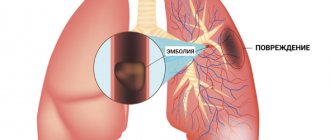
Pulmonary embolism (PE) is a serious, potentially life-threatening condition. Pulmonary embolism occurs due to blockage of blood vessels by thrombotic masses in the lungs. Pulmonary embolism (PE) can cause symptoms such as chest pain and/or shortness of breath. Also, in some cases, PE may have no symptoms at all, and it is difficult to detect without specialized examination. Massive pulmonary embolism can cause collapse and death. PE usually occurs due to the formation of a thrombosis in the lower extremity - deep vein thrombosis (DVT).
Pulmonary embolism (PE)
Prompt treatment is important and can save lives. Pregnancy, various medical conditions and medications, immobility, and major surgery all increase the risk of PE. Anticoagulant therapy, including heparin drugs, direct oral anticoagulants, or warfarin, is the usual treatment for PE.
General information
Thrombosis is the formation of a blood clot in a blood vessel that interferes with blood flow.
Its danger lies in the fact that there is a danger of a blood clot or its fragment moving through the bloodstream - an embolism and blockage of another vessel occurs. For example, supplying the brain or heart. Pulmonary embolism often occurs, which leads to severe organ dysfunction or death. What is pulmonary embolism in medicine? Pulmonary embolism is an acute occlusion (blockage) of the pulmonary artery trunk or its branches (main, lobar or segmental). Occlusion occurs more often due to embolization of a thrombus from the right half of the heart or veins of the lower extremities. The incidence of this disease increases with age and the age of such patients is 62 years.
Pulmonary artery thrombosis is an emergency in cardiac resuscitation and is often the cause of patient death. Seriously ill patients with a high risk of pulmonary embolism and death are patients with cardiac arrhythmias, cancer pathology , and thrombosis of the veins of the lower extremities . Diagnosis is often difficult, so the disease is often not recognized. Early diagnosis and initiation of intensive treatment are important in the prognosis of this disease. The code for pulmonary embolism according to ICD-10 is I 26.
Scintigraphy and angiography for pulmonary embolism
The multicenter PIOPED study (1990) examined the use of ventilation-perfusion scintigraphy and pulmonary angiography; It was found that normal results of ventilation-perfusion scintigraphy practically exclude PE, and changes with a high degree of confidence practically confirm the diagnosis. However, the diagnosis of pulmonary embolism was confirmed or excluded only in 174 patients out of 713 (24%) – in those whose clinical symptoms clearly correlated with changes on the scans. In most patients, including those who had cardiopulmonary diseases underlying the occurrence of pulmonary embolism, the results of ventilation-perfusion scintigraphy were questionable or had no diagnostic value, which required additional studies. Based on this, in patients with pathological changes on chest radiographs, CT rather than scintigraphy is the preferred primary screening diagnostic method.
Pathogenesis
The main thing in the pathogenesis of pulmonary embolism is occlusion of the pulmonary artery and its branches by embolism, which leads to disruption of hemodynamics and gas exchange. During occlusion, the pressure in the pulmonary artery increases by almost 50% due to vasoconstriction associated with the release of serotonin and thromboxane A2 . A sharp increase in pulmonary resistance causes expansion of the right ventricle, stretching of myocytes and tension of the ventricular walls. Lengthening the time of its contraction towards diastole causes protrusion of the septum into the left ventricle. Therefore, left ventricular filling becomes more difficult, cardiac output decreases, and systemic hypotension .
Massive infiltrates are found in the ventricular myocardium, which further destabilizes hemodynamics. Right ventricular failure due to pressure overload is a leading cause of death. Respiratory failure is also associated with hemodynamic disturbances. A decrease in cardiac output causes desaturation of venous blood. The appearance of zones of reduced blood flow causes a mismatch between perfusion and ventilation. Small emboli in the periphery do not affect hemodynamics, but cause pulmonary hemorrhage with hemoptysis, pleurisy and pleural effusion (“pulmonary infarction”).
Classification of pulmonary embolism
The 2008 classification of the European Society of Cardiology identifies:
- Low risk pulmonary embolism.
- Intermediate.
- Tall.
Clinical classification takes into account the caliber of the pulmonary arteries and the percentage of pulmonary involvement:
- Massive (cessation of blood flow in less than 50% of the pulmonary bed). It is of great clinical importance because it is accompanied by shock or decreased pressure, pulmonary hypertension. With occlusion of the trunk, pronounced cardiorespiratory disorders develop. Under such conditions, the right ventricle cannot perform the function of a pump and its cavities quickly expand, and tricuspid valve insufficiency develops. The septum between the ventricles shifts to the left (towards the left ventricle), which is accompanied by poor filling in diastole. Due to the cessation of blood flow, the pulmonary parenchyma is not supplied with blood, but is ventilated. In the affected area, obstruction of the bronchi occurs, the alveoli collapse and surfactant is not formed in them, which contributes to the development of atelectasis (collapse) of the lungs on days 1-2 of embolism. Such hemodynamic disturbances and impaired pulmonary function often lead to the death of the patient.
- PE of small branches of the pulmonary artery (cessation of blood flow is noted in less than 30% of the pulmonary bed). In this case, there is occlusion of lobar and segmental small branches. The disease is not severe without hemodynamic disturbances and patients only need anticoagulant therapy. The pulmonary circulation has compensatory capabilities and there is a possibility of independent dissolution of blood clots when fibrinolysis .
- Submassive—thromboembolism of the branches of the pulmonary artery (cessation of blood flow in less than 30-50% of the bed). It manifests itself as right ventricular failure, and hemorrhagic infarctions form in the lungs.
Causes of pulmonary embolism
Considering the causes of pulmonary embolism, it is necessary to highlight a number of diseases and conditions that are accompanied by the formation of blood clots, which are the basis of embolism:
- Varicose veins and phlebothrombosis of the deep veins of the lower extremities. This pathology causes thromboembolism in 90% of cases. The threat is posed by floating blood clots, which are freely located in the lumen of the vessel and are connected to the vein wall in only one part.
- Congestive heart failure , overstretching of the right ventricle, which creates conditions for the formation of blood clots in the cavity of the right ventricle.
- The use of oral contraceptives and pregnancy , which are associated with an increased risk of blood clots.
- Antiphospholipid syndrome (autoimmune thrombotic vasculopathy ), manifested by venous and arterial thrombosis . Vessels of different sizes may be affected - capillaries and large arterial trunks. Deep vein thrombosis of the lower extremities is a typical manifestation of antiphospholipid syndrome. Repeated pulmonary embolisms are typical.
- Central venous catheterization.
- The presence of malignant diseases.
- Carrying out chemotherapy .
- Spinal cord injury.
- Forced immobilization after surgery, during a stroke , fractures of the pelvis and limbs.
- Hip replacement.
- Atrial fibrillation.
- Polytrauma and extensive surgery.
- Hereditary predisposition to thrombosis ( thrombophilia ), which is caused by mutations of certain genes, deficiency of antithrombin III, deficiency of protein C and S.
Considering pulmonary thrombosis , it is necessary to note the risk factors for this condition:
- Damage to the venous endothelium.
- Obesity.
- Age.
- Hypercoagulation.
- Slowing of venous blood flow.
- Infection.
- Blood transfusions.
- Migraine.
- The combination of several factors is accompanied by a high risk of thrombus embolism .
Venous thromboembolic complications
Pulmonary embolism (PE) is part of a group of clinical problems collectively known as venous thromboembolic events (VTE).
Thrombosis is the blockage of a blood vessel by a blood clot (thrombus). Embolisms occur when part or all of a blood clot displaces from where it formed. The thrombotic masses then move with the bloodstream until they get stuck in narrower blood vessels in other parts of the body. In this case, the blood clot is called an embolus. Deep vein thrombosis (DVT) is the cause of venous thromboembolism, including pulmonary embolism (PE). Thrombosis most often occurs in the veins of the lower extremities.
Symptoms of pulmonary embolism (PE)
Making a diagnosis in the early stages is difficult, only in the acute course the symptoms of pulmonary artery thrombosis appear manifestly and suddenly: shortness of breath , chest pain , tachycardia , decreased blood pressure. In this case, the patient has risk factors for thromboembolism - varicose veins and phlebothrombosis .
Hypotension and shock indicate central thromboembolism . Severe acute shortness of breath also develops with central pulmonary embolism. With the same localization, chest pain is angina-like in nature . Massive thromboembolism is accompanied by signs of right ventricular overload - this is the bulging of the jugular veins in the neck and the right ventricular gallop rhythm.
Signs of PE in subacute cases are not very specific. Respiratory and right ventricular failure progresses, infarction pneumonia and hemoptysis appears. With a recurrent course, repeated attacks of shortness of breath , there are signs of pneumonia and periodic fainting occurs. If the thrombus blocks small arteries in the periphery, shortness of breath is minor and transient. Only in the case of chronic lung pathology or heart failure does shortness of breath worsen. Sometimes PE is asymptomatic.
The clinical picture depends on the caliber of the blocked vessel and, accordingly, the degree of involvement of the pulmonary vascular bed. Massive pulmonary embolism (most often thrombosis of the main branch ) occurs with symptoms of shock or a decrease in pressure by 40 mm Hg. Art. for a short time that is not associated with arrhythmia , sepsis , or decreased blood volume. Characteristic shortness of breath , cyanosis , and sometimes fainting .
Submassive pulmonary embolism (obstruction of lobar or segmental branches) is manifested by right ventricular failure (swelling of the neck veins, pallor, cyanosis), but without arterial hypotension. The patient develops shortness of breath , tachycardia , pulmonary infarction (fever, cough, pulmonary-pleural pain, sputum streaked with blood). In non-massive cases, there are no signs of right ventricular failure, and the pressure is normal.
Computed tomography (CT) in the diagnosis of the body
The technical development of the CT method, especially the emergence and introduction of multidetector devices (MSCT), has led to the fact that computed tomography has become an important diagnostic method for suspected pulmonary embolism. Contrast-enhanced CT is increasingly used as the primary method of investigation for pulmonary embolism, especially in those patients in whom pathological changes were detected on chest x-ray and the results of scintigraphic examination are not of diagnostic value.
CT pulmonary angiography can visualize emboli directly and is non-invasive and easy to use. In recent years, computed tomography scanners have been installed in almost all large hospitals, so the method is relatively affordable. Also, CT allows you to obtain additional information regarding an alternative diagnosis, which is a great advantage of this diagnostic method over classical pulmonary angiography and scintigraphy.
Contrast-enhanced spiral CT allows you to contrast the lumen of the pulmonary vessels and see a thrombus in their lumen. After an intercontinental flight, a young man developed acute chest pain and breathing problems. CT scan visualizes thrombus in the anterior segment artery of the left upper lobe ( LA 2) and the anterior segment artery of the right upper lobe ( RA 2).
CT scan for chronic thromboembolism in a 69-year-old patient with pulmonary arterial hypertension. The tomogram visualizes a parietal thrombus with the presence of point calcifications, located parallel to the anterior wall of the right lower interlobar artery.
In most cases where CT is positive for pulmonary emboli, the emboli are multiple and intraluminal filling defects (enhancement defects) are seen in the larger central arteries and in the segmental and subsegmental vessels. Most often, emboli are found on both sides and are localized in the lower lobe arteries. An obvious filling defect in a single segmental or (especially) subsegmental vessel can be difficult to recognize. It must be taken into account that artifacts associated with the partial volume effect may be mistaken for a filling defect in the subsegmental artery.
Since deep vein thrombosis and pulmonary embolism are partial aspects of a single disease, after CT angiopulmonography, CT venography can be performed without additional administration of a contrast agent. In this case, the research time will increase by only a few minutes.
Get a CT angiopulmonography in St. Petersburg
Tests and diagnosis of pulmonary embolism
Instrumental diagnostics of this condition includes:
- Chest X-ray . In the lungs, discoid atelectasis , a raised dome of the diaphragm, or pleural effusion . All these signs are nonspecific, but exclude various causes of pain and shortness of breath.
- ECG.
- Echocardiography . It is of key importance in diagnosis, especially in patients with unstable hemodynamics. This test directly detects blood clots in the right side of the heart, and blood clots in the large arteries of the lungs (trunk and main branches). An indirect sign of pulmonary embolism detected by echocardiography is overload of the right chambers. An indirect sign of pulmonary artery thrombosis is identified - overload of the right ventricle: expansion of the ventricular cavity, unusual movement of the septum between the ventricles and a D-shaped left ventricle.
- Ultrasound of the deep veins of the extremities.
- A special research method is ventilation-perfusion scintigraphy . This is a safe study that involves the intravenous administration of albumin microspheres that are labeled with technetium. Albumin microspheres block pulmonary capillaries and lung perfusion is assessed based on this feature. This study is complemented by a ventilation study, which increases the specificity of the examination (in case of thrombosis, ventilation in segments of the lungs in which there is poor blood supply remains normal). That is, a discrepancy between ventilation and perfusion is detected. Radioactive radiation during scintigraphy is lower than during CT angiography. Perfusion scintigraphy alone can be performed in patients with a normal radiograph.
- CT angiography . This study has become the method of choice for diagnosing pulmonary vascular pathology. The pulmonary arteries can be viewed down to the segmental branches. The specificity of the method is 96%. The technique is optimal for diagnosing a patient whose condition is stable. In another case, the patient cannot be transported from intensive care.
- Pulmonary angiography is considered the gold standard for diagnosing PE. However, it is rarely performed since a less invasive test, CT angiography, has become available. Diagnosis is based on identifying a blood clot that causes a defect in the filling of the pulmonary artery branch or is completely absent. The method allows you to obtain images of peripheral pulmonary arteries and detect blood clots of 1-2 mm in the smallest arteries. A contrast agent is injected for the study.
ECG signs of PE and radiographic signs
Blood tests:
- D-dimer is a fibrin degradation product. Its increase in plasma is observed during acute thrombosis - this is explained by the activation of fibrinolysis and coagulation. A normal D-dimer level makes the diagnosis of PE unlikely. Also, this indicator is nonspecific, since fibrin overproduction is observed during inflammation, bleeding, myocardial infarction , aortic aneurysm , oncological processes, trauma, and surgery. The specificity of D-dimer for thrombosis decreases with age. This indicator is paid attention to during treatment, since an increase in its level after the end of anticoagulant treatment indicates a risk of relapse.
- of brain natriuretic peptide increases , which is associated with distension of the right ventricle. The degree of increase is proportional to the severity of the patient. However, an increase in this marker is nonspecific, since it can be observed with myocardial ischemia , left ventricular hypertrophy , sepsis and tachycardia . However, the absence of a significant increase indicates a favorable prognosis for pulmonary embolism.
X-ray of the lungs with pulmonary embolism
Pathological changes on chest x-rays are detected in most cases of pulmonary embolism, but are not specific. The most commonly detected pathological changes on radiographs include atelectasis (collapse) of part of the lung, pleural effusion, decreased transparency of the lung tissue, and high standing of the right or left dome of the diaphragm. Classic X-ray signs of pulmonary infarction are the presence of a wedge-shaped (triangular) darkening, with a wide base facing the pleura, the apex of which is directed towards the root of the lung (Hampton's tubercle), or a decrease in the severity of the vascular pulmonary pattern in the zone of thromboembolism (Westermarck's sign).
Other changes on radiographs detected with pulmonary embolism are expansion of the central pulmonary artery with its sharp break - “cut off roots”, an increase in the size of the heart (especially its right parts), as well as signs of pulmonary edema. These changes can be combined with acute clinical symptoms of cor pulmonale. The absence of changes on the chest x-ray in a patient with severe respiratory distress and hypoxemia, but without signs of bronchospasm or atypical blood flow in the heart, is highly suspicious for PE. In general, chest radiography cannot be used to confirm or refute the diagnosis of PE; however, radiography and ECG may be helpful in confirming alternative diagnoses.
In children
Thrombosis in children occurs much less frequently. For their occurrence, a combination of acquired and congenital factors is necessary. Of the hereditary ones, it is worth noting the hereditary decrease in the activity of antithrombin III and proteins C, S, which are natural anticoagulants. Acquired factors:
- severe somatic diseases
- infectious diseases;
- systemic connective tissue diseases;
- malignant tumors;
- connective tissue dysplasia ;
- hemolytic anemia;
- antiphospholipid syndrome.
The most important factor in thrombosis is the use of an intravascular catheter. Up to 80% of thromboses are associated with catheterization. In children, pulmonary embolism is often associated with deep vein thrombosis. Venous thrombosis in children is preceded by infection, thrombocytopenic purpura , antiphospholipid syndrome , and in some, excessive insolation. Recurrent pulmonary embolism also occurs, but the source of thrombosis cannot be identified. PE in children is often mistakenly regarded as pneumonia .
Prevention of pulmonary embolism
- Primary prevention of embolism after surgery on the pelvic organs and orthopedics.
- If the risk of thromboembolism is low, prevention involves the use of compression stockings or elastic compression with an elastic bandage of the lower extremities.
- enoxaparin or fondaparinux or dabigatran until discharge .
- If the risk is high, these recommendations should be followed for a month.
- New oral anticoagulants are registered for primary prevention after orthopedic surgery.
There are also non-surgical patients (chronic heart failure class III-IV), who are also at risk for pulmonary embolism. Their choice of anticoagulant is the same. The standard of treatment is enoxaparin .
Secondary prevention is the prevention of recurrent thromboembolism. Without prophylaxis, recurrence of pulmonary embolism within the first three months is observed in 20-45% of patients. With prevention, the number of relapses is reduced to 1%. Warfarin is used for prevention , and for the first six months, cancer patients are prescribed low molecular weight heparins ( Fraxiparin , Fraxiparin Forte , Clexane , Enixum , Fragmin , Daltep ), then they are transferred to oral medications ( vitamin K antagonists - Warfarin , Warfarex ).
How long should I take them? To do this, the risk of bleeding is assessed based on risk factors (age 65 years or older, history stroke malignant tumor , renal and liver failure , diabetes mellitus , low platelet levels, anemia and others). The risk of bleeding is high if more than two risk factors combine.
It is also possible to use “new” anticoagulants for secondary prevention, which are included in the American Guidelines for the prevention of pulmonary embolism. Rivaroxaban ( Xarelto ) is superior in long-term treatment and can be prescribed for 6-12 months with a low risk of bleeding, providing protection against relapse.
Prevention of venous thrombosis and pulmonary embolism
Prevention of venous thrombosis and pulmonary embolism (PE) is based on determining the degree of risk of their occurrence for each individual patient and assigning it to one of three risk categories: low, moderate or high.
The category of risk of venous thrombosis is determined depending on the presence of risk factors for the development of venous thrombosis in each patient, which include: malignant neoplasms, heart failure, myocardial infarction, sepsis, dilated cardiomyopathy, atrial fibrillation, stroke, broncho-obstructive diseases, erythremia, inflammatory bowel diseases, obesity, nephrotic syndrome, surgery, trauma, age over 40 years, estrogen intake, prolonged immobility, pregnancy, varicose veins of the lower extremities, history of venous thrombosis, bed rest for more than 4 days, thrombophilia.
During surgical interventions, the degree of risk of venous thromboembolism is determined by an assessment of the severity of the surgical operation and the patient’s condition. The basis for the prevention of venous thrombosis in this category of patients is their early activation, elastic compression of the lower extremities and heparin therapy.
Elastic compression of the lower extremities affects blood stasis and the hemodynamic factor of thrombosis, being a method of nonspecific prevention and includes the following methods.
Elastic compression socks and stockings
unlike bandages, they create the compression necessary to normalize venous outflow and provide physiologically distributed pressure along the entire length of the limb.
To prevent deep vein thrombosis and thromboembolic complications, a special type of medical compression hosiery is used, called “anti-embolic” or “hospital” hosiery. In our practice, we use hospital knitwear from Tyco Healthcare/Kendall. In this case, the maximum pressure is created at the level of the ankles and measured in mm Hg, appropriate for each compression class, followed by a gradual decrease in the proximal direction, which eliminates the threat (as in the case of elastic bandages) of iatrogenic venous stagnation.
These products retain their compression properties for a long time, are processed, and are easy to put on and take off, which saves the time of medical personnel. The use of anti-embolic knitwear increases the effectiveness of specific anticoagulant prevention of pulmonary embolism by 3-4 times. “Lastoshir” compression garments have 3 compression classes and a wide range of modern colors (flesh, bronze, blue, gray, black, brown).
Contraindications for the use of compression hosiery include the following cases:
- Progressive atherosclerosis of the vessels of the lower extremities (degree of ischemia above grade IIA);
- Ankle circumference more than 35 cm;
- After surgery for autodermoplasty, plastic surgery with a rotational flap or on a free pedicle to close varicose ulcers;
- Severe leg deformity.
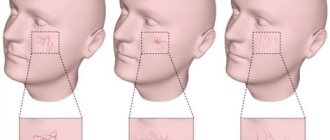
The size of compression hosiery is selected strictly individually, taking into account the morphometric data of the patient. When using compression socks, four parameters are measured - foot length, ankle circumference, maximum calf circumference, calf length (Fig. 1).
When choosing a stocking size, six measurements should be taken: foot length, ankle circumference, maximum calf circumference, maximum thigh circumference, thigh length.
It should be noted that this type of medical product practically does not cause a contact reaction of the skin, which is very important for most patients, since it does not contain latex.
A new generation of hospital knitwear - anti-embolic stockings T.E.D. — have a more reliable fit on the leg due to the presence of a flexible silicone elastomer along the edge of the product. The silicone coating is comfortable on the skin and holds the stockings in place perfectly. For a long time, anti-embolic products maintain constant graduated compression on the leg.
Rice. 1. Differential pressure distribution in TED compression garments (Tyco Healthcare / Kendall)
This allows you to increase blood flow by 138%. The mosaic circular knitting, directed to one side, correctly distributes pressure along the leg, and the absence of pressure in the popliteal vein allows blood to flow freely through this critical area. Ergonomics are also created by the interrupting bandage and the two-layer V-shaped insert in the fixing elastic band of the stocking, which prevents the effect of a tourniquet on the femoral vein. 3 types of T.E.D. compression hosiery are available: knee socks, stockings, stockings with a belt, and 27 possible sizes allow you to choose the knitwear for most patients. High-precision manufacturing techniques and strict quality control ensure correct pressure distribution and no defects.
The advantages of hospital knitwear over “classic leg bandaging” are obvious. Almost all clinics are aware that it is impossible to create dosed vertical (foot-shin-thigh) and horizontal (front surface of the leg - back surface of the leg, popliteal fossa) using elastic bandages. However, knowledge has not universally developed into beliefs, and especially into clinical practice, which was the reason for writing this section in sufficient detail.
Intermittent pneumatic compression
is carried out using a special compressor and cuffs divided into several chambers. Consecutive inflation of the chambers creates a “traveling wave” effect, which is especially useful in the absence of your own active muscle contractions. As a result, even in immobilized patients, the speed of venous blood flow increases significantly, i.e. one of the key factors of thrombogenesis is eliminated. Intermittent pneumocompression is most effective when using devices with microprocessor control and electronic timing (for example, the fifth generation pneumocompression device SCD, Fig. 2), which allows you to individually set the cuff filling time and maintain different levels of pressure on individual venous segments.
Rice. 2. SCD Response adjustable pneumatic compression device (Tyco Healthcare / Kendall)
With a digital display and special devices for fixing the device to the bed, intermittent pneumocompression becomes a relatively simple, safe and highly effective way to prevent venous thrombosis, which can be used during surgery, in the postoperative period, as well as in the intensive care unit for patients in hospital. critical condition. In cases where, due to a high risk of bleeding or for other reasons, the use of direct anticoagulants (surgeries on the brain and spinal cord, organs of vision and hearing, acute hemorrhagic stroke, etc.) is contraindicated, intermittent pneumatic compression in the modern version of its implementation is a method choice.
Specific prevention includes the perioperative use of pharmacological drugs of various groups.
Low molecular weight heparins (LMWHs) (ardeparin, dalteparin, nadroparin, parnaparin, reviparin, sandoparin, tinzaparin, certoparin, enoxaparin, etc.) are increasingly used for thromboprophylaxis in surgical patients, of which nadroparin calcium salt (fraxilarin) is currently the most widely used. , enoxaparin sodium salt (Clexane, Lovenox) and dalteparin sodium salt (Fragmin). Compared with UFH, these drugs have high bioavailability (more than 90%), a longer half-life (40 - 90 minutes for UFH and 1 90 - 270 minutes for LMWH) and antithrombotic effect (8 - 12 hours and 17 - 24 h), are less likely to bind to acute phase proteins, i.e. retain their effect against the background of endogenous intoxication, have a more predictable dose-dependent anticoagulant effect, do not stimulate, but weaken platelet aggregation, less often (less than 0.5%) cause thrombocytopenia and do not require laboratory monitoring.
These properties of LMWH are due to the chemical composition of the drugs. Heparin molecules, consisting of 25 - 50% of less than 18 saccharides (with a molecular weight of less than 5400 Da), are not able to bind ATIII and thrombin simultaneously, therefore they cannot accelerate the inactivation of the latter, but retain the ability to catalyze the process of factor Xa inhibition. As a result, the ratio of anti-Xa and anti-IIa activity in commercial LMWHs is not 1:1-2, as in UFH, α3-4:1; they have less anticoagulant activity than UFH, but have more pronounced antithrombotic properties. Since the composition of each of the commercial LMWHs is different, they differ, as we previously noted, from each other in physicochemical, biological and pharmacokinetic properties, are not interchangeable and have different clinical efficacy and safety.
Nadroparin calcium (Fraxiparin) is an official solution in pre-filled syringes with a volume of 0.3 - 0.6 -0.8 - 1.0 ml containing 2850 - 5700 - 7600 - 9500 MEanti-Xa activity, respectively. After a single subcutaneous administration of anti-Xa factor activity reaches a maximum after approximately 4 - 6 hours. Activity against Pa changes slightly and is maximum 3 hours after administration of the drug.
The main indications for use are: prevention of thromboembolic complications in general surgery, orthopedics, gynecology, as well as in other surgical and non-surgical patients who have a high risk of venous thrombosis due to acute respiratory or heart failure; patients being treated in the intensive care unit; patients with surgical infection. The drug is also used in the treatment of patients with acute coronary syndrome, the treatment of thromboembolic complications and for the prevention of blood clotting during hemodialysis.
The prophylactic use of nadroparin involves daily administration of the drug in a dose of 0.3 ml 1 time/day. before and after surgery in the form of a course lasting at least 7 days or until the patient’s motor activity is completely restored. During planned operations, the first dose is administered 2–4 hours before surgery; in emergency surgery, administration begins 1–2 hours after the end of surgery.
Enoxaparin sodium (Clexane)
- low molecular weight heparin, consisting of short mucopolysaccharide chains with an average molecular weight of 4500 daltons, produced in the form of an aqueous solution for injection containing 100 mg/ml in ready-made syringes of 0.2-0.4-0.6-0.8- 1.0 ml. 1 mg of enoxaparin corresponds to 100 MG of anti-Xa activity. The peak of anti-Xa factor activity reaches a maximum approximately 3 - 5 hours after subcutaneous administration and persists for 24 hours.
To prevent venous thrombosis and thromboembolism, surgical patients begin to administer enoxaparich before surgery: at a moderate risk, 20 mg (0.2 ml) 2 hours before surgery, at a high risk, 40 mg (0.4 ml) 1 2 hours before surgery and continue in the postoperative period for 7-10 days, one injection per day in the same doses. The use of higher doses of enoxaparin for the prevention of thrombosis in surgical patients is inappropriate, since increasing the dose is accompanied by a statistically significant increase in both anti-Xa activity and anti-lla, which significantly increases the incidence of hemorrhage. Therefore, higher doses of enoxaparin are used only in the treatment of deep vein thrombosis, as well as for the prevention of thrombosis during extracorporeal hemocorrection and hemodialysis operations. In the treatment of thromboembolic complications, the best safety/activity ratio of enoxaparin is achieved at a dose of 1 mg/kg, which is administered subcutaneously twice a day after 1-2 hours. For hemodialysis, enoxaparin is administered initially at a dose of 1 mg/kg for a 4-hour procedure. For patients with a high risk of bleeding, the dose is reduced to 0.5 - 0.75 mg/kg. If there are signs of fibrin deposition and a threat of thrombosis of the system, during a longer procedure, an additional 0.5 - 1 mg/kg can be administered.
At doses used for the prevention of venous thrombosis, enoxaparin has virtually no effect on bleeding time, blood clotting time, aPTT, and has no effect on platelet aggregation. In the first days of treatment with enoxaparin, moderate transient asymptomatic thrombocytopenia may appear. An asymptomatic and reversible increase in the number of platelets and an increase in the level of liver transaminases are possible. If the platelet count decreases by 30–50% of the initial value, treatment with enoxaparin should be discontinued.
Enoxaparin should be prescribed with caution to patients with a potential risk of bleeding, hypocoagulation, and patients with severe liver disease.
Consequences and complications
- Hemodynamic disturbances (increased vascular resistance in the pulmonary artery system and increased afterload on the right ventricle).
- Respiratory failure.
- Hypoxia.
- Pulmonary-pleural complications ( atelectasis , pleurisy , lung abscess , pulmonary infarction , pneumonia , pyopneumothorax , pleural empyema ).
- Post-embolic pulmonary hypertension .
List of sources
- Vatutin N.T., Sklyannaya E.V., Eshchenko E.V. /Pulmonary embolism. Review of recommendations of the European Society of Cardiology for diagnosis and treatment (2014 // Practical Angiology. - 2015. - No. 1 (68). - P. 5-18.
- Russian clinical recommendations for the diagnosis, treatment and prevention of venous thromboembolic complications // Phlebology. — 2015.— No. 4, issue 2. 46 p.
- Vatutin N.T. Emergency cardiology. - Donetsk, 2011. - 236 p.
- Vatutin N.T., Kalinkina N.V., Perueva I.A. /Pulmonary embolism // Practical angiology. – 2011. – No. 2. – P. 32-40.
- Vatutin N.T., Kalinkina N.V., Eshchenko E.V. and others. Pulmonary embolism (basic information and own observation) // Heart and Vessels. – 2013. – No. 1. – P. 120-123.