest, see Atrial septal defect.
• Radionuclide angiocardiography (first pass method or equilibrium) •• Registration of pathological flow from the pulmonary veins and its quantitative assessment •• Diagnosis of concomitant dysfunction of the contractile function of the right ventricle.
• Probing of the cavities of the heart •• Pressure in the cavities of the heart is usually within normal limits or slightly increased in the right atrium •• With the supracardial type of APPV - increased pO2 and blood oxygen saturation in the superior hollow; in the case of subcardial - in the inferior vena cava •• Changes in blood oxygen saturation in the right chambers of the heart are not specific for ADLV •• The most specific sign is passing a probe from the inferior vena cava or right atrium into the pulmonary vein •• Diagnosis of concomitant ASD - see Atrial septal defect .
• Right atriography and ventriculography, phlebography of the superior vena cava, left atriography, angiopulmonography •• Receipt of contrast that has passed through the pulmonary circulation into the vena cava or right atrium (practically the only sensitive method for diagnosing total ADPV) •• The most specific sign is that the contrast of the orifice is abnormal drained pulmonary vein when a catheter is inserted into its lumen •• Diagnosis of concomitant ASD - see Atrial septal defect.
Drug therapy: not developed, there is no need for the prevention of infective endocarditis.
Surgery
• Indications: see Atrial septal defect.
• Contraindications: see Atrial septal defect.
• Methods of surgical treatment •• In case of partial ADPV - complete anatomical correction of the defect through numerous options for replantation of the mouths of the pulmonary veins, their common collectors, additional superior vena cava or coronary sinus draining the pulmonary veins into the left atrium •• Elimination of concomitant ASD - see Defect interatrial septum •• With the cardiac variant of partial ADLV and with total ADLV - the creation of an artificial or expansion of a natural ASD with subsequent isolation using an autopericardial or synthetic patch of the created defect and the mouths of abnormally drained veins into the left atrium.
Specific postoperative complications: sick sinus syndrome, increased pulmonary venous hypertension with inadequate provision of outflow tracts from the pulmonary veins.
Prognosis • With partial ADPV is similar to that with ASD (see Atrial septal defect) • With total ADPV, less than 50% of children survive to 3 months and less than 20% to 1 year • Patients who survive the first few weeks usually have severe cardiomegaly, cardiac insufficiency and pulmonary hypertension • Patients who survive up to 1 year without surgery usually have very large ASDs and survive until 10–20 years, after which some of them develop irreversible pulmonary hypertension • Presence of obstruction in the outflow tract from abnormally draining pulmonary veins reduces life expectancy by 3.5-5 times • Overall hospital mortality with surgical correction of total ADLV - 6-25% (on average 13%), partial ADLV - 2-4% • Hospital mortality with radical correction of subcardial type of ADLV in newborns - 30–40%, cardiac and supracardial - 5–10% • In 85% of patients, complete correction of hemodynamics occurs within 1 year after surgical treatment of partial ADLV • Long-term mortality after correction of total ADLV almost completely depends on the immediate (hospital) period, subsequent lethal cases are caused by other diseases.
Synonyms: Abnormal drainage of the pulmonary veins; Anomaly of the connection of the pulmonary veins; Complete anomalous drainage of the pulmonary veins; Incomplete anomalous drainage of the pulmonary veins.
Abbreviations. APLV is anomalous drainage of the pulmonary veins.
ICD-10 • Q26 Congenital anomalies [malformations] of large veins
How is hemodynamics disrupted?
During intrauterine development, this heart defect does not manifest itself in any way, since the intracardiac circulation in the fetus is accompanied by communication between the left and right atria through the open foramen ovale. ADLV begins to make itself felt after the birth of a child, and the severity of hemodynamic disturbances will be determined by the type and variant of this anomaly. In addition, the severity of the symptoms of the defect may be influenced by the presence of concomitant defects in the development of the heart and blood vessels.
With total ADPV, oxygenated blood entering the right atrium mixes with venous blood. Next, some of it enters the right ventricle, and the other enters the left atrium through an existing atrial septal defect or patent foramen ovale. In such cases, total ADLV is compatible with life, since communication between the systemic and pulmonary circulation is not lost. Such hemodynamic disturbances lead to overload of the right chambers of the heart, increased pressure in the pulmonary vessels and a decrease in the oxygen content in the blood, which leads to oxygen starvation of organs and tissues.
With partial ADLV, hemodynamic disturbances occur in the same way as with interatrial defects. The severity of the patient's condition with this type of anomaly is determined by the volume of pathological arteriovenous discharge.
"Riding" tricuspid valve
An obligatory component of the defect is a VSD and displacement of the valvular apparatus of the tricuspid valve into the cavity of the left ventricle.
EchoCG criteria
One-dimensional echocardiography:
- Small size of the right ventricle.
- The anterior leaflet of the tricuspid valve crosses the IVS during diastole.
- Visualization of echo signals from the valve apparatus of the tricuspid valve in the cavity of the left ventricle.
- Interruption of the echo signal from the interventricular septum.
- Dilation of the left ventricle and left atrium.
Two-dimensional echocardiography:
- Hypoplasia of the right ventricular cavity.
- Displacement of the right atrioventricular ring and subvalvular apparatus into the left ventricle.
- Direct visualization of a ventricular septal defect.
- Detection of associated anomalies.
Doppler echocardiography:
- Assessment of the state of pulmonary blood flow.
- Estimation of the magnitude of the systolic gradient between the ventricles.
Differential diagnosis:
Open common atrioventricular canal.
Symptoms
Signs of ADLV make themselves known immediately after the birth of the child. With a total defect, which is not accompanied by the presence of defects in interatrial communications, blood circulation between the small and large circles becomes completely impossible, and the newborn quickly dies. In such cases, only an emergency cardiac surgery using the Rashkind method, such as balloon endovascular atrioseptostomy, can save the child’s life in such cases.
In other cases, the severity of clinical manifestations of ADLV is determined by its anatomical variant, the size of atrial septal defects and the nature of hemodynamic disorders. Typically, parents of children with such a congenital defect notice the following manifestations of this pathology:
- fast fatiguability;
- the appearance of shortness of breath after physical activity (feeding, crying, etc.);
- pain in the heart area (the child sleeps poorly, is restless, cries loudly and stretches his legs);
- pale skin;
- mild cyanosis;
- cough;
- nausea and vomiting;
- slow weight gain;
- frequent ARVI and pneumonia.
By palpating the pulse, the doctor can detect its rapidity and arrhythmia. Upon examination, deformation of the chest and an increased heartbeat are determined.
Mild cyanosis that occurs in the first weeks of life may disappear or become less pronounced over time. As a rule, cyanosis is more pronounced during physical activity.
When listening to heart sounds, specific signs of this defect are not detected. Can be heard:
- loud I tone over the heart;
- splitting of the second tone with increased pulmonary component;
- heard III sound above the apex of the heart (in many patients);
- soft systolic murmur over the pulmonary artery with variable duration and intensity.
The ECG determines the overload of the right parts of the heart - high voltage of the P wave in the right leads and deviation of the electrical axis to the right in standard leads. Overload of the right atrium is indicated by a high P wave in the right chest and standard leads. Often there are signs of incomplete blockade of the right bundle branch.
If there is a large defect in the interatrial septum, the child may develop normally and then, at an older age, complaints arise of a significant decrease in exercise tolerance, shortness of breath, pallor and poor general health. Later, such children develop signs of right ventricular failure.
Congenital aortic stenosis
Fig.94.
Valvular aortic stenosis. Poststenotic dilatation of the ascending aorta (scheme). Aortic stenosis is divided into the following forms:
- Valvular congenital stenosis (Fig. 94).
- Subvalvular aortic stenosis
- A. discrete membranous stenosis.
- B. discrete fibromuscular subaortic stenosis.
- B. tunnel subaortic stenosis.
- Supravalvular aortic stenosis.
- Bicuspid aortic valve with stenosis.
In 20% of cases, congenital aortic stenosis is accompanied by PDA, coarctation of the aorta, VSD, and pulmonary stenosis.
Congenital valvular stenosis (see aortic valvular stenosis)
Note:
if all 3 semilunar leaflets are fused, then a narrow central opening is determined; when 2 leaflets are fused (more common), the opening in the aortic lumen is located asymmetrically.
Subvalvular aortic stenosis discrete membranous
Immediately below the aortic valve there is a thin fibrous membrane (Fig. 95).
Fig.95.
EchoCG criteria
One-dimensional echocardiography:
- Partial early systolic closure of the aortic valve.
- Systolic flutter of the aortic valve.
- The presence of additional signals in the left ventricular outflow tract.
- Narrowing of the left ventricular outflow tract.
- Anterior systolic movement of the anterior mitral leaflet.
- Left ventricular hypertrophy.
- Delayed opening of the mitral valve.
Two-dimensional echocardiography:
- A fibrous membrane just below the aortic valve prolapses into the left ventricular outflow tract in diastole (Fig. 96).
- Narrow left ventricular outflow tract.
Doppler EchoCG:
- Increase in peak systolic flow in the ascending aorta over 1.5 m/s.
- The presence of a systolic gradient (over 5 mm Hg).
Fig.96.
Subvalvular perimembranous aortic stenosis.
Differential diagnosis:
- Asymmetric hypertrophic cardiomyopathy with obstruction of the left ventricular outflow tract (idiopathic hypertrophic subaortic stenosis): with discrete stenosis, partial early systolic closure of the aortic valve is usually observed (on average after 0.05 s), with IGSS - mid-systolic (on average after 0.14 s from the moment opening of the aortic valve)
Subvalvular aortic stenosis discrete fibromuscular
Formed by a fibromuscular ring, it is usually located more distal to the aortic valve than in discrete membranous stenosis, approximately 1 cm or more.
Diagnostics
One of the methods that helps detect and verify ADLV is cardiac ultrasound.
For the first time, ADLV can be detected in the fetus during an ultrasound examination in the third trimester of pregnancy.
To clarify all the data about such a congenital heart defect, the following instrumental research methods are prescribed:
- radiography;
- ECG;
- Echo-CG (for adults and older children, transesophageal echo-CG is prescribed);
- MRI;
- probing of the heart chambers and angiocardiography.
Treatment
The only way to eliminate ADLV is its cardiac surgical correction. Before surgery, the child is recommended to limit physical activity and is prescribed medications to prevent acute heart failure (diuretics, cardiac glycosides). Parents of a child should be aware that even crying or temperature discomfort can significantly worsen the state of health with such a heart defect. If the child is already an adult, then they must constantly monitor his leisure time - he should not run, lift weights and overwork.
Methods of surgical correction
The method of performing cardiac surgical correction for ADPV is determined by the type and variant of the defect.
With total drainage, a critically ill child under 3 months of age can undergo palliative surgery, which consists of increasing interatrial communication using balloon atrioseptomy using the Rashkind technique. This correction promotes blood flow into the left atrium and restoration of blood circulation in the systemic circle.
To radically eliminate ADLV, interventions are carried out aimed at creating a wide anastomosis between the left atrium and the pulmonary veins, stopping the pathological drainage of the pulmonary veins with other venous vessels and eliminating the atrial septal defect. It is preferable to perform such interventions at an early age. The radical correction technique is selected depending on the anatomical variant of the ADLV.
Corrective interventions are quite effective, however, despite the development of modern cardiac surgery, the percentage of deaths occurring during surgery or in the postoperative period remains high. The result of radical correction largely depends on the skill of the cardiac surgeon, technical equipment, and the type and variant of the defect. In addition, the highest risk of death is observed among newborns and young children suffering from severe pulmonary hypertension. Most often, this manifestation of the defect is observed among patients with the subcardial variant of the anomaly.
Long-term results among children who survived radical correction of ADLV are quite satisfactory. Only some patients may develop sinus node weakness and pulmonary hypertension, which are difficult to respond to drug therapy.
Complications
- Obstruction of anastomoses (5-10% of cases)
Complete anomalous drainage of the pulmonary veins into the right atrium (type II, cardiac), combined with an atrial septal defect. X-ray of the chest in direct projection. Enlargement of the right heart and increased vascularization of the central parts of the lung. MRI with contrast enhancement of the chest. Partial anomalous drainage of the pulmonary veins, the left pulmonary vein drains through the vertical vein (arrow) into the innominate vein (*).
Forecast
Successfully performed radical cardiac surgical correction of ADLV significantly improves the prognosis of the outcome of this heart defect. However, the risk of death during or after surgery remains high.
With the total form of ADPV and the absence of emergency cardiac surgical correction, about 80% of children die before the age of one year. If the anomalous drainage of the pulmonary veins is partial, then patients can live up to 20-30 years. Without surgical treatment, such patients die from heart failure or pulmonary infections.
Anomalous drainage of the pulmonary veins is a rare congenital heart defect and always requires cardiac surgical correction. In some cases, the condition of a newborn child becomes critical due to significant hemodynamic disturbances, and such operations are performed on an emergency basis. After successful radical cardiac surgery, the patient’s condition improves significantly, and the prognosis for the outcome of the disease becomes favorable.
Clinical manifestations
Typical symptoms:
Pulmonary vein obstruction:
- acute emergency situation in the first few hours or days of life
- Cyanosis
- Dyspnea
- Pulmonary hypertension.
Without pulmonary vein obstruction:
- Increased pulmonary blood flow
- Pulmonary hypertension
- Tachypnea
- Signs of heart failure
- Dystrophy.
A slight increase in pulmonary artery pressure may cause only a minimal number of symptoms; Cyanosis may be absent or mildly expressed.