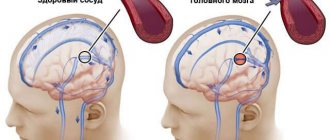
Signs and symptoms of cerebral ischemia in newborns
The disease manifests itself with obvious symptoms that attract attention.
- The child is easily excitable, cries for no reason, sleeps poorly, shudders, and has tremors.
- Muscle tone is reduced, the baby moves little, has difficulty sucking and swallowing.
- The fontanel is enlarged, intracranial pressure is increased due to the fact that fluid accumulates in the brain.
- Convulsions, twitching of the limbs and head, as well as comatose states with loss of coordination of movements and consciousness occur.
- The newborn's skin takes on a marbled hue.
- The functioning of the gastrointestinal tract is disrupted - bloating, constipation, and diarrhea are observed.
Cerebral ischemia in prematurity and in full-term infants
At term and at prematurity, the nature of brain damage is different. If a baby is not carried to term for 9 months, he or she is at risk of periventricular leukomalacia (PVL). This means that the white matter of the brain, located near the ventricles, dies and cysts form there.
PVL causes dementia and cerebral palsy in those born before the 31st week of gestation.
And for those who spent the entire period in the mother’s belly, damage mainly occurs to the cerebral cortex, that is, to the gray matter. The consequences for the child will depend on where the necrotic zone is located and how large it is. In acute and severe asphyxia (suffocation), damage to the brain stem is likely. Therefore, the child will have problems with heartbeat and breathing, which in some cases causes death, that is, death.
How does cerebral ischemia occur?
In 70% of cases, ischemia occurs in the fetus in the womb and is associated with the formation of a blood clot in one of the vessels supplying the brain, or with insufficient development of the vessel. Most often, the disease is diagnosed in premature babies whose vascular system is not yet fully formed.
As a result, an insufficient amount of blood enters the vital organ, and with it, oxygen. Delay in providing medical care leads to damage to larger areas of the brain, cerebral hemorrhage and other serious consequences.
Development mechanism
Violation of the metabolism of brain cells during ischemia leads to irreversible processes of destruction of nerve structures and disruption of their activity. As a rule, the mechanism of formation of the pathological process is based on a rapid decrease in the level of adenosine triphosphoric acid, which is the main energy source in the body.
Impaired cerebral circulation creates favorable conditions for the formation of ischemia
For the full functioning of neurons, a balance is required between the concentration of intracellular and extracellular ions penetrating through the membranes. Long-term oxygen deficiency creates an imbalance of transmembrane components of the brain, which subsequently dramatically changes the concentration of potassium and sodium. This leads to a decrease in membrane potentials. In parallel with these processes, there is an increase in calcium levels, which allows the release of the neurotransmitter glutamate, which overstimulates brain receptors, thereby promoting structural and functional changes in the brain.
The body's first response to perinatal oxygen deficiency is to increase blood supply to the main organs. Subsequent arterial hypotension leads to a throughput decrease in cerebral blood flow.
Pathological changes in the brain in a newborn
Thus, the following stages are distinguished in the mechanism of development of pathology:
- Intrauterine oxygen deficiency.
- Decreased oxygen supply to the brain and increased carbon dioxide concentration.
- Increased acid-base environment in the fetus.
- Swelling of intracellular components.
- Increase in brain tissue volume.
- Local decrease in cerebral circulation.
- General cerebral edema.
- Increased level of cranial pressure.
- General blood flow disorders.
- Death of brain cells.
The mechanism of ischemia formation depends on the date of birth of the child, which is associated with age-related characteristics of the structure of the brain.
Causes
In the vast majority of cases, the causes of cerebral ischemia in newborns are various disorders of gestation in recent weeks, as well as non-standard situations during childbirth.
- Detachment of the placenta or disruption of blood flow in it.
- Compression of the umbilical cord, suffocation of the fetus.
- Congenital heart defects.
- Circulatory problems.
- Intrauterine hypoxia.
- Infection during childbirth.
- Openness of the ductus arteriosus.
- Acute placental insufficiency.
Risk factors
Various vascular and neurological pathologies, problems with blood pressure (especially hereditary) in the mother should alert the doctor who is managing the pregnancy. Also, risk factors for cerebral ischemia in a child are:
- mother's age is more than 35 years;
- endocrine diseases;
- premature, prolonged labor;
- multiple pregnancy;
- late toxicosis;
- failure of the mother to follow a healthy lifestyle;
- exacerbation of chronic or acute diseases in the mother during pregnancy.
Diagnostics
The disease is usually diagnosed within the first few hours.
The presence of pathology is indicated by deviations in checking reflexes and a general blood test . Typically, the analysis shows an increased level of carbon dioxide in the body.
If obvious symptoms of a serious illness are detected magnetic resonance imaging , as well as electroencephalography , which reveals hidden convulsions and other abnormalities in the functioning of the brain.
Diagnosis of myocardial infarction in children
Myocardial infarction is an acute disease caused by the occurrence of one or more foci of ischemic necrosis in the heart muscle due to absolute or relative insufficiency of coronary blood flow. Most pediatricians and cardiologists believe that myocardial infarction in childhood belongs to the section of casuistry. Atherosclerosis of the coronary arteries, which is the main cause of myocardial infarction in adults, practically does not occur in children, with the exception of cases of familial hyperlipidemia [3, 6]. That is why many pediatricians are not wary when making this diagnosis. It is interesting to note that the US National Library of Medicine specifically records every case of myocardial infarction in children described in the world medical literature [5].
The incidence of myocardial infarction in children is unknown. However, this disease is much more common than is commonly believed. It is known, for example, that with congenital heart defects, even in the absence of structural abnormalities of the coronary vessels at autopsy, infarct areas in the myocardium were detected in 75% of cases, and half of them could be diagnosed clinically [4].
If in adults the main cause of myocardial infarction is atherosclerotic damage to the coronary arteries, then in children this etiological factor ranks last in terms of prevalence. The most common causes of myocardial infarction are inflammatory diseases of the coronary arteries - coronaritis and anomalies of the coronary arteries. Let us list the main causes of myocardial infarction in children.
- Coronaritis, including: non-rheumatic carditis, infectious diseases, Kawasaki disease, Takayasu disease, systemic lupus erythematosus, periarteritis nodosa, nonspecific aortoarteritis.
- Anomalies of the coronary arteries, including: the origin of the left coronary artery from the pulmonary artery (Bland-White-Garland syndrome), anomalies in the number of coronary arteries, other anomalies of the coronary arteries.
- Trauma to the heart and coronary arteries.
- Pheochromocytoma.
- Congenital heart disease (supravalvular aortic stenosis).
- Hypertrophic cardiomyopathy.
- Heart tumor.
- Infectious endocarditis.
Currently, myocardial infarction with a Q-wave (transmural myocardial infarction) and myocardial infarction without a Q-wave (fine-focal, subendocardial, intramural) are distinguished. In the first case, a pathological Q wave or QS complex is formed on the ECG; in the second, changes concern only the T wave and the ST segment.
The diagnosis of myocardial infarction also indicates the features of its course (primary, repeated, recurrent) and complications.
According to localization, they are distinguished: anterior (apical, lateral, septal, widespread anterior), inferior (diaphragmatic), posterior and inferobasal. These locations refer to the left ventricle, which is most often affected by this disease. Right ventricular infarction is extremely rare.
Clinic
The clinical manifestations of myocardial infarction of any etiology in children are similar: these are attacks of sudden anxiety in young children and a typical anginal status in older ones. Much less often, a heart attack occurs without pain. When examined in children, as a rule, pale skin, cyanosis, cold extremities, sweating, tachypnea or dyspnea, and arterial hypotension are noted. Some children may experience signs of gastrointestinal dysfunction that are of reflex origin - abdominal pain, nausea, vomiting, diarrhea. Signs of circulatory failure develop mainly in the pulmonary circulation (tachycardia, shortness of breath, cough). Somewhat less often, patients exhibit liver enlargement, less often - swelling of the legs, which indicates the addition of right ventricular failure.
Some children may develop cardiogenic shock (cold gray-pale skin covered with sticky sweat, oligoanuria, thready pulse, decreased pulse pressure less than 20-30 mmHg, decreased systolic pressure). The decrease in coronary blood flow that occurs during cardiogenic shock aggravates the decrease in the pumping function of the heart and the course of cardiogenic shock, the development of pulmonary edema - the main causes of death in myocardial infarction.
The course of myocardial infarction can be complicated by the occurrence of arrhythmias (extrasystole, atrial fibrillation, paroxysmal tachycardia, ventricular fibrillation), thromboembolism, the development of acute and the formation of chronic cardiac aneurysm.
Diagnostic criteria
The diagnosis of myocardial infarction is confirmed using instrumental and laboratory research methods.
It is known that electrocardiography is of greatest importance for diagnosing ischemia [5]. An ECG in the ischemic stage shows a rise in the ST segment in the so-called “straight” leads (in these leads a pathological Q wave or QS complex will subsequently form) and a reciprocal decrease in the ST segment in leads in which there will be no change in the QRS complex. In the acute phase, in the “direct” leads during transmural myocardial infarction, the R wave sharply decreases or completely disappears and the QS complex is formed. With less depth of necrosis of the myocardial wall, a pathological Q wave appears in the “direct leads” (equal in amplitude to 1/3 of the R wave or more, lasting 0.04 s or more). Subsequently, the ST segment returns to the isoline and a negative “coronary” T wave is formed in the “straight” leads (Fig. 1).
| Figure 1. ECG of a patient with transmural myocardial infarction of the inferior wall of the left ventricle and reciprocal changes in the precordial leads |
In case of subendocardial infarction, ECG changes are limited to ST segment depression and T inversion in the “straight” leads (Fig. 2).
When necrosis is localized in the area of the anterior wall of the left ventricle, changes on the ECG are characteristic in leads I, aVL, V1-6: for infarction of the lateral wall - in leads I, aVL, V5,6; if the septal area is affected, changes are detected in leads V1,2( 3), with a heart attack in the region of the apex of the heart - in leads V3,4. Infarction of the inferior wall is characterized by changes in leads II, III, aVF.
| Figure 2. ECG (chest leads) for anterior myocardial infarction without a Q wave |
A sign that suggests the presence of an aneurysm is the so-called “frozen” ECG: persistence of ST segment elevation in combination with the QS complex in the “straight” leads, with a possible T wave.
Resorption-necrotic syndrome during myocardial infarction is confirmed by the results of general clinical and biochemical blood tests: leukocytosis with a shift in the leukocyte formula to the left, an increase in ESR from the third to fifth day, an increase in the blood activity of creatine phosphokinase (CPK) and its MB-fraction, aminotransferases (especially aspartic and to a lesser extent alanine) and lactate dehydrogenase (general) and its first and second isoenzymes. Assessing the state of the hemostasis and fibrinolysis system allows us to identify hypercoagulable changes.
In recent years, new markers have been widely used: troponin T, troponin I and myoglobin. Troponin is a protein complex that regulates muscle contraction and consists of 3 subunits - troponin T, troponin C and troponin I. In the early 90s. Immunological methods have been developed that make it possible, using monoclonal antibodies, to distinguish troponins T and I of cardiomyocytes and other striated muscle fibers. It is believed that troponins T and I are the most informative and sensitive markers of cardiac muscle necrosis. Their level increases in the blood within 2-3 hours after myocardial infarction, can increase 300–400 times compared to normal and remains elevated for 10–14 days. Unfortunately, these methods are still very rarely used in the diagnosis of myocardial infarction in children.
The diagnosis of myocardial infarction is confirmed by echocardiography. Diagnostic criteria are the presence of zones of akinesia (area of necrosis), hypokinesia, asynchrony of contractions of individual segments of the left ventricle in the area of ischemic damage. In the future, some patients may develop a left ventricular aneurysm. In areas of undamaged segments, phenomena of dyskinesia or hyperkinesia of a compensatory nature are determined. In cases where it is not possible to clearly visualize the initial sites of origin of the left coronary artery, one can think of an anomaly of the coronary arteries. Chest X-ray is not very informative for diagnosing myocardial infarction. Cardiomegaly indicates an underlying disease (carditis, congenital heart disease, anomalous origin of the coronary arteries) and may also be associated with a cardiac aneurysm. In patients with left ventricular failure, an increase in the vascular pattern is observed.
It is known that coronary angiography and ventriculography provide the most accurate diagnosis of damage to the coronary arteries [2]. These studies make it possible to clearly establish the location, nature and extent of damage to the coronary arteries, to identify a decrease in myocardial contractility, and sometimes a left ventricular aneurysm. Unfortunately, they are underutilized in pediatric cardiology.
Recently, myocardial scintigraphy or myocardial positron emission tomography has been used to diagnose myocardial infarction. These methods make it possible to non-invasively assess myocardial perfusion, the volume and localization of destructive changes, and identify metabolic disorders.
Inflammatory diseases of the coronary vessels
One of the most common causes of myocardial infarction in children is inflammatory diseases of the coronary arteries - coronaritis. The true prevalence of coronaritis due to the complexity of diagnosis is not known; in most cases, this diagnosis is made at autopsy. The inflammatory process often involves three vessel membranes simultaneously [1]. Coronaritis often accompanies carditis and can lead to the development of myocardial infarction [4]. Thus, according to our data, in 6 young children, a clinical picture of a typical myocardial infarction developed against the background of acute carditis.
Acute coronaritis can occur with various infectious diseases (influenza, scarlet fever, typhus, etc.). We observed a boy S., 6 months old, with a generalized form of salmonella infection, who after 2 weeks. From the onset of the disease, transmural myocardial infarction of the left ventricle was observed, followed by the development of a left ventricular aneurysm. Figure 3 shows an electrocardiogram of patient S., 6 months old, with transmural myocardial infarction, the cause of which was coronaritis that arose against the background of a generalized Salmonella infection.
| Figure 3. ECG of patient S., 6 months |
Often the cause of myocardial infarction can be Kawasaki disease. The lack of statistical data regarding this disease in Russia is due to the lack of awareness among pediatricians. Kawasaki disease is included in ICD-10 under the name “mucocutaneous lymphonodular syndrome” (code 178.010). Currently, Kawasaki disease is considered as a vasculitis of unknown etiology with a fever syndrome and predominant damage to the coronary arteries, which occurs more often in young children [2, 7].
The main criteria for diagnosing Kawasaki disease are fever over 38°C or higher for 5 days or more in combination with at least 4 of the 5 symptoms listed below:
- polymorphic exanthema;
- damage to the mucous membranes of the oral cavity (diffuse erythema of the oral mucosa, catarrhal tonsillitis and/or pharyngitis, “strawberry” tongue, dryness and cracked lips);
- bilateral catarrhal conjunctivitis;
- acute non-purulent cervical lymphadenitis;
- changes in the skin of the extremities (hyperemia and/or swelling of the palms and feet, peeling of the skin of the extremities).
The listed symptoms are observed in the first 2–4 weeks. diseases. Heart damage can be of the type of myocarditis and/or coronaritis with the development of multiple aneurysms and occlusions of the coronary arteries, which can subsequently lead to myocardial infarction. We observed 3 children with Kawasaki disease, coronary artery disease, and myocardial infarction.
The causes of coronaritis can be nonspecific aortoarteritis, periarteritis nodosa, infective endocarditis, hemorrhagic vasculitis.
Nonspecific aortoarteritis (Takayasu's disease, pulseless disease, multiple obliterating panarteritis) is characterized by inflammatory and destructive changes in the wall of the aortic arch and its branches, accompanied by their stenosis and ischemia of the blood supplying organs. In this case, damage to the coronary arteries is often noted. At the onset of the disease, weakness, weight loss, dizziness, pain in the chest and limbs, anemia, fever, pericarditis, iridocyclitis, and edema occur. In the future, complaints of numbness of the extremities may appear, and in some cases neurological symptoms are added. The examination reveals the absence of a pulse, most often in the area of the radial, ulnar and carotid arteries. Pressure asymmetry is characteristic. Auscultation of the carotid, subclavian arteries and abdominal aorta helps diagnose arteritis. Persistent pain in the heart, ischemic and cicatricial changes on the ECG help to suspect concomitant coronary artery disease.
In the pathogenesis of myocardial infarction in Takayasu's disease, along with coronaritis and subsequent stenosis of the left or right coronary arteries, arterial hypertension, as well as relative coronary insufficiency due to myocardial hypertrophy, are important.
We observed 2 cases of myocardial infarction in children with aortoarteritis. An 11-year-old boy was sent to the hospital with suspected pheochromocytoma. The examination did not confirm the diagnosis. Nonspecific aortoarteritis was detected with damage to the aortic arch, occlusion of the celiac trunk, left subclavian and both renal arteries. A transaortic endarterectomy was performed from the aorta (supra-, inter- and infrarenal sections), celiac trunk, superior mesenteric and both renal arteries, and plastic surgery of the aorta with a synthetic patch. After 3 weeks After the operation, complaints of “squeezing” pain in the heart appeared, the child was again hospitalized. Death occurred from extensive myocardial infarction of the posterolateral wall of the left ventricle, resulting from damage to the coronary arteries.
With aortoarteritis, the valvular apparatus of the heart may also be involved in the process. Thus, a 10-year-old girl had aortic regurgitation, which arose as a result of inflammation of the aorta and aortic valve ring, damage to the mitral valve, the phenomenon of myocarditis with subsequent transformation into dilated cardiomyopathy, with signs of circulatory failure of degree IIB. Myocardial infarction occurred against the background of malignant arterial hypertension caused by stenotic and thrombotic occlusion of the carotid and renal arteries.
Damage to the coronary arteries can occur with diffuse connective tissue diseases. We observed a girl S., 15.5 years old, with systemic lupus erythematosus (sick for 7 years). The patient experienced pressing pain in the heart area, which the clinic doctor explained as osteochondrosis. And only 2 days later an ECG was done, which revealed changes indicating subendocardial myocardial infarction of the lateral wall and apex of the left ventricle. The examination excluded the presence of antiphospholipid syndrome as a possible cause of myocardial infarction in a patient with systemic lupus erythematosus. In all likelihood, myocardial infarction in this case was associated with chronic inflammatory changes in small or large coronary vessels, which could lead to gradual narrowing of the lumen and thrombosis. It should be noted that a year after myocardial infarction, complete normalization of the ECG pattern was noted.
Anomalies of the coronary arteries
Another most common cause of myocardial infarction in children is congenital anomalies of the coronary arteries. They can occur in isolation and in combination with congenital heart defects (stenosis and coarctation of the aorta, tetralogy of Fallot, etc.). Frequently occurring (0.3% of the total number of unselected autopsies) anomalies of the coronary arteries are represented by an unusual number of vessels, their mouths, and the location of the main trunks. An explanation for the high frequency of congenital anomalies can be found in the characteristics of the embryonic development of the coronary system. Differences in the development of coronary arteries in comparison with vascular formations in any other organs are the fusion of proximal (from the aortopulmonary trunk) and distal (secondary and tertiary vessels from myocardial sinusoids) arteries, as well as the dependence of vessel growth on the growth of the myocardium itself. The occurrence of myocardial infarction with congenital anomalies of the coronary arteries is caused by: direct deficiency of myocardial perfusion; the phenomenon of “stealing” (steal phenomenon); functioning of the coronary artery as a venous drainage (coronary fistula).
The most common congenital pathology of the coronary vessels is the anomalous origin of the left coronary artery from the pulmonary artery (ALCA from PA) - Bland-White-Garland syndrome. The frequency of this syndrome, according to the literature, is 0.25–0.5% of all congenital heart defects [1, 4]. According to one of the hypotheses, this pathology is based on the incorrect formation of one of the coronary arteries in that part of the trunk from which the pulmonary artery is subsequently formed; the division of the main trunk is not disturbed. We observed 7 patients aged from 2 months. up to 6 years of age, the cause of myocardial infarction in whom was anomalies of the coronary arteries, of which 5 children had an anomalous origin of the left coronary artery from the pulmonary artery - Bland-White-Garland syndrome.
Issues of hemodynamics in AOLCA from PA still remain controversial. It was previously thought that the only factor causing myocardial ischemia was the decrease in pulmonary artery pressure after birth, leading to a drop in perfusion pressure in the anomalous left coronary artery. Using coronary angiography, it was possible to show that blood into the left coronary artery does not come from the pulmonary artery, but through intercoronary anastomoses from the right coronary artery arising from the aorta, i.e., a discharge occurs from the area of high pressure (aorta, right coronary artery) to the area lower pressure (left coronary artery, pulmonary artery). In this regard, the survival of patients with this pathology determines the collateral blood flow in the myocardium at the time of birth and thereafter. It should be noted that well-developed intercoronary anastomoses are not always able to prevent myocardial ischemia due to low perfusion pressure as a result of blood flow through collaterals from the right to the left coronary artery and further to the pulmonary artery (coronary steal syndrome). With severe “steal syndrome,” the subendocardial blood flow is especially affected. This is one of the causes of endomyocardial fibroelastosis in this disease.
According to clinical and instrumental indicators and prognosis, there are 2 types of Bland-White-Garland syndrome: “infantile”, with poorly developed collaterals of the coronary arteries, and “adult”, in which there is a large number of intercoronary anastomoses that ensure long-term survival.
The first clinical manifestations in most patients can be noted already in the first 3 months. life, less often in the second half of the year. All the children we observed with AOLCA from LA had attacks of sudden anxiety, shortness of breath, sweating, threadlike pulse, and a pained expression on their face. Such attacks are called “angina pectoris” in the literature due to their similarity with the clinical picture of angina pectoris in adults [1]. Between attacks, the children looked calm, but shortness of breath persisted. Children were lagging behind in physical development. On examination, a left-sided cardiac hump was noted. The borders of the heart were expanded to the left, the apical impulse was diffuse, weakened, displaced into the sixth-seventh intercostal space. Heart sounds were muffled, a murmur of mitral valve insufficiency was heard, the cause of which was most likely associated with ischemia or infarction of the papillary muscles, dilatation of the left ventricular cavity, and deformation of the mitral valve leaflets.
An important place in the diagnosis of AOLCA from PA is given to the electrocardiography method. As a rule, the ECG shows a deviation of the electrical axis of the heart to the left due to blockade of the anterior left branch of the His bundle and characteristic electrocardiographic changes are revealed: a deep, widened Q wave in leads I, aVL, V5-6, maximally in aVL (Fig. 4). In the stage of decompensation, changes are often combined with a rise in the S-T segment above the isoline by 3-6 mm and a decrease in the amplitude of the R wave, which corresponds to the picture of an acute infarction. A “dip” in the amplitude of the R wave in leads V3 and V4 (the morphology of the ventricular complex becomes rS, QS, Qr), indicating a previous infarction, has diagnostic significance in case of AOLCA from PA.
| Figure 4. ECG of child G., 3 months, with AOLCA from PA and apical-lateral myocardial infarction |
On a chest x-ray in children with AOLCA from PA, cardiomegaly is noted, mainly due to the left parts. Indirect evidence of anomalous origin of the coronary arteries is the inability to clearly visualize the initial sections of the left coronary artery during echocardiography [2].
The diagnosis is confirmed using selective coronary angiography (a contrast agent is injected through the dilated trunk of the right coronary artery, while retrograde filling of the left coronary artery system through intercoronary anastomoses is visible, followed by contrasting of the pulmonary artery).
The prognosis for AOLCA from LA without surgery is in most cases unfavorable. Life expectancy is determined by the severity of intercoronary anastomoses, “steal syndrome,” cardiosclerosis and fibroelastosis. If patients survive a critical period of life (1-2 years), then later they undergo surgery. Children over 2 years of age tolerate ligation of the left coronary artery well. Radical surgery for AOLCA from PA consists of transplanting the left coronary artery into the aorta directly or through a prosthesis. This operation is more physiological, as it preserves the bicoronary blood supply system of the heart. However, due to anatomical features, it is not always feasible in young children.
Trauma to the heart and coronary arteries
The cause of myocardial infarction in children can be closed injuries to the heart and coronary arteries. We observed 2 such children. Thus, a 3-year-old boy developed a myocardial infarction 7 days after his chest was compressed by a huge dog. In a state of extreme severity, the child was taken to the hospital by ambulance with an erroneous diagnosis of pneumonia. The ECG recorded signs of transmural myocardial infarction of the anterior wall of the left ventricle. The pathogenesis of the development of myocardial infarction during cardiac injury is most likely associated with hemorrhage into the myocardium, which could spread to the coronary arteries with the subsequent development of sclerotic changes and stenosis. Thus, the second boy, 13 years old, developed subintimal hemorrhage with moderate occlusion of the left coronary artery after a closed chest injury in a car accident. A feature of this case was the long-term (after 3 years) development of myocardial infarction during intense physical activity. According to the literature, complete or partial ruptures of the coronary arteries with the formation of an arterial aneurysm or coronary fistula are also possible [8]. The narrowing of the lumen of the coronary arteries during trauma is facilitated by spasm and increased platelet aggregation [9].
It should be remembered that with a closed heart injury, the conduction system may be damaged, although often signs of its damage (intraventricular block, complete and incomplete atrioventricular block) may appear only several months or years after the injury. This dictates the need for regular examination by a doctor over a long period of time (including electrocardiographic examination) of children with chest injuries.
Sometimes myocardial infarction can occur in patients with pheochromocytoma. The development of myocardial infarction in these cases is facilitated by prolonged hypercatecholaminemia, which is accompanied by high arterial hypertension, left ventricular myocardial hypertrophy, and thickening of the walls of the coronary arteries. The phenomena of coronary spasm and hypercoagulable changes associated with hypercatecholaminemia also have a certain significance in the development of myocardial infarction. According to our observations, pheochromocytoma was the cause of myocardial infarction in an 11-year-old boy.
Myocardial infarction often occurs in children with congenital heart defects (aortic stenosis). It may occur due to a deficiency of coronary blood flow. We observed for several years a patient with Williams-Beuren syndrome, a congenital heart defect (supravalvular aortic stenosis), who developed a fatal myocardial infarction at the age of 13.
We also observed several patients in whom myocardial infarction complicated the course of such serious diseases as hypertrophic cardiomyopathy, heart tumor, and infective endocarditis. Thus, in a 4-year-old girl with infective endocarditis and large vegetations on the aortic valve, the course of the disease was complicated by embolism of the left coronary artery and the development of acute transmural myocardial infarction. Myocardial infarction due to a heart tumor (left atrial myxoma) developed in a 6-year-old boy.
Thus, other diseases can complicate the development of myocardial infarction, which, in turn, can influence the course of many serious diseases. Therefore, increased attention is required from doctors in such cases.
For questions regarding literature, please contact the editor.
L. V. Tsaregorodtseva , candidate of medical sciences, associate professor of Russian State Medical University, Moscow
Cerebral ischemia grade 2
A dangerous form of the disease. It is characterized by:
- severe apnea (stopping breathing during sleep);
- decreased grasping and sucking reflexes;
- weak muscle tone;
- increase in head shape due to fluid accumulation;
- lack of coordination;
- loss of consciousness;
- changes in skin color.
Most often, grade 2 ischemia manifests itself in the first day of a newborn’s life, and symptoms can be observed for 2-4 weeks. At this time, the child is carefully monitored by doctors, and he undergoes a course of therapy. If necessary, surgery is performed to remove the blood clot.
Cerebral ischemia grade 3
The most severe form, in which:
- the baby has no reflexes;
- the child falls into a coma;
- heart rhythm is disrupted;
- blood pressure rises sharply;
- there are problems with independent breathing;
- strabismus is observed.
An experienced doctor can already determine the presence of signs and symptoms of grade 3 cerebral ischemia in the first 5 minutes of a newborn’s life. In this case, the child is sent to intensive care and, if necessary, connected to a ventilator.
Treatment of cerebral ischemia in newborns
The goal of treatment is to restore normal blood circulation in the brain tissue, prevent pathological changes and eliminate the consequences of ischemia. For stage 1 disease, treatment usually involves prescribing massage to improve blood circulation.
For diseases of the 2nd and 3rd degrees, drug therapy and surgery are used to remove a blood clot in the vessel and restore the structure of the vascular bed. In difficult cases, the baby undergoes a rehabilitation course of intensive therapy.
Treatment
To date, specific therapy that “revives” dead brain cells has not been developed. There are medical methods that stop the process of hypoxia and death so that the child does not die.
Treatment of acute ischemic period
As already noted, severe and moderate cerebral ischemia makes itself felt immediately, the symptoms are specific. If there is no spontaneous breathing in the first 2 minutes of life (as well as in the absence of breathing with an oxygen mask), resuscitation must be performed. do intubation and mechanical ventilation. If small areas of the brain are damaged, then 2-3 minutes after the start of resuscitation the baby is already breathing on his own. Then the child is given to the mother. Such a small patient is being observed.
If resuscitation takes longer, the baby is taken to the intensive care unit. There you need to constantly monitor the readings of the body’s condition, including the amount of glucose and hemoglobin in the blood, the level of gases, the processes of blood circulation and the beating of the newborn’s heart. There is special equipment for this.
Anticonvulsant therapy consists of prescribing special drugs, for example, phenytoin. The dose is selected by the doctor individually. This helps stop the seizures and prevents further brain damage. Since ischemia has a negative effect not only on the brain, but also on the heart, special therapy is needed for this organ. To prevent the little patient’s heart from stopping, he is injected with dobutamine and dopamine.
Today, research data is relevant that a temperature that is 3-4 degrees lower than normal can save parts of the brain from dying, which prevents disability and death. This method is called hypothermic therapy. It has been practiced not so long ago, only since 2010. It is necessary that the process be supervised by experienced doctors. The patient is warmed up gradually.
Treatment of consequences
As mentioned above, there are always consequences for a child with severe and even moderate cerebral ischemia. It could just be attention deficit disorder. But in other cases, mental retardation and cerebral palsy are noted. If there are convulsions, special drugs are used to stop them. Prescribed by a doctor in an individual dosage; self-medication is strictly prohibited.
If the baby has severe cerebral palsy with spasticity of the limbs, then muscle relaxants are taken to alleviate the situation. Whatever the consequences, treatment will not be limited to medications. Daily rehabilitation is important. Children with cerebral palsy are given special massage courses. This should be done by a specialist. At home, you can do only part of the exercises that experts will show parents. Older children who are diagnosed with cerebral palsy due to cerebral ischemia need exercise therapy.
Correction of incorrect postures is carried out using special devices: strollers, bolsters, splints, special chairs. If there is spasticity, the arms and legs of a sick baby may become unphysiological. It's better to avoid this.
In our country there are few specialists who can adequately treat the consequences of cerebral ischemia in newborns. There is overdiagnosis. Children under 6 months of age may have Graefe's sign, increased muscle tone and shuddering. And doctors may think that these are manifestations of encephalopathy. Accordingly, they will prescribe treatment that is actually unnecessary.
Newborns should not be examined when they are drowsy or frightened in some way. Then it is also possible to diagnose neurological pathologies that do not exist. Unnecessary medications that may be prescribed in error include:
- vascular
- nootropics
- homeopathy
Prognosis and consequences of cerebral ischemia
It is much more effective to eliminate ischemia itself after the birth of a baby than to treat its complications. Among the consequences of cerebral ischemia of the 2nd degree:
- sleep disorders;
- headache;
- irritability;
- isolation;
- physical inactivity.
- Stage 3 disease has severe consequences:
- cerebral palsy;
- attention deficit disorder;
- mental retardation;
- Graefe's symptom, etc.
If all actions to eliminate ischemia were carried out by doctors in a timely manner, then the symptoms disappear during the rehabilitation period, which usually lasts 6-12 months.
About prevention
To prevent the development of ischemic manifestations in the unborn baby, a woman must:
- Plan your pregnancy carefully.
- Undergo the necessary examination procedures during pregnancy.
- If required, take iron supplements as prescribed by your doctor.
- Completely give up bad habits.
- Do moderate exercise.
- Eat a balanced diet, follow your daily routine, and get plenty of rest.
- If the pregnancy is difficult, you should be treated in a hospital.
This is the only way to minimize the likelihood of cerebral ischemia in the child.