How does blood clotting occur?
The actual clot is formed by creating a fibrin network that strengthens and stabilizes it. This is due to the activation of the coagulation cascade - inactive blood coagulation factors circulating in the blood plasma begin to activate each other.
Under the influence of mechanical damage, platelets secrete thrombokinase, which triggers a number of processes leading to the formation of the corresponding factor that initiates blood clotting - calcium ions and plasma protein factors are important in this process.
Blood coagulation diagram
As a result of the blood clotting cascade, factor X together with factor Va forms a complex called prothrombinase, which converts prothrombin into thrombin. Thrombin, in turn, converts fibrinogen (a plasma protein that circulates in the blood) into fibrin (a water-insoluble protein), which forms a network of fibers that form the backbone of the clot.
Causes of blood clots
Thrombosis is promoted, in particular:
- surgical intervention;
- extensive injuries;
- respiratory failure;
- heart failure;
- oral contraceptives;
- use of certain hormonal drugs;
- phlebeurysm;
- obesity;
- stroke;
- age > 40 years.
The risk of thrombosis increases significantly during pregnancy and the postpartum period.
The risk of developing thrombosis during pregnancy is many times higher in people with congenital or acquired thrombophilia. It is estimated that 20-30% of cases of thromboembolic disease are associated with a genetic predisposition to its development.
The main genetic causes of the disease are the carrier of the Leiden variant of the factor V gene of the blood coagulation system (F5 gene, mutation p.Arg534Gln) or a mutation of the prothrombin gene (F2 gene, mutation c. * 97G>A). Other known predispositions are hereditary deficiencies of blood clotting inhibitors (antithrombin, protein C or protein S) and hereditary hyperhomocysteinemia (associated, for example, with mutations in the MTHFR gene). In families with thrombosis, factor V Leiden mutation and prothrombin gene mutation are detected in approximately 60% of cases.
Diagram of blood clot formation
Material and methods
We analyzed the course of pregnancy, childbirth, the postpartum period and perinatal outcomes in 7 pregnant women with CVT who were hospitalized at the Moscow Regional Research Institute of Obstetrics and Gynecology.
Diagnosis and treatment of pregnant women, women in labor and postpartum women with CVT were carried out by a multidisciplinary team of specialists: obstetricians-gynecologists, anesthesiologists, resuscitators, neurologists, neurosurgeons, ophthalmologists, neonatologists-resuscitators, ultrasound and radiation diagnostics doctors, rehabilitation specialists. To verify the diagnosis of CVT in pregnant and postpartum women, the following diagnostic methods were used: MRI (Fig. 1) , CT, MRI venography (Fig. 2) , ophthalmoscopy;
electroencephalography (EEG); 24-hour blood pressure monitoring and electrocardiography; ultrasound examination and duplex scanning of extra- and intracranial vessels. Rice. 1. MRI of the brain of patient D. The arrow indicates a focal lesion in the right hemisphere of the brain. Rice. 2. Magnetic resonance angiogram of the brain of patient D. Thrombosis of the left transverse, sigmoid, superior sagittal and direct sinuses;
bilateral venous infarctions of the subcortical nuclei (indicated by arrows). A pregnancy management plan, method of delivery and anesthesia were collectively developed. Since these pregnant women were at high risk of developing thrombotic complications, the hemostatic system was corrected using anticoagulants and antiplatelet agents were prescribed. Perinatal outcome was assessed by the nature of the early neonatal period.
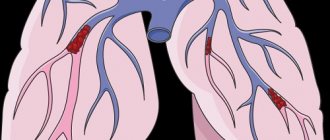
Why is a blood clot dangerous?
Thrombosis is life-threatening when a blood clot breaks away from the wall of a vein. A clot that travels through the bloodstream may travel into the atrium or ventricle or into the pulmonary artery, causing shock, cardiac arrest, and respiratory arrest due to a heart attack or pulmonary embolism. These conditions pose a direct threat to the life of a pregnant woman and can cause her sudden death.
Other consequences of thrombosis include: post-thrombotic syndrome, which manifests itself as changes in skin color, pulmonary hypertension and leg ulcers. In pregnant women, thrombosis can cause miscarriage, and pregnancy and the postpartum period significantly increase the risk of its occurrence.
The risk of thrombosis during pregnancy is associated with pressure on the iliac veins, which increases as the baby grows. In this case, embolism most often develops in the iliofemoral region of the deep veins, and in 90% of women, symptoms appear only on the left leg.
Statistics on morbidity and mortality of pulmonary embolism
Statistics show that about 0.1% of the world's population dies every year from pulmonary embolism. In terms of the frequency of deaths, this pathology is second only to coronary heart disease, strokes and some cancers.
The main cause of mortality in pulmonary embolism is late diagnosis. About 90% of patients do not receive the necessary treatment in the early stages of this acute condition.
The difficulty in diagnosing thromboembolism is that this condition can masquerade as a large number of heart and lung diseases. Many patients begin receiving therapy for heart attack or asthma. As a result, valuable time is lost.
Another danger of pulmonary embolism is suddenness. It affects not only patients with cardiovascular diseases, but also women during childbirth, and practically healthy people after injuries. Most often, after a diagnosis of pulmonary embolism is made, only 70% of patients can be saved. But if the pathology was diagnosed in a timely manner and optimal treatment was started, then this figure can increase to 98% percent.
Causes of thrombosis during pregnancy
During pregnancy and the perinatal period, the cause of thrombosis can be:
- hormonal changes necessary to maintain pregnancy (hormones cause vasodilation and stagnation of blood in the veins, increase blood viscosity, which contributes to the formation of blood clots);
- difficult outflow of blood from the legs due to pressure on the veins by the enlarging uterus;
- C-section;
- prolonged immobilization after childbirth, contributing to venous stagnation.
Stagnation of blood in the veins
The above risk factors, coexisting with an individual genetically determined predisposition to thrombosis, significantly increase the risk of thrombosis during pregnancy and its dangerous consequences.
Symptoms of thrombosis in pregnant women
Thrombosis in pregnant women is often asymptomatic. The symptoms that may appear with thrombosis during pregnancy can be ambiguous and can occur with other diseases, and when they do occur, they are often underestimated and considered typical pregnancy symptoms.
The most common symptoms that appear several days after a clot forms include:
- swelling, usually in one leg, around the ankle, then swelling in the calf or the whole leg (usually one leg is affected);
- pain in the legs, aggravated by walking, with increased pain and tenderness when touched, disappearing when the limb is immobilized;
- redness and increased warmth of the skin of the painful limb.
Dangerous symptoms of thrombosis appear when a blood clot breaks off and migrates along with the bloodstream. During complications of thrombosis (pulmonary embolism), difficulty breathing occurs with shortness of breath and chest pain. Difficulty in breathing progresses rapidly and can kill the patient within seconds.
Diagnosis of thrombosis
Diagnostic tests for thrombosis include blood chemistry tests (especially D-dimer levels) and duplex Doppler ultrasound of the venous vessels, which will determine the correct venous blood flow in the extremities and the presence of blood clots. These tests can confirm the occurrence of thrombosis only when symptoms first appear.
Ultrasound examination of venous vessels
A test to determine the risk of thrombosis is to perform DNA tests that look for abnormal gene mutations, and most often mutations that predispose to congenital thrombophilia, especially during pregnancy and the puerperium.
Determining the risk of the disease in women during the precontraceptive period will allow the doctor to begin appropriate prevention and treatment of thrombosis during pregnancy. To determine whether you are at risk of developing thrombosis, it is necessary to carry out thrombophilia tests, that is, tests that detect mutations in the genes encoding: factor V Leiden (Leiden-FVL mutation), prothrombin, methylenetetrahydrofolate reductase (MTHFR), factor PAI-I. / SERPINE1 Factor V R2.
In pregnant women, thrombosis most often occurs in the form of FVL mutation (2-10%), mutation of the methylenetetrahydrofolate reductase gene MTHFR (8-16%), mutation of the prothrombin gene (2-6%), deficiency of proteins C and S (0.2-1% ) and the presence of anticardiolipin antibodies (1-7%).
Both congenital (hereditary) thrombosis and acquired thrombosis increase the risk of pregnancy loss. An estimated 15% of pregnancies end in miscarriage, and this affects 0.4–2% of couples. The results of a clinical study conducted by the Nimes Obstetrics and Hematology Department (NOHA) showed a strong correlation between unexplained pregnancy loss and the presence of prothrombin and factor V mutations in the heterozygous system.
The Leiden mutation is a genetic defect that affects approximately 5% of white people, is associated with a 3- to 7-fold increased risk of venous thrombosis, and is predominantly inherited.
This means that a person who has a mutation in one copy of the gene (known as a heterozygote) has an increased risk of thrombophilia. The risk is even higher for people who are homozygous, i.e. have a mutation in both copies of the F5 gene. Heterozygotes for the Leiden mutation have a 2-3 times increased risk of pregnancy loss and other complications during pregnancy (for example, eclampsia, fetal malnutrition, premature placental abruption).
Thrombophilia associated with factor V Leiden mutation and thrombophilia associated with resistance to active protein C are inherited in an autosomal dominant manner. The resistance of factor V to activated protein C accelerates the blood clotting process and does not inhibit the growth of a blood clot.
Activated coagulation factor V is a component of the factor X enzyme complex that converts prothrombin to thrombin during blood clotting. Factor V deficiency is inherited in an autosomal recessive manner and promotes a slower blood clotting process due to decreased amounts of factor V.
Factor II of the prothrombin F2 gene (G20210A) causes thrombophilia associated with thrombin deficiency. The mutation increases the concentration of prothrombin in the blood and is inherited in an autosomal dominant manner. Prothrombin is activated to thrombin during blood clotting, which allows fibrinogen to be converted into fibrin; the presence of mutations disrupts this process.
The MTHFR gene encodes the enzyme methylenetetrahydrofolate reductase, which catalyzes the formation of 5-methyltetrahydrofolate, which is necessary for the conversion of the potentially toxic amino acid homocysteine to methionine by methionine synthase. This mutation is manifested by an increase in the concentration of homocysteine in the blood serum. Excess homocysteine in the body can damage the endothelium of blood vessels and, as a result, lead to atherosclerosis and venous and arterial thrombosis.
Thrombophilia associated with prothrombin is predominantly inherited and is characterized by symptoms of venous thromboembolism, in adults mainly deep vein thrombosis or pulmonary embolism. Thrombophilia associated with the presence of the c.20210G>A variant of the F2 gene has been shown to increase the risk of pregnancy loss.
Deep vein thrombosis
The risk of thrombosis during pregnancy and pulmonary embolism is reduced by any measures to improve blood flow and prevent its stagnation. In pregnant women with a genetically confirmed predisposition to the disease, prophylactic anticoagulants (low molecular weight heparins) are used under strict medical supervision. These women should wear special tights, maintain a healthy body weight, eat a healthy low-fat diet, hydrate regularly, be physically active and avoid prolonged sitting.
By performing genetic tests for thrombophilia and gene mutations that cause thrombosis, a pregnant woman can prevent serious complications (mainly pulmonary embolism and miscarriage) by administering prophylactic or anticoagulant treatment in close collaboration with her doctor. Only this procedure guarantees the correct course of pregnancy, childbirth and the postpartum period for both mother and child.
Once performed, genetic testing will provide lifelong information and enable you to make better decisions, including preventative measures and reducing symptoms of thrombosis during pregnancy and throughout the fertile period.
MAKE AN APPOINTMENT
[contact-form-7 id=”296" title=”Untitled”]
Abortion and contraception clinic in St. Petersburg - department of the medical gynecological association "Diana"
Make an appointment, tests or ultrasound via the contact form or by calling +8 (812) 62-962-77. We work seven days a week from 09:00 to 21:00.
We are located in the Krasnogvardeisky district, next to the Novocherkasskaya, Ploshchad Alexander Nevsky and Ladozhskaya metro stations.
The cost of a medical abortion in our clinic is 3,300 rubles. The price includes all pills, an examination by a gynecologist and an ultrasound to determine the timing of pregnancy.
Causes and symptoms of pulmonary embolism
The development of pulmonary embolism has several main causes:
- Acute deep vein thrombosis of the ileofemoral segment.
- Deep vein thrombosis of the leg.
- Thrombosis of the venous plexuses of the pelvis.
- Blood clots in the right cavities of the heart.
- Thrombophlebitis of the superficial veins (a fairly rare cause).
- Septic generalized process.
- Cardiovascular diseases that predispose to the formation of blood clots (ischemic heart disease, rheumatism with the presence of mitral stenosis, endocarditis, cardiomyopathy, etc.).
- Oncological diseases.
- Thrombophilia (impaired vascular hemostasis, changes in blood clotting properties).
- Antiphopholipid syndrome (formation of antibodies to phospholipids of platelets, endothelial cells and nervous system).
The greatest danger to the patient’s health is posed by so-called “floating” blood clots. They have a single small point of fixation, so they can be damaged quite easily and move into the pulmonary circulation.
Risk factors for the development of pulmonary embolism
When diagnosing an acute condition, the presence of risk factors that could provoke thrombus formation must be taken into account. The most significant are:
- fracture of the upper femoral neck;
- hip or knee replacement;
- undergone major operations;
- history of serious injuries;
- brain damage.
The following should also cause caution regarding thrombosis:
- arthroscopy of the knee joint;
- installation of a central venous catheter;
- history of chemotherapy;
- oncological diseases;
- taking hormonal therapy (including combined oral contraceptives);
- history of strokes;
- pregnancy, childbirth and the postpartum period;
- thrombophilia.
The presence of cancer and concomitant chemotherapy significantly increases the risk of developing pulmonary embolism. About 15% of patients with a malignant neoplasm die due to blockage of the pulmonary artery by a thrombus or embolus. Chemotherapy treatment increases the risk of thrombosis by 47%.
In more rare cases, the development of thromboembolism is facilitated by:
- long-term immobilization of a limb or bed rest (more than 3 months);
- frequent air travel;
- elderly and senile age;
- phlebeurysm;
- history of laparoscopic surgery.
Also, risk factors characteristic of the formation of any thrombosis can lead to the development of pulmonary embolism:
- smoking;
- obesity;
- physical inactivity (sedentary lifestyle);
- diabetes;
- increased blood cholesterol and triglyceride levels (especially LDL and VLDL);
- regular psycho-emotional overload;
- poor nutrition.
With age, the risk of developing pulmonary embolism increases. A hereditary predisposition to the development of the disease has also been proven. Therefore, if close relatives have had episodes of pulmonary embolism, you need to carefully monitor your health.
Symptoms of pulmonary embolism
The course of the disease is multifaceted and varied. There are no specific symptoms characteristic only of PE. Therefore, for correct diagnosis, the professionalism of the doctor and modern medical equipment are important, which will help differentiate the pathology in the shortest possible time.
The first manifestations of PE (acute) may be chest pain (similar to myocardial infarction), shortness of breath, cough, hemoptysis, decreased blood pressure, cyanosis and loss of consciousness (fainting).
In most cases, the diagnosis of pulmonary embolism is made by exclusion. First of all, it is necessary to differentiate it from myocardial infarction.
A characteristic feature of PE is the suddenness of shortness of breath. For example, habitually climbing the stairs to the 2nd floor can provoke it.
If small pulmonary vessels are affected, the symptoms may be blurred and non-specific. Signs of pulmonary infarction appear only 3-5 days after the development of the disease: pain in the chest, cough and hemoptysis. Pleural effusion accumulates in the chest. The fever can last from 2 to 12 days.
The full range of symptoms can be found only in every seventh patient. Most people have only 1-2 nonspecific symptoms. Particular difficulties are caused by making a diagnosis in cases where small branches of the pulmonary artery are affected. The manifestations of the pathology are erased, so often the correct diagnosis can be made only on the 3-5th day.
For patients with pulmonary embolism, it is important to make a timely diagnosis. This helps reduce the symptoms of the disease, as well as improve overall well-being. To speed up diagnosis, special scales have been developed. For inpatient settings, the PSWells model is used, and for outpatients, the Geneva model is used.
In parallel with establishing the diagnosis of pulmonary embolism, it is important to identify the cause of thrombosis. This is also quite difficult to do, since blood clots formed in the veins tend to be asymptomatic.
How does pulmonary embolism develop?
The main link in pathogenesis is venous thrombosis. There are a number of factors that contribute to the development of thrombosis. Among them:
- decreased blood flow speed in the veins;
- increased blood viscosity;
- turning off passive contraction of the venous wall;
- compression of veins by space-occupying formations.
The main prerequisites for the development of venous thrombosis are:
- disorders of the blood coagulation system. May develop due to internal factors in the body, as well as when taking certain medications. Patients taking hormone replacement therapy, including combined oral contraceptives, should regularly take a blood coagulogram. The latter can cause the development of thrombosis and pulmonary embolism in young women.
- Damage to the vascular wall Can develop for many reasons: trauma, surgical interventions on the vascular wall, viral damage, thrombophlebitis, hypoxia, etc.
Blood clots are dangerous formations that can cause sudden death. Therefore, it is important to carry out high-quality diagnostic measures to identify them. Ultrasound is considered the main way to detect thrombosis.
Floating (moving) blood clots pose a particular health hazard. They are loosely attached to the wall of the vessel and are able to move in its lumen. The detachment of such formations occurs very easily, and they quickly move with the blood flow and are accompanied by impaired pulmonary circulation.
Changes in hemodynamic parameters during pulmonary embolism appear when 30-50% of the vascular bed is blocked. In the pulmonary circulation, pressure increases and the load on the right ventricle of the heart increases. As a consequence, acute right ventricular failure develops.
Oxygen transportation suffers, the concentration of carbon dioxide in the blood increases, and hypercapnia develops. Coronary blood flow is disrupted, blood pressure decreases and systemic hypotension develops. All this leads to fainting, collapse, cardiogenic shock, or even clinical death.
Stabilization of blood pressure during pulmonary embolism is a misleading indicator of hemodynamic stability. Normal numbers after 24–48 hours may again be significantly reduced due to repeated thromboembolism and ongoing thrombosis of the arterial vessels of the lungs. A so-called “vicious circle” is formed when some symptoms aggravate others.
Classification of pulmonary embolism
The classification of pulmonary embolism is varied: according to the volume of the lesion, the severity of the process, the speed of development, etc.
According to the volume of damage to the vascular bed
The following options for TELA are distinguished:
- Massive Localization of the thrombus - the main trunk of the pulmonary artery or large vessels adjacent to it. Damage to the vascular bed reaches 50-75%. The patient's condition is extremely serious and urgent medical attention is required. There is a significant decrease in blood pressure and increasing tachycardia. Cardiogenic shock and acute right ventricular failure develop. Patients with massive pulmonary embolism have a high mortality rate.
- Embolism of lobar, segmental pulmonary arteries Damage to the vascular bed varies between 25-50%. The patient's condition is moderate. All the main symptoms of the disease are expressed, however, blood pressure is reduced only slightly.
- Embolism of small branches of the pulmonary arteries Damage to small vessels is characterized by a blurred clinical picture and an asymptomatic course. Up to 25% of the vascular bed is affected. The condition has a relapsing course, and therefore also requires careful monitoring of the patient.
According to the clinical course
The following variants of the course of pulmonary embolism are distinguished:
- Acute (“lightning-fast” development of symptoms) Instant and complete blockage of the pulmonary arteries (main trunk). As a result, acute respiratory failure and respiratory arrest develop. The patient enters a state of collapse and ventricular fibrillation begins. With this course, death can occur in just a few minutes.
- Acute In this condition, obstruction of the lobar branches of the pulmonary artery, as well as its main trunk, rapidly increases. The disease begins suddenly and progresses rapidly. Symptoms of respiratory and cerebral insufficiency increase. In the absence of adequate treatment, death occurs within 3-5 days.
- Subacute (protracted course of symptoms) Subacute course is characterized by the development of multiple pulmonary infarctions. The large and medium branches of the pulmonary artery become blocked. May last for several weeks. Progression is slow, gradual. Symptoms of respiratory and right ventricular failure become more pronounced as the disease progresses. It is characterized by the risk of developing repeated thromboembolism and exacerbation of symptoms in the absence of treatment.
- Chronic (recurrent) Thrombosis affects the lobar and segmental branches of the pulmonary artery. The main manifestation of the pathology is repeated pulmonary infarctions, gradually increasing arterial hypertension of the pulmonary circulation and symptoms of right ventricular failure. However, most often patients with a chronic course first present with complaints to a pulmonologist, where a diagnosis of “bilateral pleurisy” is made. Also, this type of pathology often develops in the postoperative period or against the background of existing diseases (cardiovascular, cancer, etc.)
The main influence on the rate of development of disease symptoms is the percentage of damage to the vascular bed of the pulmonary circulation. For each type of course, treatment is selected individually. In the case of a fulminant and acute form, you need to seek emergency medical help, and in the case of a chronic course, together with your doctor, choose the most gentle treatment tactics that will not have a significant impact on your lifestyle.
By severity
Pulmonary embolism has several degrees of severity:
- mild severity of the disease develops in 15–27% of patients;
- moderate severity is observed in 45–57% of patients with pulmonary embolism;
- severe degree is recorded in 16–35% of patients.
The prognosis of the course of the disease in patients with pulmonary embolism is based on the use of special scales. They include 11 indicators that help identify the risk of 30-day mortality for a given condition.
Complications of pulmonary embolism
The main danger of the disease in its acute course is sudden death due to cardiac arrest. If the disease develops gradually, pulmonary hypertension appears.
Chronic thromboembolic pulmonary hypertension is a pathology in which blockage of small and medium branches occurs with thrombotic masses. This leads to an increase in the load on the heart (especially its right parts: the atrium and ventricle). This condition can be treated with both conservative and surgical methods. The most informative way to confirm the diagnosis is pulmonary artery catheterization. There is pressure in the pulmonary artery above 25 mm Hg, an increase in pulmonary vascular resistance above 2 Wood units, emboli during long-term anticoagulant therapy (3-5 months).
The most severe complication of chronic thromboembolic pulmonary hypertension is right ventricular failure. It is characterized by:
- general weakness;
- feeling of heartbeat;
- decreased exercise tolerance;
- accumulation of fluid in the abdominal cavity (ascites);
- accumulation of fluid in the chest (hydrothorax);
- accumulation of fluid in the heart sac (hydropericardium).
A feature of shortness of breath in right ventricular failure is its absence at rest in a horizontal position. Long-term circulatory disorders and hypoxia cause atrophy of internal organs, protein imbalance and weight loss. The prognosis for this condition is unfavorable. Drug therapy can only temporarily stabilize the condition. As symptoms progress, cardiac reserves become depleted. Life expectancy for right ventricular failure with poorly selected therapy does not exceed 2 years.
How is pulmonary embolism diagnosed?
Diagnosis of pulmonary embolism is carried out according to the following algorithm:
- Estimation of clinical probability (pre-test probability).
- Determination of D-dimer level (threshold values previously adjusted for age and level of clinical probability of pulmonary embolism are taken into account).
- Contrast-enhanced computed tomography of the pulmonary artery. It is a highly evidence-based method for diagnosing pulmonary embolism. It can be used to visualize both small and large branches of the pulmonary artery. It is not possible to perform the study during pregnancy, intolerance to iodine-containing contrast agents and some other conditions.
- Lung scintigraphy. A study of pulmonary blood flow is performed. To do this, a small amount of radioactive substance is injected into the body. And then, using a gamma camera, the distribution of this substance in organs and tissues is visualized. The method is effective and recommended for the vast majority of patients. However, lung scintigraphy is not readily available.
- Angiopulmonography. An invasive test that is also designed to evaluate pulmonary blood flow. To do this, an X-ray contrast agent is injected into the vessels of the lungs. Using this technique, it is possible not only to determine the fact of embolism, but also the extent of damage to the vascular bed.
- Magnetic resonance imaging.
- Echocardiographic examination (bedside, if there is a high probability of pulmonary embolism).
- Compression ultrasound examination of venous vessels.
To assess the clinical likelihood of pulmonary embolism, the following risk factors are taken into account:
- surgeries and fractures in the previous month;
- history of malignant neoplasms;
- age over 65 years;
- tachycardia (high heart rate);
- pain in one of the lower extremities;
- hemoptysis.
Tests to diagnose pulmonary embolism
To effectively diagnose this pathological condition, a number of studies are carried out:
- Determination of D-dimer One of the most effective and informative methods for diagnosing pulmonary embolism. However, even this laboratory test is not completely specific. Increased results may also occur in pregnant women, the elderly, cancer patients, and during atrial fibrillation.
- Troponin levels increase as ischemia of the right ventricle (in rare cases, the left) increases.
- H-FABR A cardiac protein capable of binding fatty acids. Provides information in acute pulmonary embolism.
- The level of sodium diuretic peptide increases with right ventricular failure.
Electrocardiographic study for pulmonary embolism
An ECG is a medical procedure that is mandatory for every patient with suspected pulmonary embolism. The popularity of the technique is associated with its availability in any medical institution, and any emergency medical team has a portable ECG device. On the film for pulmonary embolism the following is recorded:
- acute overload of the right atrium and ventricle;
- complex rhythm disturbances;
- signs of coronary blood flow insufficiency.
All this allows you to suspect the correct diagnosis, choose the appropriate treatment tactics and reduce the risk of developing severe complications.
EchoCG is also performed to diagnose PE. This study allows you to obtain important information about the state of blood flow and the level of pressure in the pulmonary artery. EchoCG allows one to differentiate PE from pericardial tamponade, aortic dissection and other cardiac pathologies. Despite its high information content, the technique is rarely used in emergency patients. This may be due to the impossibility of organizing a 24-hour ultrasound service, the lack of a transesophageal sensor, the patient’s obesity, etc.
Ultrasound for diagnosing pulmonary embolism
Ultrasound has important diagnostic value for pulmonary embolism. It can be used to examine the veins of the lower extremities. It is carried out at four points: in the groin and popliteal region on both sides. The larger the study area, the higher the diagnostic value of the technique.
How is pulmonary embolism treated?
Preserving life and maximum health is the main priority in the treatment of PE. Doctors’ efforts are also aimed at preventing the development of chronic pulmonary hypertension.
When pulmonary embolism is detected, it is first necessary to eliminate the thrombus or embolus that is preventing blood flow in the vessels of the pulmonary circulation.
Emergency condition: methods of treating pulmonary embolism in the acute phase
For the treatment of thromboembolism in the acute phase, the following are used:
- Drug therapy aimed at eliminating right ventricular failure.
- Life support. If necessary, patients are connected to a ventilator (artificial lung ventilation).
- Mechanical circulatory support.
- Extracorporeal membrane oxygenation (saturation of blood with oxygen).
- Anticoagulant therapy Injectable forms of anticoagulants are used. This is a group of drugs that prevents the formation of new blood clots. New generation anticoagulants (NOACs) are presented in tablet form. However, at present they are used quite rarely to relieve acute conditions.
- Oral vitamin K antagonists.
- Systemic thrombolysis. This procedure is aimed at dissolving existing thrombotic masses in the area of the systemic circulation. In each individual case, the doctor individually selects the drugs and the required dosages.
- Percutaneous catheter therapy.
- Use of compression orthopedic garments “for the entire leg.” Elastic bandages can be used as an alternative.
For each patient, treatment tactics are selected individually. The clinical severity of the condition, symptoms and medical history must be taken into account.
Surgical techniques for the treatment of pulmonary embolism
Surgical treatment of pulmonary embolism is not uncommon in modern medical practice. Surgical intervention is indicated for massive lesions, when it is necessary to restore the patency of almost completely blocked vessels. Thrombectomy is used to normalize hemodynamics.
To determine the degree of risk and select a treatment strategy in patients with stable hemodynamics, specialized scales are used.
| Options | PESl scale | sPESl scale |
| Age (in years) | Age in years | 1 (if age over 80 years) |
| Male | +10 | — |
| Presence of malignant neoplasms | +30 | 1 |
| CHF (chronic heart failure) | +10 | 1 |
| Chronic lung diseases | +10 | — |
| Heart rate more than 110 beats/min | +20 | 1 |
| Systolic pressure level is less than 110 mmHg. | +30 | 1 |
| Respiratory rate more than 30 per minute | +20 | — |
| Body temperature less than 36.0 degrees Celsius | +20 | — |
| Impaired consciousness | +60 | — |
| Blood oxygen saturation level less than 90% | +20 | — |
| 30-day mortality risk level | ||
| Class I (≤65 points) Very low risk 0–1.6% | 0 points – risk 1% Confidence interval: 0-2.1% | |
| Class II (66–85 points) Low risk 1.7–3.5% | ||
| Class III (86–105 points) Moderate risk 3.2–7.1% | ≥1 point – risk 10.9% Confidence interval: 8.5–13.2% | |
| Class IV (106–125 points) High risk 4.0–11.4% | ||
| Class V (≥126) Very high risk 10.0–24.5% | ||
Thrombolytic therapy in the treatment of pulmonary embolism
Thrombolytic therapy has been shown to be effective in 92% of patients with pulmonary embolism. With its help, it is possible to achieve an improvement in basic hemodynamic parameters. This method of treatment, when started in a timely manner, significantly improves the prognosis of the disease, with a small number of contraindications to the technique.
The main disadvantage of thrombolytic therapy is time limitations. It is effective only in the first 2 days after the development of pulmonary artery thrombosis. After this period, the effectiveness of the drugs decreases, and hemorrhagic complications make the treatment more dangerous than effective. In low-risk patients, thrombolysis is not indicated.
Implantation of venous filters
In cases where it is impossible to prescribe anticoagulants or they are ineffective, venous filters are installed. Special devices are installed in the inferior vena cava that trap thrombotic clots and emboli from peripheral veins.
Alternative Treatments
If the patient has contraindications to systemic fibrinolysis, then the transcatheter thrombus fragmentation technique can be used. During this manipulation of blood clots, they are destroyed, and their contents are immediately aspirated (removed).
If the patient has a thrombus or embolus in the central pulmonary vessels, surgical removal is recommended. Especially with the development of refractory cardiogenic shock to therapy, the presence of contraindications to fibrinolytic therapy or its low effectiveness.
Features of anticoagulant therapy
Anticoagulant therapy in patients with acute venous thrombosis continues for at least 3 months. All medications are taken strictly as prescribed by the attending physician in appropriate dosages. Dynamic monitoring of the state of the blood coagulation system is carried out.
Treatment of acute conditions begins with parenteral (intravenous) unfractionated heparin. With its help, it is necessary to increase the activated partial thromboplastin time by 1.5–2 times in comparison with the initial laboratory values.
After stabilization of the condition, a transition to maintenance anticoagulant therapy is necessary. Intravenous administration of the drug is abandoned in favor of subcutaneous injections. In parallel, warfarin is prescribed to achieve normal INR (international normalized ratio) values. In modern clinics, warfarin is replaced with more modern drugs: Pradaxa, Xarelto, etc. Compared to warfarin, they have an extremely important advantage: they do not require constant monitoring of the INR and can be used in oral forms even at home.
In some cases, an increase in the duration of anticoagulant therapy is required. This is especially often necessary for patients with repeated episodes of pulmonary embolism, right ventricular dysfunction, antiphospholipid syndrome, etc. The duration of taking medications is prescribed by the doctor individually. Self-medication is prohibited, as it can lead to the development of a number of serious complications (for example, bleeding from internal organs and varicose veins).
Additional recommendations for pulmonary embolism
PE is a serious condition that requires not only drug treatment, but also lifestyle adjustments.
Diet therapy for pulmonary embolism
With the development of pulmonary artery thrombosis and after treatment, strict adherence to the diet is required. It is necessary to exclude from the diet:
- spicy food;
- fatty foods;
- foods high in glucose.
It is also necessary to limit the consumption of foods containing vitamin K. These include: broccoli, milk, cottage cheese, cabbage, olive oil, etc. This is necessary in order to avoid stimulation of the blood coagulation system.
Pregnancy and pulmonary embolism
PE occurs in pregnant women with a frequency of 0.3–1 case per 1000 births. Diagnosis of this condition is complicated by the fact that complaints of shortness of breath can be caused not only by pathological processes, but also by physiological changes in the woman’s body. Thus, often an increase in the height of the diaphragm dome is one of the most common causes of shortness of breath in pregnant women. And in the absence of additional symptoms, it is most often accepted by doctors as a normal condition.
Also, for women carrying a child, indications for X-ray examinations, computed tomography and magnetic resonance imaging are strictly limited. This creates diagnostic difficulties.
The main method for diagnosing pulmonary embolism is ultrasound of the lower extremities. If thrombotic masses are detected, anticoagulants are prescribed. If during an ultrasound examination it was not possible to visualize the formation, and the symptoms rapidly increase, then the woman is additionally sent for a CT examination of the chest.
The main treatment for PE in pregnant women is low molecular weight heparins. Their advantage is that they do not penetrate the placental barrier and do not cause fetal development abnormalities. They are prescribed over a long course (from 3 months) and can be used until childbirth. New oral anticoagulants are not indicated for pregnant women.
Vitamin K antagonists may be used with caution in the second trimester. In the first and third trimester of pregnancy, their use is highly discouraged due to the increased risk of bleeding.
Anticoagulant therapy is also continued for three months after birth.
Pulmonary embolism in children
Thrombosis is extremely rare in childhood. They can develop in cases against the background of oncohematological diseases.
Prevention of acute pulmonary embolism
Preventive measures to prevent pulmonary embolism include:
- Reducing the duration of immobilization and bed rest This is necessary in cases where the patient's condition allows movement. However, modern experience in the treatment of serious illnesses shows that early activity (even warming up in bed) increases the speed of rehabilitation after illnesses.
- Lifestyle changes It is necessary to give up smoking (both active and passive), devote time to sports and walks in the fresh air.
- The use of compression garments “for the entire leg” in the postoperative period
- Use of anticoagulant therapy after surgery
- Regular ultrasound examination of the veins of the lower extremities It is especially advisable to carry it out for patients who are at risk: women taking COCs, cancer patients, people with a sedentary lifestyle and frequent air travel, people with a genetic predisposition. Also, these groups of people can be prescribed anticoagulants on a routine basis.
To prevent the development of thrombosis during air travel, it is necessary to maintain a drinking regime, get up and walk every 1.5 hours.
If venous thrombosis has already developed, then it is possible to prevent pulmonary embolism using surgical techniques. For this the following can be used:
- implantation of a filter into the inferior vena cava;
- endovascular catheter thrombectomy (during the operation, the blood clot is removed from the vein using a catheter inserted into it);
- ligation of the great saphenous or femoral vein (these vessels are the main source of thrombotic masses in the body).