Recently, heart disease is increasingly being diagnosed in childhood. The most common pathology of the heart muscle in children is cardiopathy, a disease of non-infectious origin that manifests itself in changes in heart tissue. How is cardiopathy manifested and treated in children?
Classification of cardiopathy
Cardiopathy is diagnosed in children of different ages. Most often, this disease is detected in the first 2 weeks of a newborn’s life, at 7–9 years old and in adolescence. This pattern is explained by the fact that in the first days of a child’s life they are carefully examined. Particular attention is paid to those children who have poor heredity or prerequisites for congenital pathologies. By the age of 7-9, the child becomes old enough to be able to explain what is bothering him, and the detection of the disease in adolescents is due to a sharp increase in hormonal activity, which leads to the first or secondary exacerbation of hidden pathologies. The types of cardiopathy are described in the table.
| Classification sign | View | Description |
| Pathology detection period | Congenital | Appears due to intrauterine developmental disorders or genetic predisposition |
| Acquired | Occurs as a consequence of the negative impact on the body of various factors (diseases, stress, physical activity) | |
| Causes of the disease | Functional | It manifests itself with systematic physical tension and stress that the child’s body cannot cope with. |
| Secondary | It is a consequence of acute and chronic diseases. | |
| Changes in the heart muscle | Dilatational | It is expressed in stretching of the muscles of the left ventricle. The walls of the heart do not change in thickness, but due to pathology, the speed of blood flow and the elasticity of the cardiac tissues decrease. |
| Dysplastic | In some places, muscle tissue is replaced by connective tissue, so the heart cannot relax normally. The causes of pathology in children are unknown. | |
| Hypertrophic | The walls of the ventricles of the heart thicken, the volume of the heart chamber decreases. This makes it difficult for blood to flow in and out of the heart. |
Magazine "Child's Health" 2(5) 2007
Dilated cardiomyopathy (DCM) is a serious disease that is characterized by a decrease in the contractile function of the myocardium, caused by a primary internal defect of damaged cardiomyocytes, cardiomegaly due to pronounced dilatation of the cavities of the heart, especially the left ventricle [20]. This is accompanied by progressive chronic heart failure, often refractory to therapy [6, 16, 20]. The disease is characterized by a severe course, often leads to disability and is associated with a high risk of death [6, 20]. The incidence of DCM, according to various authors, varies significantly, which is probably due to differences in methodological approaches, research methods used, and the lack of specific diagnostic criteria [1]. However, in the last decade, many authors have noted an increase in the incidence of DCM in children [29]. In the Donetsk region alone, 28 children with DCM aged from 2 to 18 years have been under our supervision for 5 years.
Chronic heart failure in idiopathic DCM and other heart diseases is a complex syndrome, in the pathogenesis of which neuroendocrine activation of catecholamines, natriuretic peptides and components of the renin-angiotensin system plays a significant role. In recent years, the attention of researchers has been drawn to studying the role of a number of other peptides: endothelins, neuropeptide Y and cytokines, chromogranin A, etc. It has been established that in heart failure in patients with DCM, the expression of genes of many classes is altered, which leads to changes in the organization of the cytoskeleton and myofibrils, disruption of signal transmission, protein metabolism and energy in the myocardium [32].
In most children, it is difficult to determine the onset of the disease, since it often has a long, almost asymptomatic course. A number of patients experience increasing weakness, a lag in body weight gain and/or physical development [20], and there is a tendency to fainting, syncope, and recurrent pneumonia. Sometimes the only sign of the disease is changes in the ECG in the form of disturbances in intraventricular and atrioventricular conduction, and extrasystole. An important feature of DCM is the tendency to form blood clots in all cavities of the heart, more often in the left ventricle, with subsequent thromboembolic complications [1, 20]. However, their frequency is significantly lower than in the adult population.
On examination, marked pallor of the skin, tachycardia, and orthopnea are noted. The pulse is weakly filled, often arrhythmic, and a decrease in systolic and pulse blood pressure is observed. The apex beat is weakened, diffuse, and sometimes the cardiac beat and precordial pulsation are pronounced. The borders of the heart are shifted in all directions, especially to the left, the first tone is weakened and muffled, the second tone is moderately accentuated, the second tone is sometimes bifurcated above the pulmonary artery. With decompensation, a gallop rhythm and a pathological III tone are heard. At the apex of the heart and at the V point, a systolic murmur of relative mitral insufficiency is often heard, caused by expansion of the mitral valve ring, as well as non-closure of the mitral valve leaflets due to their retraction together with the subvalvular apparatus inward with significant dilatation of the left ventricular cavity. There are often signs of left ventricular failure, stagnation of blood in the pulmonary circulation, followed by decompensation of the right ventricle [1, 20].
The lack of specific criteria makes early diagnosis of DCM difficult, and the frequent manifestation of the disease against the background of ARVI or pneumonia further complicates its detection [16, 20].
The electrocardiogram records changes characteristic of severe metabolic and dystrophic changes in the myocardium. Signs of moderate hypertrophy of the left ventricle, less often of both ventricles and left atrium overload are often observed [1]. Along with this, low voltage QRS complexes can be recorded in standard leads, which allowed Japanese researchers to develop one of the ECG criteria for DCM: the ratio of the amplitude of the RV6 wave to the maximum amplitude of the R wave (Rmax) in one of the standard leads (RV6/Rmax > 3) [ 20]. In children with DCM, various heart rhythm disturbances can be detected: from polytopic and polymorphic extrasystole to attacks of atrial fibrillation and unstable ventricular tachycardia [20]. Conduction disturbances manifest themselves in blockades of the branches of the atrioventricular bundle, most often the anterosuperior branch, which is accompanied by a sharp deviation of the electrical axis of the heart to the left, or in atrioventricular block of the I–II degree. The development of left bundle branch block is associated with the frequent detection of fibrous foci in the subendocardial layer of the myocardium at the site of the left bundle branch [28]. The frequency of its detection correlates with the severity of left ventricular dilatation and the degree of heart failure and is a poor prognostic sign [28]. Characteristic changes in the repolarization phase are in the form of depression of the ST segment, flattening or inversion of T waves in the left leads without any positive dynamics, depending on the therapy [16, 20, 28].
An X-ray of the chest of a child with DCM shows an increase in the pulmonary pattern due to venous congestion, and in 1/3 of the patients there are moderate signs of pulmonary hypertension. The shape of the heart is often spherical, mitral or trapezoidal, the increase in the cardiothoracic index is more than 60–65%. In lateral and oblique projections, an increase in all cavities of the heart is detected with predominant dilatation of the left ventricle and atrium [20, 35].
An echocardiographic (EchoCG) study reveals a sharp expansion of the cavities of the heart, especially the left ventricle. In rare cases, the right ventricle is predominantly affected [6]. Dilatation of the cavities of the heart often leads to the development of relative insufficiency of the atrioventricular valves and dysfunction of the papillary muscles. An important echocardiographic indicator characteristic of DCM is a significant decrease in the left ventricular ejection fraction, which can decrease in children with DCM to 50–30% (with a norm of 65–70%), which indicates the complete ineffectiveness of the Frank-Starling compensatory mechanism and a sharp decrease myocardial contractility [6]. In the case of DCM, there are clear diagnostic signs of heart disease: the left ventricle has a spherical shape, all chambers of the heart are usually dilated, the wall thickness of the left ventricle is normal or reduced, during systole all segments of the left ventricle contract, although cases of scar changes in the myocardium are possible [16]. Enlargement of the right heart can be either primary due to involvement of the right ventricle, or a consequence of developing pulmonary hypertension. At the same time, cardiac output may be close to normal values due to the often observed sinus tachycardia and large end-diastolic volume, which provides sufficient stroke volume values even with low ejection fraction [17, 25]. When examined in Doppler mode, in approximately one third of patients, left ventricular thrombi are detected in the apex, sometimes in the right ventricle and atria [16, 25]. An important prognostic factor is the state of contractility of the left ventricle and hemodynamics of the pulmonary circulation (pressure in the pulmonary artery, right atrium and end-diastolic pressure in the right ventricle).
Endomyocardial biopsy reveals widespread (over 30%) irreversible alteration of the myocardium with replacement sclerosis or relatively low severity of compensatory hypertrophy and the absence of exudative and proliferative manifestations of the activity of the inflammatory reaction, atrophy of over 50% of viable cells of the contractile myocardium with a relatively short duration of the disease. Characteristic features include universal damage to the nuclear apparatus of cells, diffuse deposition of deposits of calcium salts in the mitochondrial matrix, and widespread ruptures of nexuses in various parts of the myocardium [28].
Coronary ventriculography in the case of DCM often reveals gross changes in segmental contractility, its heterogeneity, the absence of hypercontraction, a significant increase in the functional volumes of the LV, a sharp decrease in its hemodynamic performance with the possibility of thrombus formation, and a clear deterioration in diastolic function. A significant decrease in the difference between the systolic and diastolic volumes of the left ventricle, typical of small ejection syndrome, is observed [10, 20]. Children with DCM have disturbances in the functional state of platelets in the form of an increase in their Ca2+ content and their aggregation. Autoimmune reactions in DCM are characterized by a high titer of circulating antibodies to contractile (actin, myosin of the myocardium of the ventricles, atria and skeletal muscles) and regulatory proteins (troponin and tropomyosin) [24].
As a biochemical marker of myocardial damage, an increase in the activity of the cardiac-specific isoenzyme MB fraction of creatine phosphokinase can be detected. The disease is characterized by a recurrent, progressive course with periodic worsening of heart failure [20, 23]. It has now been established that in heart failure, the expression of the NO synthetase gene decreases and, as a consequence, the content of endomyocardial nitric oxide decreases, which, in turn, leads to impaired diastolic function of the left ventricle. Perhaps the inactivation of nitric oxide occurs due to increased oxidative stress and the formation of free oxide radicals [32]. Determining the plasma concentration of endothelin-1 helps establish the diagnosis of heart failure in the early stages of the disease, and the concentration of cerebral natriuretic peptide in the blood is a predictor of the risk of death in patients with DCM [32].
Based on the predominant localization of the lesion in the muscle, six clinical variants of DCM are currently distinguished:
— Option I — with isolated damage to the left ventricle;
- Option II - with damage to the left atrium and left ventricle;
- Option III - with a predominant change in the right side of the heart;
— IV option — with dilatation of both ventricles;
— V option — with dilatation of all four chambers of the heart;
- Option VI - with significant dilatation of both atria in the presence of minimal changes in the morphofunctional state of the ventricles of the heart.
Long-term follow-up observations indicate the need to distinguish between DCM with a slow, rapidly progressing and favorable course [25]. The rate of progression of the disease is determined not so much by the nature of the therapy administered, but by the severity of the pathological process in the heart muscle.
Since in most cases the cause of the disease cannot be determined, treatment is aimed at combating heart failure and low cardiac output, and preventing complications [28]. Therapy for a severe disabling disease in a hospital setting should not only be selected to achieve immediate improvement in the condition, but also lay the foundation for further outpatient treatment. Hospital specialists formulate the principles of outpatient management developed for a given patient [28]. In turn, outpatient treatment should ensure the continuity of adequately selected treatment not by formally maintaining previously prescribed drugs and their dosages, but by constant active monitoring of the patient’s condition, appropriate correction of therapy, regular laboratory monitoring and timely consultations. It is extremely important to achieve mutual understanding between the doctor and the patient when solving emerging problems. It is important to provide psychological support to the patient and his relatives. The doctor should avoid a rash abrupt transition from one drug to another without making sure of possible flaws in the dosage, regimen, and the absence of the possibility of unaccounted for drug interactions. The patient must be warned about the need to categorically avoid inappropriate physical activity and carefully follow the intended therapy. The patient should be explained the importance of limiting the consumption of salt and animal fats, and warned of his actions in the event of possible side effects of the drugs. Compliance with such seemingly obvious principles of treatment of heart failure is especially important in patients with DCM, since their implementation really helps to prolong life [28]. In case of congestive cardiac decompensation, limit salt and fluid intake. In case of excess body weight, the calorie content of the diet is adjusted mainly due to carbohydrates, and in the presence of protein-vitamin deficiency or anemia, proteins and vitamins are included in the diet. In case of arrhythmic syndrome, the diet is enriched with foods containing potassium and magnesium salts (raisins, dried apricots, prunes, bananas, dates, nuts, buckwheat, etc.) [20].
The main efforts in therapy are aimed at reducing the activity of the renin-angiotensin-aldosterone system using ACE inhibitors. Any of the drugs in this group can be used, but enalapril is currently used more widely than others [15]. The dosage is always individual (0.1–0.4 mg/kg/day), in two doses under blood pressure monitoring. Captopril is prescribed in non-hypotensive doses of 0.5 mg/kg/day. and requires three doses. When taking captopril, potassium supplements and potassium-sparing diuretics should not be prescribed. The choice of a specific drug is determined by tolerability, blood pressure response, and side effects [14, 20, 28]. The appearance of atrial fibrillation significantly worsens the hemodynamics of patients with DCM. In case of atrial fibrillation, to regulate the pulse rate and eliminate tachysystole, it is necessary to select cardiac glycosides in a dose that ensures the optimal frequency of the ventricular response [20]. They are also necessary for sinus heart rhythm, although one cannot count on a significant inotropic effect, which was previously considered as the main factor in the action of glycosides. Cardiac glycosides are initially used in maintenance doses, since patients with DCM are very sensitive to them. They are prescribed 5 days a week for a long time. When congestive heart failure worsens, the synthetic β1-receptor stimulator dopamine or its analogue dobutamine [30] can be prescribed in the form of short-term courses (3 days) of intravenous infusions at a rate of 2–5 mcg/kg/min to enhance myocardial inotropism. The use of catecholamines has a more powerful cardiotonic effect than the use of glycosides, however, exceeding the dosage can cause tachyarrhythmias and arterial hypotension [20].
An important place in the treatment of children with DCM is the use of small doses of β-blockers. Currently, this group of drugs is considered not only as a means of controlling heart rate and preventing rhythm disturbances, but also as an important stabilizer of neurohumoral regulation, which undergoes significant changes against the background of heart failure in DCM. However, treatment with β-blockers should be started only after the patient’s condition has been stabilized during therapy with ACE inhibitors, diuretics and glycosides. Begin treatment with minimal doses, slowly increasing them every 1–2 weeks and bringing them to the desired doses (titration method). The effect of β-blockers is detected no earlier than 3 months from the start of treatment and increases as the course of therapy is prolonged. Metoprolol is prescribed to children over 12 years of age at an initial dose of 5 mg/day. in 2 doses. If well tolerated, the dose can be increased, observing whether signs of heart failure increase [20]. The most effective in the treatment of congestive heart failure against the background of DCM are 3rd generation drugs (carvediol, etc.), which have a vasodilating effect. The use of the drug carvedilol, a β-α-blocker, which has a positive antioxidant effect on the myocardium that is unique for this group, is promising. It is prescribed at an initial dose of 6.25 mg/day. in 2 doses, increasing the dose to 50 mg/day. Bisoprolol is prescribed to children over 12 years of age at a dose of 0.625 mg per day, increasing the dose to 2.5 mg/day. [20].
As in the treatment of heart failure caused by other diseases, diuretics are traditionally used with constant monitoring of potassium and magnesium levels in the blood, cardiac output, EF and blood pressure [20, 28].
When managing children with DCM, great importance is attached to means of preventing arrhythmias and thromboembolic complications. When choosing antiarrhythmic drugs, it should be remembered that they should not inhibit the contractile function of weakened myocardium and aggravate conduction disorders [18]. Therefore, at present, in the treatment of rhythm disturbances in patients with DCM, preference is given to drugs of the third class of the modern classification of antiarrhythmic drugs - amiodarone and sotalol [40]. During an attack of paroxysmal tachycardia, it is better to administer amiodarone intravenously in 150–200 ml of 5% glucose solution at the rate of 5 mg/kg body weight once a day, switching to oral administration of 10 mg/kg/day after obtaining the effect. In combination with sotalol (40 mg/day in 2–3 doses), it has not only a therapeutic, but also a preventive effect [34].
A high probability of thrombus formation determines the need for anticoagulants. Modern monitoring of the effect of anticoagulant dosages should be carried out using coagulogram parameters, for example, maintaining the international normalized ratio within the range of 2.0–3.0. Warfarin is prescribed to older children, starting at 2.5–5 mg/day. for 3 days, and then adjust the dose according to the prothrombin time, which should exceed the norm by 1.5–2 times (to ensure a therapeutic anticoagulant effect). Phenyline is taken at a dose of 1 mg/kg/day. in 2 doses. Prothrombin time is determined before the start of treatment, after 2–3 days of therapy, and subsequently 1–2 times a week [20, 28].
The drug coenzyme Q10, replenishing the deficiency of this coenzyme, normalizes ATP synthesis in cardiomyocytes, improves the course of energy processes in mitochondria, and has an antioxidant effect, blocking lipid peroxidation [20]. The drug is prescribed to school-age children in the form of ubiquinone, 1–2 capsules per day, the course of treatment is 4–6 weeks [20].
Currently, various areas of surgical correction of heart failure in DCM seem promising, in particular physiological dual-chamber cardiac pacing (PAC) of the heart in the DDD mode. The electrodes of the implantable pacemaker are placed in the right atrium and in the area of the apex of the right ventricle. ECS is performed in patients with DCM with severe heart failure, preserved sinus rhythm in the absence of bradycardia. Electrocardiostimulation is carried out with an individually selected optimal atrioventricular delay (interval) equal to about 100 ms, providing a shorter than normal time interval between contraction of the atria and ventricles. This probably reduces the degree of mitral and tricuspid regurgitation, preserves the kinetic energy of the volume of blood ejected by the atria, increases the efficiency of the Frank-Starling mechanism, which is accompanied by an increase in the ejection fraction, improving the well-being of patients and their tolerance to physical activity [20, 34].
Another direction in the treatment of CHF, based on improving the pumping function of the heart, is the electrophysiological method, called cardiac resynchronization (HRS), which consists of implanting a three-chamber pacemaker, which can be equipped with a cardioverter-defibrillator function. For this, a three-chamber cardiac stimulation scheme is used, when one electrode is in the right atrium, the second in the left, and the third (through the coronary sinus) in the LV. Such a system makes it possible to establish the optimal atrioventricular delay for each patient (a pause between the imposed contraction of the atria and ventricles) and eliminate asynchrony in the work of the ventricles (by simultaneous stimulation of them). The mechanisms of improvement during resynchronization therapy are achieved by:
- improvement of systolic function (increase in ejection fraction);
— coordination of contraction of the left ventricle;
- shortening the time of contraction of the left ventricle;
- improvement of diastolic function;
- prolongation of left ventricular filling time;
- reduction of mitral regurgitation;
- possible remodeling of the ventricles of the heart.
Installation of an implantable cardioverter-defibrillator is necessary for patients with CHF and life-threatening ventricular cardiac arrhythmias (gradations IV and V according to Lown - Wolff). Special studies for patients with CHF have not yet been carried out, however, in patients who have previously suffered an acute myocardial infarction, this procedure can significantly improve the prognosis in comparison with antiarrhythmic therapy [32].
In the terminal stage of development, when drug support becomes insufficient to prolong the patient’s life, only surgical treatment remains, which significantly improves the prognosis of life compared to non-operated patients [20, 22].
Indications for surgical correction of DCM are: 1) severe heart failure of NYHA functional class IV; 2) the severity of concomitant arrhythmic and thromboembolic syndromes; 3) lack of effect from all long-term modern methods of intensive drug therapy; 4) unfavorable prognosis for the next year of life [36].
Palliative operations of cardiomyoplasty and ventriculoplasty are like a bridge to subsequent radical heart transplantation (in patients awaiting a donor heart) or are performed in patients for whom radical transplantation is contraindicated for a number of reasons (presence of severe concomitant diseases, renal or hepatic failure, uncontrolled infections etc.) [22, 36, 42].
The cardiomyoplasty method involves mobilizing the latissimus dorsi muscle (while maintaining its blood supply), moving it to the mediastinum and “enveloping” both ventricles with this muscle. In this case, the skeletal muscle graft covers about 70% of both ventricles and is fixed to the myocardium and pericardium. The latissimus dorsi muscle graft is pre-trained using programmed cardiac synchronized electrical stimulation. Electrical neurostimulation of the muscle graft using stitched electrodes is synchronized along the R wave of the cardiac signal with contractions of the heart itself, which is accompanied by improved systolic emptying of the ventricles and elastic support of the dilated heart in diastole. This also leads to a reverse development of ventricular remodeling due to a decrease in wall tension and changes in diastolic function [20].
Ventriculoplasty, or Baptiste's operation, involves reducing the volume of the left ventricle by excision of a significant portion of its lateral wall in combination with mitral valve replacement using annuloplasty. As a result of the operation, the effective stroke volume and ejection fraction of the left ventricle increase [15].
In recent years, the introduction of a method of assisted circulation, which in Russia has received the name “artificial left ventricle” (ILV), has begun. Its essence lies in the fact that while the heart is beating, an outflow line is sewn into the apex of the LV, connected to a pumping device that sends blood through a second line to the aorta. As a result, the heart works in a “sparing” mode, since an artificial pump additionally operates in parallel with the patient’s LV. This method has been proposed for patients with critical hemodynamic deterioration awaiting a donor for heart transplantation. However, given that in some cases it was necessary to wait a long time for a donor heart, in some patients the ILV worked for a long time for several weeks or even months. It was found that in some cases the “rested” left ventricle of the patient gradually restored its propulsive ability. This has led to a change in concept from “bridge to transplant” to “bridge to recovery.” The idea arose to use long-term (several months) placement of ILV as a treatment method for patients with critical CHF. Currently, three types of devices are the most popular: the Heart Mate rotary pump, the Toratec diaphragm pump and the Jarvic 2000 high-speed turbine. Typically, these devices are placed in the abdominal cavity subdiaphragmatically and connected to external power sources, which are becoming smaller in size and require increasingly infrequent charging [ 32].
Mechanical methods of treating CHF today are essentially reduced to the use of a restrictive external elastic mesh that limits cardiac dilatation. Initial clinical observations have shown the safety of this procedure, but thorough clinical studies must be conducted before its widespread introduction into practice [32].
Heart transplantation is a radical, most proven and effective method of surgical correction of DCM in cases of severe heart failure refractory to drug therapy. In the largest cardiac surgery centers in the world, in terms of the number of heart transplants performed, patients with dilated cardiomyopathy occupy second place after patients with ischemic cardiomyopathy and make up more than 50% of recipients. However, heart transplantation is associated with certain medical and social problems, in particular with the psychological stability of the recipient, the presence of donor hearts, graft rejection, stenotic lesions of the coronary arteries of the allograft, the consequences of powerful postoperative immunosuppressive therapy (malignant tumors, uncontrolled infections, toxic damage to many organs, etc. .), as well as the high financial cost of all surgical and therapeutic measures performed [20].
Despite significant clinical variability in the course of the disease, its prognosis in children is usually unfavorable [24]. This is due to the development of treatment-refractory congestive heart failure, life-threatening arrhythmias and often thromboembolic complications. Foreign authors propose to use an increase in the level of L-carnitine in blood plasma and urine as a marker of the severity of congestive heart failure in DCM, reflecting the degree of myocardial damage and dysfunction of the left ventricle.
Determining the reserves of myocardial contractility in patients with congestive heart failure is of great clinical importance. This, on the one hand, largely determines the prognosis, and on the other hand, it is necessary to determine the indications for heart transplantation, the urgency of its implementation, the advisability of managing patients before transplantation with conservative therapy or using mechanical means of circulatory support. Stress echocardiography with dobutamine loading is used to determine the reserves of contractility of the left ventricle of the heart. The ejection fraction and sphericity of the left ventricle are determined before and at the peak of the load with dobutamine. An increase in ejection fraction and peak systolic velocity after dobutamine loading is the most optimal predictor of possible improvement in left ventricular function. Of interest is the emergence of a new non-invasive indicator that allows assessing systolic and diastolic dysfunction, as well as predicting outcome in patients with DCM - Tei-index. The parameters for its calculation are determined using Doppler echocardiography. Tei-index = (IRT – – ICT)/ET, where IRT is the isovolumic relaxation time, ICT is the isovolumic contraction time and ET is the ejection period. With a Tei-index value of more than 1.14, the risk of death increases sharply. Tei-index does not depend on heart rate, blood pressure, changes in left ventricular geometry and the degree of mitral regurgitation [32].
The cause of death in patients with DCM is often life-threatening cardiac arrhythmias. Late potentials on signal-averaged electrocardiograms and a history of episodes of ventricular tachyarrhythmias are predictors of arrhythmia and an increased risk of cardiac death in patients with DCM [32].
In children with damage to the left side of the heart, it seems possible to stabilize the patients' condition in cases of continuous treatment. With combined damage to the ventricles or all chambers of the heart, DCM has a malignant course, accompanied by the development of congestive heart failure refractory to therapy, the development of life-threatening arrhythmias and thromboembolism, which causes high mortality [20]. In young children, with persistent and long-term treatment, a significant improvement in hemodynamics often occurs, and in some cases, complete normalization of the morphofunctional state of the heart is noted [41].
Thus, dilated cardiomyopathy is a serious disease with the progressive development of heart failure, decreased quality of life and frequent disability of patients, unfavorable prognosis, the possibility of complications in the form of syncope and sudden cardiac death. Despite dissatisfaction with the existing effectiveness of currently carried out therapeutic interventions, the search for new ways to optimize the management of this contingent of patients continues and gives hope for an improvement in the prognosis for the pathology under discussion.
Symptoms of pathology in children
In infancy, it is difficult to notice cardiac abnormalities. Kids cannot explain what causes them discomfort. You can suspect cardiopathy in an infant based on the following symptoms:
- with strong crying, the area of the nasolabial triangle turns blue;
- the rhythm of breathing is lost;
- the baby gets tired quickly.
Older children experience more extensive symptoms. With cardiopathy, children often complain of pain in the area of the heart or chest. The disease is also accompanied by:
- high fatigue;
- shortness of breath;
- dizziness and fainting;
- pale skin;
- increased sweating.
The manifestation of symptoms is due to the fact that due to improper functioning of the heart, the delivery of blood to other organs is disrupted. With some types of disease, health worsens even with such physical activity that a healthy child does not feel.
The dilated form of cardiopathy, in the absence of timely treatment, leads to the appearance of a bulge in the area of the heart. This occurs because the heart muscle expands significantly and causes displacement of nearby tissue.
The most striking sign of functional cardiopathy is cardiac arrhythmia. If physical activity is not reduced, it leads to a significant deterioration in heart condition.
Secondary cardiopathy is accompanied by the same symptoms that are inherent in the underlying disease. In this regard, secondary cardiopathy is difficult to recognize.
Characteristics of the disease
The main function of the heart muscle is to contract rhythmically, which pushes blood through the vessels and fills the cavities of the organ. This ability is provided by cardiomyocytes. Their continuous functioning maintains metabolism at the level necessary for work.
Under the influence of unfavorable factors, over time the processes presented are disrupted. This is manifested by the formation of structural changes, which ends with a weakening of contractility.
Diagnostic methods
If cardiopathy is suspected in a child, the doctor conducts a careful examination and asks the parents about the presence of symptoms. The initial examination is performed by a pediatrician, but a more thorough diagnosis is carried out under the supervision of a cardiologist.
Pediatric cardiology uses the following methods to diagnose cardiac pathologies:
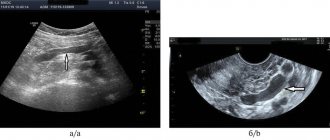
- Echocardiography. The study consists of examining the chest using an ultrasound machine. With its help, the specialist determines the parameters of the heart muscle and its structural elements. Another name for the procedure is cardiac ultrasound. The procedure allows you to identify dysplastic pathology without the use of other methods.
- ECG. An electrocardiogram records the electrical signals produced by the heart. The device records the heart rate, describes the physical condition of the heart, and indicates the presence of arrhythmic movements.
- Phonocardiography. This method is in addition to ultrasound and ECG. It allows you to record the noise that appears during the work of the heart muscle.
Additionally, the child may be prescribed blood tests to determine genetic abnormalities. In some cases, it is necessary to determine the cause of the pathology. To do this, he is given a referral for a general blood test, CT scan, and MRI.
Let's try to figure it out. Let's start in order
Worry? No, let's first go to a pediatric cardiologist. The pediatrician will give you a referral, and in it there will be a number of completely incomprehensible terms and abbreviations. Let's try to decipher them.
- Functional cardiopathy (FCP) or FIS – functional changes of the heart – is one of the most common diagnoses in pediatric cardiology. The use of this term is not always justified. In fact, FIS refers to any set of minimal deviations from the norm (based on the results of a clinical examination, ECG, EchoCG, etc.) that cannot be combined into any other, more serious diagnosis.
- FSS - functional systolic murmur - in this case, the word “functional” means “not caused by any serious disease,” which is most often found in pediatric practice.
A “heart doctor” – a specialist in heart and vascular diseases – will examine your baby.
He will ask if there are any specific complaints - how the child eats, if he gets tired when sucking, if he turns blue, if he has attacks of shortness of breath, how he gains weight. If the child has grown up and can already speak, is there any pain in the heart area, palpitations, or dizziness? Was there any fainting? He will look carefully to see if there is cyanosis (blueness), shortness of breath, deformation of the chest, fingertips and nails, swelling, or pathological pulsation of blood vessels. He will percuss (knock) to see if the boundaries of the heart are expanded. Palpates (feels) peripheral vessels, the heart area, liver and spleen. He will listen carefully to the heart - how and where it makes noise, what the heart sounds are, where the noise is directed, whether there are any accompanying sounds, and count the frequency of breathing and pulse. And, most likely, he will say that your baby has no signs of circulatory failure (or hemodynamic disorders, or heart failure, which, in general, is almost the same thing). This means that at the moment the child’s heart is doing its job well. Hooray? Hooray. But it’s too early to part with the doctor. Most likely, he will recommend conducting the necessary additional research. For what? And in order to determine where this noise comes from, how to monitor the child, what to fear and in what case to begin treatment.
Forecast
With the development of dysplastic cardiopathy, the prognosis is often unfavorable. This is due to the emergence of difficulties in establishing a diagnosis. In most patients, it is almost impossible to detect the disease at an early stage. Some patients do not complain during the entire period of progression of cardiopathy (asymptomatic form). Establishing a diagnosis in them is possible only after the addition of complications.
By the time the pathology is detected, in most cases, cardiovascular failure has lasted for a long time. After confirmation of the diagnosis, the survival rate of such patients in 30% of cases does not exceed 5 years. If a heart transplant is achieved, the life expectancy increases to 10 years.
In childhood and adulthood, with early diagnosis, it is possible to stabilize the condition and ensure the absence of manifestations of pathology. To maintain quality of life, all patients should be observed by a cardiologist, independently monitor their well-being and constantly take medications according to the prescribed regimen.
Complications
A minor anomaly in the development of the heart (cardiopathy) is dangerous not only for its manifestations, but also for its complications. They can form due to late diagnosis and lack of treatment. With dysplastic cardiopathy, the following consequences are possible:
- angina pectoris;
- cardiac ischemia;
- arterial hypertension;
- pericarditis;
- pulmonary edema;
- arrhythmia;
- thrombosis;
- chronic heart failure.
The most common complication is arrhythmia. It appears in almost 10% of the total number of sick children. Cardiopathy leads to disruption of the normal conduction of electrical impulses in cardiomyocytes. As a result, heartbeats become irregular. Most often there is an acceleration in the number of beats per minute.
When the ventricles dilate, when the disease lasts for a long time, the blood in the cavities stagnates. Conditions are created for the formation of clots.
The greatest danger is represented by blood clots that enter the bloodstream (emboli) and over time they can penetrate into narrow-diameter vessels. This feature is characteristic of lung and brain tissue. Blockage of the lumen in them will lead to throboembolism.