Shortness of breath, constant weakness, unexplained swelling, chest pain - grounds for a cardiological examination. The listed phenomena may indicate a risk of chronic heart failure. The first mild symptoms should not be ignored. Without appropriate therapy, the condition will worsen. The IMMA medical clinics provide therapeutic and cardiological appointments.
In our clinics you can:
- Get a consultation with a cardiologist;
- Take an ECG and get a professional interpretation of the results;
- Complete the ABPM procedure;
- Undergo Holter monitoring;
- Take advantage of other services of our clinics.
For more details and any questions, please contact the number listed on the website
Congestive heart failure - decreased contractions of the heart muscle. This pathological condition is not an independent disease, it is a consequence of various disorders of the cardiovascular system.
The prevalence of heart failure among the population is constantly growing, the disease is considered a social problem along with oncology and AIDS. The reasons for the spread are different: poor environmental conditions, sedentary lifestyle, ignoring healthy eating rules, bad habits. Inattention to health leads to various damage to the circulatory system. Heart failure is a common complication.
Introduction
The heart is an organ that works throughout life without stopping. Over 70 years, it makes about 3,000,000 contractions. To perform such colossal work, it needs nutrition and oxygen, which are supplied through a network of coronary arteries. If disturbances occur in this network, the heart muscle begins to “starve”, this leads to unpleasant sensations and poses a danger to life.
In the last 100 years, the incidence of cardiovascular diseases has increased due to the consumption of large quantities of sweet and fatty foods, fast food, decreased mobility, smoking and stress.
In the practice of a cardiologist, one has to deal with myocardial hypoxia in patients. Most complain of squeezing pain behind the sternum. The doctor examines, identifies the causes, prescribes additional diagnostics and selects treatment: medicinal, surgical or complex, depending on the degree of development of hypoxia.
Etiology of occurrence
The development of the disease is influenced by several reasons, grouped together:
- Hypoxic. It is characterized by a low concentration of oxygen in the room where a person is located.
- The cardiovascular system. The disease occurs as a result of blockage of blood vessels or when the heart malfunctions due to diseases such as a heart attack.
- Histotoxic. This is poisoning with toxic chemicals, heavy metal salts and other chemicals. To prevent poisoning, enzymes responsible for the distribution and absorption of oxygen are blocked.
- Mechanical. This includes damage to the respiratory system caused by suffocation or trauma.
- Hematological. Anemia, atherosclerosis, smoking, carbon dioxide poisoning contribute to the development of the disease. All this leads to the death of red blood cells.
- Respiratory system - pneumonia, swelling of the respiratory tract.
- Physical. Myocardial hypoxia occurs against the background of overload caused by unusual stress during hard work or sports, when the necessary oxygen does not reach the heart.
Indirect factors include:
- diabetes;
- poor diet;
- Anomalies in the central nervous system;
- high cholesterol.
According to statistics, hypoxia of the heart muscle is most often diagnosed in men. In most cases, there is a lack of oxygen in the tissues of the left ventricle. Hypoxia of the right ventricle is diagnosed much less frequently.
Causes
- Cardiac ischemia. A common cause of cardiac hypoxia. An atherosclerotic plaque appears in the walls of the heart vessels, which partially or completely blocks the lumen of the arteries. The organ does not receive oxygen and nutrition, which leads to hypoxia.
- Thrombus. Formed when atherosclerotic plaques rupture. They block the artery and lead to sudden and severe ischemia of the muscle, threatening a heart attack. Less commonly, a blood clot enters the heart from other organs.
- Spasm of the arteries of the heart. Temporary contraction of the muscles in the wall of the vessel slightly reduces or blocks the flow of blood to the organ. This is a rarer cause of ischemia.
A person experiences unpleasant manifestations of hypoxia when:
- severe emotional stress;
- inadequate physical activity;
- cold ambient temperature.
I had to examine patients who complained of chest pain when going outside in winter, at a temperature of -20ºC.
It is important to know the possible causes of hypoxia in order to avoid the occurrence of severe heart pathologies.
Risk factors for the development of myocardial hypoxia
- Smoking. Nicotine damages the inner wall of blood vessels. A cholesterol plaque forms in the lumen, which leads to a slowdown in blood flow. This bad habit increases the risk of blood clots in the coronary vessels. Keep in mind that passive smoking also carries risks.
- Studies have shown an association between type 1 and type 2 diabetes and an increased risk of myocardial ischemia.
- Arterial hypertension. Increased pressure in blood vessels aggravates atherosclerosis. Hypertension is often inherited. If your parents or grandparents have had similar episodes, monitor your condition carefully and see a specialist regularly.
- High levels of cholesterol in the blood , which is the main component of atherosclerotic plaque. An increase in the “bad” fraction (low-density lipoproteins, LDL) occurs with inherited metabolic disorders or a diet high in cholesterol and saturated fat (fast food).
- High levels of triglycerides and other fats in the blood. This is a risk factor for the development of atherosclerosis of the heart vessels.
- Obesity. Leads to the development of arterial hypertension, increases the chance of developing diabetes, increases the level of “bad” cholesterol in the blood and leads to myocardial hypoxia.
- Waist circumference. More than 89 cm in women and 102 cm in men is a sign of an increased risk of heart disease.
- Lack of physical activity. The absence or small amount of this component of health has been proven to affect cholesterol and triglyceride levels in the blood. Aerobic exercises (fast walking, running, swimming) are recommended to reduce the risk of myocardial hypoxia and heart attack. Sports activity reduces arterial hypertension.
Additional preventative measures
The first and surest remedy is regular walking; fresh air has a beneficial effect on the body and will provide the necessary amount of oxygen. It is also important to constantly ventilate the living space.
Traditional medicine is also used to help; tinctures of woodlice or hawthorn, as well as birch sap (about one liter per day), are considered effective.
You definitely need to change your lifestyle: replace sedentary activity with active sports (jogging, race walking, cycling), get rid of bad habits (including passive smoking).
Fact! Calmness and emotional balance are the last, but very important stage in the treatment and prevention of cardiac hypoxia!
Kinds
- Ischemic hypoxia (cardiac) – caused by a decrease or disruption of blood flow in the arteries.
- Systemic hypoxia (cardiac) occurs when the body does not receive enough oxygen. For example, in case of obstruction of the respiratory tract or in high altitude conditions.
- Anemic hypoxia is an option when there is enough oxygen, but the ability of the blood to carry it is reduced. For example, with iron deficiency anemia. In such situations, no special treatment for hypoxia is required, in addition to correcting iron levels with the necessary drugs.
- Histotoxic hypoxia is an option when, with normal blood oxygen levels and the intact ability of hemoglobin to carry it, the functions of cardiac muscle cells are impaired and oxygen is not used. For example, in case of potassium cyanide poisoning.
Disease prevention
As a rule, any disease is easier to prevent. This also applies to hypoxia of the heart muscle. To prevent this from happening, it is important to follow a few simple recommendations:
- Take regular walks in the fresh air. If possible, go to the sea or mountains once a year.
- Customize your lifestyle. Play sports and avoid bad habits.
- You must follow a proper diet. This may help prevent the formation of cholesterol plaques.
- Taking folk remedies - herbal infusions and decoctions.
The human condition is the main thing that you need to pay special attention to. Lack of emotional stress, balanced work, timely treatment of diseases - all this helps to maintain health and significantly prolong life.
Parents of teenage boys are often interested in the question of whether they will join the army with such a diagnosis. It’s difficult to answer unequivocally. If the course of the disease is not life-threatening, the boy can be taken away. However, you will need to evaluate his overall health.
In severe forms, the boy, of course, will not be allowed to serve, since increased physical activity can only aggravate the situation.
Diagnostics
To identify myocardial hypoxia, the doctor will conduct an examination. First, he will collect anamnesis, then examine you.
After this, he will recommend the following diagnostic measures:
- Taking a cardiogram (ECG). Special suction cups are placed on the skin and the electrical activity of the heart is recorded. Some changes in the film, such as diffuse changes in the apical region of the myocardium, indicate disturbances in blood flow (such as left ventricular hypoxia).
- Echocardiogram (EchoCG). A special ultrasound machine is placed on the chest, and the doctor receives a video image of the heart. Using the method, areas of diffuse changes in the myocardium and disturbances in heart contractions are identified.
- Scintigraphy (nuclear scan). This is the most informative and expensive method for diagnosing cardiac hypoxia. A small amount of radioactive substance is injected intravenously. By assessing the degree of saturation of the myocardium with the isotope, we can draw a conclusion about the depth of damage.
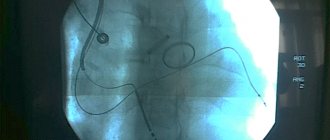
- Coronary angiography. A special dye contrast agent is injected into the blood vessels of the heart. Using an X-ray machine, a series of images (angiograms) are obtained, which analyze the blood flow in the arteries and evaluate the signs of myocardial hypoxia.
- CT scan of the heart. The presence of calcification is assessed - a reliable sign of arterial atherosclerosis. The condition of the vessels is also analyzed using CT angiography.
- Stress test. You walk on a treadmill at a fast pace or pedal an exercise bike, and machines record your breathing rate, heart rate and blood pressure levels. The heart begins to beat faster in response to physical activity and stressful conditions. As a result, it becomes possible to identify problems that would not normally be observed.
The problem of the influence of hypoxia on the heart muscle, the adaptation of the myocardium to its effects, as well as the consequences it causes, does not lose its relevance and continues to be the subject of research for many decades [1–8]. This is due to the fact that many diseases and conditions that arise both in therapeutic (chronic obstructive pulmonary disease, sleep apnea, coronary heart disease, etc.) and in pediatric practice (perinatal hypoxia, congenital heart defects, etc.) , are accompanied by a limitation of oxygen supply, which, on the one hand, can lead to the development of diseases, and on the other hand, increases the body’s resistance to subsequent effects of oxygen deficiency [1, 3, 9-15].
Of particular interest is the study of common pathological conditions of the perinatal period - intrauterine fetal hypoxia and asphyxia of the newborn [14-20]. Among their consequences, manifested by the reaction of internal organs, damage to the cardiovascular system takes 2nd place after kidney pathology and, according to foreign researchers, occurs in 25%, and according to domestic authors - in 40-70% of cases of oxygen deficiency [16 , 21]. It has been established that transient myocardial ischemia resulting from oxygen deficiency is associated with a temporary decrease or cessation of blood circulation in certain areas of the heart muscle, causing a decrease in its functional activity [17, 19]. The duration of such transient periods of acute myocardial ischemia can vary from 10 to 25 minutes per hour and depends on the severity of the hypoxia suffered [17].
It is generally accepted that the main cause of myocardial ischemia in newborns is a decrease in energy production in the myocardial cell due to perinatal “hypoxic injury” and relative coronary insufficiency, which is caused by the mismatch of the existing coronary blood flow with the functional needs of the heart, resulting from the high hemodynamic load on the ventricular myocardium during the period of postpartum adaptation blood circulation [16, 17]. The development of oxygen starvation leads to tissue hypoxia, caused by a violation of the mechanisms of oxygen utilization in heart cells, which, as is known, are very sensitive to oxygen deficiency due to their high functional load [22, 23].
Bioenergetics of the myocardium under physiological conditions. Features in newborns
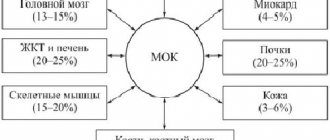
The heart muscle is the most oxygen-dependent organ of the human body: oxygen consumption by the myocardium exceeds the average level of its consumption by other tissues of the human body by an average of 25-36 times [22]. The myocardium, both fetal and adult, consumes approximately the same amount of oxygen per 1 g of tissue [22]. It is known that the human heart produces 65–104 cal/min at rest [24]. This energy production requires the delivery of 13-21 ml of oxygen every minute. The required amount of oxygen, along with a high coefficient of its extraction by the myocardium (up to 12-15 ml of O2 from 100 ml of blood), is delivered thanks to the intensive blood supply to the heart, which under conditions of muscle rest is 75-85 ml per 100 g/min (about 5% of the minute blood volume ) and with a load on the heart can increase approximately 3 times (up to 225-255 ml per 100 g/min) [22]. The ability of the heart to provide blood supply to body tissues adequately to their needs is determined by the level of myocardial blood flow and a complex of biochemical phenomena that begin from the moment oxidizable substrates enter the cardiac muscle cells and end with the interaction of a number of contractile proteins, which leads to contraction of myofibrils [24, 25].
The contractile function of the heart is steadily maintained throughout life [22, 26]. This occurs due to the close coupling of the contraction process with energy metabolism. The bioenergetics of the myocardium under physiological conditions is determined by the rate of oxidation of substrates (primarily lactic and pyruvic acids) in the tricarboxylic acid (Krebs) cycle, which ensures a yield of 36 mol of adenosine triphosphate (ATP) per 1 mol of glucose [24, 27]. However, this energy cycle is disrupted in conditions of oxygen deficiency, and lactic acid is not utilized. Another option for energy supply to the vital functions of the myocardium, implemented in the Embden-Meyerhof cycle with adequate oxygen delivery, plays only an auxiliary role, because During the oxidation of 1 mole of glucose, only 2 ATP molecules are synthesized [22]. There is another way - direct oxidation of glucose (pentose Warburg cycle), which is highly economical and can provide a sufficient amount of energy, due to the fact that about 117 ATP molecules are formed from one glucose molecule [22, 24].
The main energy substrates for the myocardium of adults and older children under conditions of aerobic metabolism are predominantly fatty acids (40-60%), the specific gravity of carbohydrates is only 35-45% [22]. The heart of the fetus and newborn child uses glucose and the main product of its metabolism, lactic acid, as the main source of energy [16]. The energetic metabolization of fatty acids by the myocardium, characteristic of older children and adults, is practically absent in them. This type of bioenergy is due to the fact that when metabolizing glucose, the efficiency of energy conversion is higher than that of fatty acids: per 1 mole of oxygen when using glucose, 14% more ATP is synthesized than when metabolizing fatty acids [16, 22]. In addition to these substrates, cardiomyocytes (CM) can also use other metabolites circulating in the vascular bed (ketone bodies, lactate, amino acids, etc.), due to which a stable level of cardiac muscle activity is maintained, unlike skeletal muscles, which can only be used by fatty muscles acids or glucose. It is noteworthy that amino acids cover only 5-7% of the total energy consumption of CM [22].
The ratio of myocardial consumption of various substrates depends on the intensity of mechanical work of the myocardium, the concentration of these substances and the oxygen content in arterial blood. The mechanical activity of the heart muscle is linearly related to the rate of oxygen absorption by the myocardium, which at rest is about 30 μl of O2 per 1 g of raw myocardial tissue every minute [28]. With an increase in the contractile activity of the heart muscle, O2 consumption also increases proportionally, which can increase tens of times and reach 300 μl/min per 1 g of raw tissue [29].
The rate of energy expenditure is closely related to the rate of ATP synthesis. To maintain the pumping function of the human heart throughout life, the body produces about 36 kg of ATP every day [22]. More than 90% of it is formed during oxidative phosphorylation in mitochondria, while the oxidation products of glucose and fatty acids burn in the cell, giving it heat and ATP [30]. From mitochondria, ATP enters the cytoplasm and is converted into creatine phosphate (CP). Its molecules are optimal for transport to structures that are energy consumers. Here CP is converted back into ATP. It is ATP and CP that represent the energy reserves of CM, which are directly used by them [24].
According to modern concepts [22], CM energy is spent on the following processes:
- contraction in myofibrils, where, under the influence of an increased concentration of Ca2+, actomyosin bonds are formed, ensuring contraction of the heart muscle;
- the work of the calcium pump in the sarcoplasmic reticulum: this structure is capable of releasing calcium ions that activate myofibrils and absorbing them back against the concentration gradient, which requires ATP energy, i.e. maintaining the calcium pump is an energy-dependent process;
- the work of a membrane sodium-potassium pump, transporting sodium ions out and potassium ions into the cell against a concentration gradient, which also requires energy costs;
— ensuring the functioning of special potassium channels (ATP-dependent potassium channels - KATP channels and Ca2+-dependent K+ channels), which, when attaching ATP molecules, close, which prevents the release of potassium ions from these channels;
— provision of synthetic processes.
All this energy is used in accordance with the total amount of ATPase activity located in the cellular organelles. Myofibrils, which have the highest total ATPase activity, use approximately 80% of the energy reserves, ion transport accounts for another 15%, and synthetic processes account for about 5% of the total energy used by the CM [22].
There are 3 phases in the energetics of the heart [22]:
The energy production phase includes the release of ionized oxygen - primarily from oxidizable carbohydrates, to a minimal extent from fatty acids and amino acids - in the main tricarboxylic acid cycle and its oxidation to water with the participation of electron transport catalysts [23];
The phase of accumulation and transport of energy is associated with its deposition in the form of ATP energy, the formation of CP and the transfer of a high-energy phosphate bond to adenosine diphosphate (ADP) of myofibrils;
The energy utilization phase is due to the transformation of energy generated from ATP dephosphorylation into actomyosin contraction and myofibril function. During cellular metabolism, ATP is broken down into ADP and adenosine monophosphate (AMP), which, in turn, are rephosphorylated into ATP under physiological conditions [22].
In this case, all high-energy phosphate compounds are in pairwise equilibrium with each other [24]. The molecular mechanisms underlying contraction are the result of a strictly determined interaction between the contractile muscle proteins actin and myosin, which form thin and thick filaments of myofibrils, respectively, and the regulatory proteins tropomyosin and troponin. In muscle, regulatory proteins are associated with actin. The calcium acceptor is a calcium-bound protein, troponin. When calcium binds to the latter, conformational changes in tropomyosin eliminate the steric blockade of actin centers, and when actin interacts with myosin, actin-myosin bridges are formed, the pulling force of which determines muscle contraction [24]. The phase of energy formation and accumulation takes place in mitochondria. The utilization phase (conjugation and contraction) is realized in the reticulum and myofibrils.
Glycolytic energy production plays an important role in maintaining the basic processes of myocardial cell homeostasis, which ensures the functioning of the reticulum calcium pump, the transport of macroergs to contractile proteins due to the activation of cytoplasmic creatine phosphokinase, the preparation of amino acids for involvement in the tricarboxylic acid cycle, and the support of the physiological duration of the CM action potential [28]. ATP, formed during glycolysis, plays an important role in energy supply for the physiological duration of the action potential of the CM membrane [24]. ATP deficiency significantly increases the risk of developing ventricular fibrillation, which was confirmed under experimental conditions. The synthesis of CP in the cytoplasm is associated with the glycolytic production of ATP [22, 28, 31].
Energy metabolism of hypoxically damaged myocardium
There are many definitions of the term "hypoxia". From the perspective of bioenergetics, hypoxia is considered as a violation of oxidative generation pathways in cells, i.e. a form of hypoergosis [4]. This definition was proposed by S.N. Efuni and V.A. Spektor to indicate the energy pathology of the cell [32].
It has been proven that hypoxia, which quickly disrupts the synthesis of ATP in mitochondria and is accompanied by a decrease in the contractile function of the heart, is the first and most important factor in the development of ischemia, which causes a number of profound disorders in the metabolism of cardiomyocytes [24, 27].
The development of hypoxia is based on an increased discrepancy between the supply of oxygen to cells and tissues and a sharp increase in the body’s need for it as a result of the intensification of aerobic metabolism at the system level, especially in newborns [33, 34]. To meet the needs of metabolism in conditions of insufficient oxygen supply to tissues, a chain of biochemical and physiological changes develops, the purpose of which is to ensure optimal functioning and, if possible, restoration of the body to its original level after the end of the period of oxygen deficiency [7, 35].
Under ischemic conditions, the cessation of oxygen delivery to the myocardium mobilizes free (about 0.07 ml of O2 per 100 g of tissue) and residual oxygen of the heart muscle, mainly associated with myoglobin, the content of which is about 0.5 g per 100 g of tissue [23]. Under physiological conditions, the function of myoglobin as an oxygen acceptor is to ensure the continuity of oxygen supply to the mitochondria during a sharp decrease in coronary blood flow during systole. However, this does not solve the problem, since this amount of oxygen is sufficient to provide energy for only 6-7 heartbeats [22]. During hypoxia, the oxygen reserve associated with hemoglobin and myoglobin is rapidly depleted, the level of oxidation substrates in tissues decreases, and enzyme activity decreases. As a result, the processes of oxidative phosphorylation in mitochondria, as well as the transport of ATP from mitochondria to places of its use, are disrupted [23, 24]. This leads to a decrease in the concentration of ATP and CP and the accumulation of metabolic products of macroergs. At the same time, the rate of decrease in the concentration of CP is significantly greater than that of ATP, which is due to its rapid consumption for the formation of ATP and impaired transport from mitochondria [22, 23]. It is typical that the rate of CP loss is faster than the rate of ATP degradation. It is interesting that under conditions of cardiac muscle ischemia, there is an increase in the amount of CP in the area bordering the ischemic area, which is compensatory in nature [36].
Despite the increase in glycolytic energy production, acute ischemia sharply slows down the rate of synthesis of macroergs, which leads to disruption of the energy supply of functions and homeostasis of ischemic bone marrow [22]. Simultaneously with the disruption of ATP synthesis in mitochondria and the accumulation of reduced forms of components of the respiratory chain in ischemic tissue, emergency compensatory energy supply mechanisms are mobilized, in particular, the glycolytic production of macroergs [37].
With prolonged and severe hypoxia, metabolism switches to the anaerobic pathway with an increase in the utilization of various substrates and the cessation of synthetic processes, as evidenced by the intensification of lipid peroxidation (LPO) [38-40]. An early transition from oxidative metabolism to anaerobic energy production is ensured by activation of phosphorylase and increased glucose transport in ischemic myocardial cells.
However, increased anaerobic glycolysis, which can be considered as a compensatory reaction aimed at replenishing energy costs under hypoxic conditions, is an ineffective mechanism, since it gives a very small energy output: only two ATP molecules are formed from one glucose molecule [24].
It is known that in the initial stage of ischemia, a universal primary reaction of mitochondria is observed—the effect of “soft uncoupling” [22]. If the oxygen tension in the heart muscle decreases below 3-5 mm Hg. not only the oxidation of tricarboxylic acid cycle substrates is inhibited, but also the associated phosphorylation, and its rate drops sharply. With prolonged ischemia, the ability of BM mitochondria to use NAD·H-dependent substrates decreases [22, 41]. The accumulation of NAD·H, lactate and protons leads to inhibition of glycolytic enzymes and disruption of the energy supply to the processes of maintaining homeostasis in ischemic BM. By the end of the 60th minute of ischemia, glycolysis is completely inhibited, the content of adenine nucleotides drops by 69%, and ATP by 94% [22]. In turn, the transport and use of ATP, which is produced in the Embden-Meyerhoff cycle due to inhibition of the activity of cytoplasmic isoforms of CP, blocks a drop in pH below 6.6 [23].
A decrease in the content of the oxidized form of nicotinamide coenzymes is the “Achilles heel” during hypoxia, because, performing the function of hydrogen carriers, NAD, NADP and their reduced forms participate in the processes of cellular respiration, which are primarily disrupted during oxygen deficiency [22, 42-45] . A change in the ratio of their oxidized and reduced forms, in turn, can serve as one of the reasons for disturbances in redox processes in the tricarboxylic acid cycle and associated oxidative phosphorylation, oxidation of fatty acids and other metabolic pathways involved in the body’s adaptation to hypoxia [24 ]. In addition to serving as a direct source of energy for most cellular metabolic processes, ATP also regulates the activity of many enzymes. A sufficient intracellular concentration of ATP is a necessary condition for ensuring cellular function and survival, but the ratio of ATP and other adenine nucleotides (“adenine energy charge” or “phosphorylation potential”) is no less important for metabolic regulation [2].
During hypoxia, the energy charge decreases because the phosphorylation of ADP to ATP is impaired [46–49]. This leads to an increase in the concentration of AMP, which is formed from ADP with the participation of adenylate kinase. Another consequence is a decrease in the total pool of adenine nucleotides due to stimulation of nucleotide catabolism. This occurs through the participation of AMP deaminase, an enzyme that is activated when the energy charge decreases. The products of this disrupted pathway are inosine monophosphate (IMP), inosine, hypoxanthine, xanthine, and uric acid. An alternative option for AMP catabolism is the primary dephosphorylation of adenosine to inosine. The significance of this pathway is that adenosine is a potent vasodilator and has been shown to play a role in the myocardial protective response to hypoxia [2, 50, 51]. Adenine nucleotides and IMP remain inside the cell, while purine nucleosides (adenosine, inosine) and derivatives (hypoxanthine, xanthine, uric acid) enter the extracellular space [35]. A small amount of AMP is dephosphorylated into adenosine, then from the myocyte enters the endothelial cell, where it is irreversibly metabolized to hypoxanthine, which is then converted to uric acid under the action of xanthine dehydrogenase and excreted by the kidneys [4].
Unlike adenine nucleotides, adenosine can leave the cell under normal conditions. Its molecule, as it were, carries out feedback, with the help of which the cell itself regulates its blood supply. If the ATP content decreases, then adenosine is formed in excess and, diffusing to the arterioles, increases capillary blood flow, as a result of which the BM receives a sufficient amount of oxygen [41, 47]. Under ischemic conditions, this molecule acts as a natural inhibitor of BM adrenoreceptors; cells become unresponsive to sympathetic stimulation, which undoubtedly preserves their energy, resulting in increased cell viability under unfavorable conditions [52].
Upon completion of hypoxia, the ratio of nucleotide concentrations - rapidly, and the sizes of their pools - more slowly, resumes. At this stage, adenosine and hypoxanthine can be utilized for nucleotide synthesis (“save pathway”) at lower energy costs than de novo purine synthesis [24].
As is known, when glycolysis is activated due to the accumulation of under-oxidized products, lactate is formed and the concentration of hydrogen ions increases [51, 53-55]. Under aerobic conditions, metabolic products are usually used in mitochondria, but when the function of these cellular organelles is turned off under ischemic conditions, under-oxidized products accumulate. Thus, the reaction of the BM medium shifts to the acidic side, which leads to the development of intracellular acidosis [56-59].
According to the literature, a decrease in pH by 0.5–1.7 after 30 minutes of ischemia causes a drop in the mechanical function of the heart by 30–100% [22]. It has been proven that severe acidosis (pH less than 6.6) is the initiating factor of cellular alteration. In the myocardium, the concentration of free calcium increases, and the presence of inorganic phosphate promotes its movement to mitochondria [24], activates the release of “myocardial” catecholamines, increases the activity of phospholipases, activates acid lysosomal proteases, induces the development of so-called peroxide stress (accumulation of H2O2, stimulation of lipid peroxidation ). This further increases the energy deficiency of the heart muscle, which leads to irreversible changes in the ischemic myocardium [42, 60-62].
As is known, acidosis suppresses the ATPase activity of myosin [56]. It causes disruption of Ca2+ uptake by the sarcoplasmic reticulum [22]. An increase in proton concentration worsens the interaction of Ca2+ with troponin and the process of its deposition in the sarcoplasmic reticulum [59]. At the same time, intracellular acidosis is accompanied by degradation of myosin due to the dissociation of its light chains and their diffusion into the blood [22]. An increase in the concentration of Ca2+ in contractile proteins leads to the fact that the detachment of myosin “heads” from actin centers becomes impossible, the process of diastolic relaxation is disrupted, and contracture characteristic of hypoxia develops [33]. The deterioration of myocardial contractility is also facilitated by the accumulation of phosphates in the cell, which is formed as a result of the uncontrolled breakdown of ATP to ADP and AMP. The accumulation of phosphates along with acidosis reduces the sensitivity of myofibrils to calcium ions.
Since oxygen is the electron acceptor at the final stage of the electron transport chain, reducing the oxygen content in mitochondria below the “critical oxygen pressure” (1-2 mmHg) leads to a slowdown in the rate of the entire process. As a result, ADP begins to accumulate because it is no longer converted into ATP. NADP stops being reoxidized, and the NAD/NADP ratio in mitochondria begins to decrease [2].
The previously existing idea of mitochondria as specialized organelles that exclusively control energy metabolism has now been supplemented by information about them as organelles containing factors that determine the fate of the cell [8, 63, 64]. It has been proven that mitochondria are responsible for the functioning and regulation of a large number of signaling pathways that provide not only mitochondrial biogenesis and cell proliferation, but also, conversely, programmed cell death by limiting redox reactions [8].
Currently, the mitochondrial ATP-sensitive potassium channel (KATP channel; ATP-sensitiveK+ channels) is the most studied of the mitochondrial factors that regulate the metabolic and functional activity of the cell. It has been established that exposure to chronic hypoxia leads to the activation of mitochondrial KATP channels in the myocardium [65], which are closed outside of hypoxia in the presence of a sufficient number of ATP molecules. If the number of ATP molecules decreases, then potassium channels open and potassium ions enter the extracellular environment. In this case, as is known, the loss of potassium ions leads to the fact that the CM loses the ability to excite.
It has been proven that moderate chronic hypoxia leads to an increase in the synthesis of the SUR2At protein (regulatory subunit of the KATP channel) and an increase in the density of these channels on the membranes of cardiomyocytes by a mechanism that does not depend on HIF-1α (hypoxia inducible factor 1 alpha) [66, 67]. The latter, as is known, is a subunit of the heterodimeric protein HIF-1, and, in contrast to its β-subunit, which is constantly expressed, the α-subunit is regulated by oxygen levels [68–70]. In a state of oxygen deficiency, the HIF-1α protein molecule is not hydroxylated, remains stable, and their accumulation occurs. Subsequently, these subunits (HIF-1α, HIF-1β) combine, and the resulting transcription protein HIF-1 in the cell nucleus binds to special DNA sequences in genes whose expression is induced by hypoxia [67, 68, 71].
Activation of KATP channels under conditions of chronic hypoxia leads to an increase in the stability of the pores that regulate mitochondrial permeability—mitochondrial transition pores. The opening of these pores provokes mitochondrial swelling, leads to the uncoupling of oxidative phosphorylation and the release of cytochrome C and the AIF protein (apoptosis-inducing factor) from mitochondria [72]. It is these substances that catalyze the conversion of inactive procaspase-9 into active caspase-9; the latter, in turn, activates the conversion of procaspase-3 to caspase-3, which ultimately leads to apoptosis [73].
The MRI pore, responsible for mitochondrial permeability, is modulated by another K+ channel: the Ca2+-dependent mitochondrial K+ channel (BKCa Big-conductance Ca2+-activated K+ channel) [74–77]. It has been established that the opening of these channels is accompanied by an increase in O2-* production.
As is known, cytochromes of the mitochondrial respiratory chain are oxygen-sensitive enzymes and the main sources of reactive oxygen species (ROS) in the cell [78, 79]. With the development of ischemia, the increase in ROS production by components of the electron transport chain increases like an avalanche [79, 80]. It is noteworthy that the highest ROS content is recorded in the myocardium during prolonged (more than 30 min) ischemia, characterized by a very low level of oxygen in the myocardial tissue [54, 79]. It is assumed that hypoxia is accompanied by an increase in the amount of reduced forms of respiratory chain carriers (NAD, coenzyme Q, etc.), which undergo autoxidation with the formation of ROS even at low oxygen concentrations [54].
It is difficult to underestimate the role of ROS in the development of myocardial ischemia [78–81]. It has been established that ROS are an important factor in ischemic myocardial damage [82–85] and arrhythmogenesis [86, 87].
An important role in preventing microcirculation disorders that develop during ischemia is played by the consistency of the NO synthase (NOS) - nitric oxide regulatory system [88-92]. In addition to the vasomotor effects of the nitric oxide radical (NO*), many of its intracellular functions have now been discovered. It has been established that NO* can competitively inhibit cytochrome oxidase, thereby participating in the regulation of the production of superoxide radical and hydrogen peroxide [55]. Nitric oxide is able to activate mitochondrial ATP-sensitive K+ channels, prevent the formation of MPT pores, and regulate mitochondrial biogenesis [93]. Nitric oxide activates soluble guanylate cyclase, which leads to the production of cGMP with subsequent activation of protein kinase C and its regulatory cascade [90].
Metabolic disorders that develop during ischemia can be characterized as the accumulation of hydrogen and phosphate ions, as well as an increased release of adenosine and potassium ions from the cell [89, 94-101]. These metabolic consequences of ischemia are actually aimed at protecting cellular metabolism, the cells' resources, from being rapidly used up. Essentially, during deep ischemia, cells enter a state of hibernation, the main purpose of which is to ensure cell viability while awaiting the possible restoration of blood flow. According to S. Rahimtoola, who gave the name to this phenomenon in 1984 [102]: “Myocardial hibernation is a subtle regulatory mechanism that adapts the functional activity of the myocardium to specific conditions of the blood supply, i.e. this is a defensive reaction of a suffering heart.” Rapid reperfusion, i.e. restoration of blood flow to the initial level in such a hibernating heart eliminates this metabolic protective mechanism and is able to restore myocardial contractile function [83]. In the figurative expression of L. Opie [29]: “The areas of the affected myocardium are as if in a sleeping state, but are able to wake up after restoration of blood flow.” Moreover, the degree and quality of its recovery depend primarily on the duration of the ischemic period. Restoration of energy metabolism after short-term ischemia (no more than 5 minutes) occurs completely. Moreover, BM acquire increased resistance to subsequent ischemic effects [3, 100, 101]. However, if blood flow is restored after a period of prolonged ischemia, then the contractile function of the heart can resume even in conditions of incomplete energy resource of the cell. The term “stunned” or reperfused myocardium is used in the literature for this condition [99, 101, 102]. The main problem of this condition is the continued damage to intracellular structures, which is largely due to the action of oxygen free radicals generated in large quantities during reperfusion of the damaged area, which returns oxygen to ischemic cells [3, 85].
The cause of a long-term decrease in myocardial contractility is considered to be reperfusion injury [3, 101]. The myocardium in the stage of reperfusion, even after short-term, reversible ischemia, reveals long-term inhibition of contractile function, which is a consequence of ultrastructural, metabolic, vascular, electrophysiological and other disorders [3, 84].
Based on this, one should distinguish between the concepts of “sleeping” / “hibernating” (hibernating myocardium) and “stunned” / “reperfusion” (miocardial stunning) myocardium. “Sleeping” myocardium occurs during chronic ischemia, “stunned” - during reperfusion, after intermittent ischemia (see table) .
The main differential diagnostic differences in the functional state of the myocardium during hibernation and “stunned”
With “sleeping” myocardium, blood flow is chronically reduced, with “stunned” it is normal or almost normal [14]. These are the most important differences. Left ventricular function is reduced in both cases. With a “sleeping” myocardium, the correspondence between changes in its function and disturbances in blood flow is maintained; with a “stunned” myocardium, this correspondence does not exist: the function of the left ventricle and contractility are impaired, ischemic dysfunction develops, at the same time, the blood flow remains normal or almost normal [63, 99]. With “stunned” myocardium, spontaneous gradual restoration of cardiac muscle function is observed. The deep inhibition of its function during the “sleeping” myocardium continues indefinitely, and if coronary blood flow does not improve, then the changes progress to the point of necrosis of the heart muscle. Left ventricular function is restored only when blood flow is normalized [14].
Thus, ischemia leads to pronounced disturbances in the processes of energy formation and damage to the cellular structures of the myocardium, especially after the resumption of oxygen supply, i.e. reperfusion. Switching to anaerobic metabolic pathways leads to a decrease in ATP reserves, because the latter is converted into ADP and AMP with a limited ability to rephosphorylate. With more severe or prolonged oxygen deficiency, maintaining sufficient levels of energy-rich phosphate compounds, especially ATP, becomes impossible. Most energy-dependent processes slow down or stop. This leads to deeper disturbances in cellular function, an inability to maintain ionic equilibrium, and ultimately to cell death.
The authors declare no conflict of interest.
Treatment
The principle of treating myocardial hypoxia is to improve blood flow in the heart muscle. The doctor selects drug therapy, surgery or an integrated approach depending on the severity of the condition.
The treatment regimen is individual, so self-medication is not recommended.
Medications
- "Aspirin". A drug that reduces the ability of blood to form blood clots. Reduces the risk of coronary artery blockage. It is used for the prevention of acute myocardial infarction, stroke, acute cerebrovascular accidents, thrombotic complications during surgical operations, and angina pectoris.
- Beta blockers. They relax the myocardium, slow the heartbeat and lower blood pressure, which facilitates blood access to the heart.
- Calcium channel blockers. They relax and dilate blood vessels, increase blood flow, and slow down the heart rate.
- Nitrates, for example, Nitroglycerin. Temporarily expand the lumen of blood vessels, improve blood flow in the arteries and veins of the heart.
- Cholesterol-lowering agents. These are statins, bile acid sequestrants, nicotinic acid compounds and fibrates.
Statins (Atorvastatin, Fluvastatin, Lovastatin and others) reduce the level of “bad” cholesterol by 25-60% and increase its “good” fraction. Most current guidelines recommend statins as the drug of choice for patients with any form of atherosclerosis, people 40–75 years of age with diabetes and high cholesterol, without diabetes, with a 10-year risk of developing atherosclerotic lesions >7.5%.
- Angiotensin-converting enzyme inhibitors (ACEIs). Relaxes blood vessels and reduces blood pressure. Most often, it is prescribed to patients with arterial hypertension or diabetes, in addition to myocardial hypoxia.
- "Ranolazin" ("Ranexa"). Relaxes the heart arteries and reduces angina.
Surgical methods
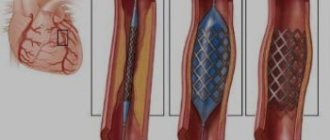
- Angioplasty and stenting. A catheter (a thin, long tube) is inserted into the narrowed area of the artery. A small balloon is inflated to expand the lumen. A wire tube (stent) is then inserted to prevent re-stenosis. The operation is not open, it is performed through vascular access.
- Coronary artery bypass surgery. The surgeon takes a vessel from another part of the body and creates an additional path for blood to bypass the blocked section of the bloodstream. The operation is indicated for significant vasoconstriction and severe hypoxia.
Therapeutic measures
If a disease such as myocardial hypoxia is diagnosed, it is necessary to select appropriate treatment. Only an experienced doctor can handle such a task. All actions should be aimed at eliminating the root cause that provoked the development of the pathology.
- Saturate the air with oxygen, connect in serious condition to an artificial respiration apparatus;
- in case of anemia, the patient is given iron and blood transfusions;
- administration of an antidote when toxic poisoning is detected;
- removal of toxins and normalization of the acid-base environment;
- therapy of pulmonary pathologies;
- restoration of blood circulation and blood viscosity.
Drug treatment
In order to increase the resistance of the heart muscle to oxygen starvation, drugs from the group of antihypoxants are used. They are conventionally divided into three types:
- direct;
- indirect;
- mixed.
Drugs of the first type help stimulate energy processes in the tissues of the heart. This is due to the fact that their action:
- Removes accumulated acids and waste;
- relieves ischemia;
- protects coronary vessels;
- restores connection with the central region of the brain.
Such drugs include:
- Neotone;
- Cytomac;
- Mildronate;
- Betimil;
- Actovegin;
- Piracetam.
They can be used both in the hospital and on an outpatient basis. Injections are usually prescribed with a transition after some time to the tablet form.
Indirect medications help reduce oxygen consumption by the heart. At the same time, all metabolic processes are reduced. Similar properties are noted in:
- sleeping pills and sedatives;
- drugs used for pain relief;
- some calcium channel blockers.
Thanks to these drugs, the patient can survive a difficult period of illness.
The third group includes vitamins (E, A, B, C) and microelements (magnesium, selenium and others). They are prescribed for chronic hypoxia.
Traditional medicine
Treatment with folk remedies gives positive results only at the initial stage of the disease.
- In order to normalize the functioning of the circulatory system, it is recommended to take hawthorn tincture. You can buy it at the pharmacy or prepare it yourself.
- In order to eliminate tissue hypoxia, a decoction of rose hips or lingonberries is used. It promotes rapid tissue regeneration due to its strong antihypoxic properties.
- Birch sap is no less effective. The daily dose should be 500 ml.
Nutrition
Particular attention should be paid to diet in the presence of oxygen starvation. Be sure to follow a diet that includes foods such as pomegranate, pork liver, green apples, and various grains. They help increase hemoglobin levels in the blood.
Doctor's advice
To reduce the likelihood of developing heart disease, doctors recommend lifestyle changes:
- Stop smoking. Discuss discontinuation strategies with your doctor and avoid a passive process.
- Improve comorbid conditions (diabetes and hypertension). These diseases increase the risk of myocardial hypoxia.
- Switch to a healthy diet. Reduce saturated fat and increase whole grains, fruits and vegetables in your diet. Control your blood cholesterol levels.
- Doing physical exercise. Consult with a specialist about safe aerobic and strength training exercises to improve blood flow to the heart.
- Maintain normal weight. If you are overweight, ask your doctor about suitable weight loss options.
- Reduce stress. Use special techniques for muscle relaxation and deep breathing.
Visit your doctor regularly for physical examinations. The main risk factors for myocardial hypoxia (high cholesterol, blood pressure and diabetes) are asymptomatic in the initial stages. The earlier pathology is detected and treatment is prescribed, the higher life expectancy and better heart health.
What is the threat of the condition?
Myocardial hypoxia increases the risk of life-threatening complications.
- Myocardial infarction (heart attack). When the blood flow in the vessels is completely blocked, the lack of blood and oxygen causes an attack, and then the death of part of the muscle. This complication is very serious and can be fatal.
See your doctor immediately if you experience symptoms of prolonged or severe chest pain! Call an ambulance. The doctor will diagnose and begin treatment as early as possible.
- Heart rhythm disturbances (arrhythmias). They lead to a weakening of contractile function and threaten life, as they increase the risk of stroke.
- Heart failure. During a heart attack, hypoxia of the left ventricular myocardium can damage the heart muscle and impair its ability to pump blood throughout the body. In this case, the organ cannot cope with the load. Swelling and shortness of breath occur, and the risk of death increases.
Acute form
Along with chronic, there is an acute form of heart failure. The reasons for its occurrence are:
- myocardial infarction;
- major stroke;
- structural defects of the heart, congenital and acquired;
- hypertension.
A distinctive feature of acute cardiovascular failure is the suddenness of the attack and the absence of the listed stages of development. The person's condition quickly deteriorates and death is possible within a few minutes. The occurrence of pathology is indicated by a sharp deterioration in condition, suffocation, severe cough with foam or red sputum, blue discoloration of the skin, and cold sweat.
In such cases, first aid is to call an ambulance. Then the victim should be taken out into the open air, the collar and other tight clothing should be unbuttoned. The best pose is considered to be sitting, with legs down. A nitroglycerin tablet should be given under the tongue. Repeat the dose every 10 minutes until the ambulance arrives.
Important! In acute heart failure, you should not take a supine position.
conclusions
Myocardial hypoxia is a leading cause of mortality worldwide. It occurs in young and old people, men, women, and people with various diseases. It is especially often provoked by coronary heart disease. Hypoxia damages the muscle and disrupts the function of pumping blood. A terrible consequence of a sudden and severe blockade of the coronary arteries is a heart attack. Other complications: serious rhythm disturbances, arrhythmias, and heart failure.
Symptoms and treatment of myocardial hypoxia are what every person who cares about their health should know. The most striking symptom is angina pectoris, severe chest pain. It feels dull, squeezing, heavy. The pain radiates to the left arm, shoulder blade, back, neck, lower jaw. The pain appears with severe stress, physical activity, and lasts 1-5 minutes.
Modern doctors have diagnostic and treatment methods with proven effectiveness in their arsenal. The goal of therapy is to restore blood flow to the heart. Medicinal and surgical methods are used: angioplasty and stenting of coronary arteries, bypass surgery.
Clinical case
In practice, there are cases when patients with moderate myocardial hypoxia do not have obvious symptoms.
Doctors call this option “silent ischemia.” But more often, patients come with complaints of dull, squeezing, severe pain in the chest, radiating to the left arm, shoulder blade, jaw, neck, or back. This is angina - which occurs when there is insufficient blood flow to the heart. Characteristic feature: when the doctor asks to describe the nature of the sensations, the person brings a clenched fist to his chest. It was precisely this symptomatology that was called “heart toad” in the old literature.
The pain often appears during physical activity and stress and lasts 1-5 minutes.
Development of the disease and the main signs of its manifestation
For this pathology, several stages of development have been identified, and these are:
- light;
- moderate;
- heavy;
- critical (deadly).
For each stage of myocardial hypoxia, symptoms and treatment are different.
The very first sign is an increased heart rate (called tachycardia). This occurs due to the fact that the heart tries to normalize the supply of oxygen to the organs. Then contractility begins to decrease. As a result, arrhythmia of the entire myocardium occurs, which can lead to ventricular fibrillation.
Characteristic symptoms for moderate myocardial hypoxia are a decrease in the body's performance, general fatigue, drowsiness, irritability, and increased sweating.
The next symptom of myocardial hypoxia is a surge in pressure: first it increases, and then there is a sharp decrease. Such a difference leads to collapse not only of the heart, but also of the respiratory system. In this case, shortness of breath and heavy breathing appear, followed by cyanosis (the appearance of a bluish appearance of the body). As a result, the body consumes less oxygen from the environment, breathing becomes less frequent and may stop.
At a critical stage, all of the listed symptoms may not appear; the attack occurs at lightning speed and leads to respiratory and cardiac arrest. This stage is the most dangerous.
We should also not forget that myocardial hypoxia can occur in two forms: acute (in which all the signs of the disease appear within a few hours and can result in death) and chronic (in which the development of the disease lasts several years).



![Rice. 1. Functioning of the glymphatic system of the brain (according to [11]) 1. Glymphatic system functioning (according to [11])](https://expert35.ru/wp-content/uploads/ris-1-funkcionirovanie-glimfaticheskoj-sistemy-mozga-po-11-fig-1-330x140.jpg)