The circulatory system in the human body is quite complex - the body has a huge number of vessels of various lengths and sizes. For convenience, the entire system is divided into several circles. For example, the Circle of Willis ensures complete blood circulation in all parts of the human brain. Its structure also includes arteries that pass in a certain place through the spinal column. And with a number of disorders, a disease such as hypoplasia of the left/right vertebral artery can develop. What is it, is it treated and how to identify this pathology?
Hypoplasia of the left/right vertebral artery - what is it?
What kind of pathology is this?
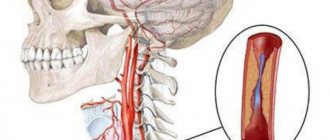
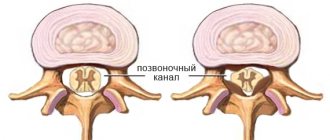
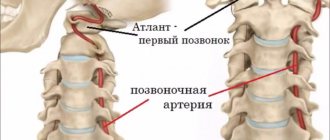
To understand what hypoplasia of the arteries of the spinal column is, you need to study a little about the blood supply system itself in this part of the body and brain. The latter receives blood precisely from the vertebral arteries, as well as the internal carotid arteries. Each vessel is responsible for delivering blood to a specific part of the brain. The vertebral arteries are connected to each other, and if a situation occurs that one of them is blocked, then the other will feed the brain, compensating for the losses.
Cerebral circulation
The vertebral arteries themselves depart from the subclavian artery and reach the human head, where they are divided into smaller vessels. They are located inside the spinal canal of the cervical spine and make several bends as they pass through it.
General information about hypoplasia
Normally, the arteries are the same, but under a certain set of circumstances, one of them - left or right - may have certain abnormalities, which is why hypoplasia occurs. This is underdevelopment of the artery or a narrowing of its diameter to 2 or less millimeters.
Hypoplasia of one of the vertebral arteries can lead to circulatory disorders, resulting in dysfunction of the heart and the rest of the blood supply system, disruption of the vestibular apparatus, etc.
One of the causes of the pathology may be osteochondrosis
Hypoplasia of the vertebral artery can be:
- right-sided;
- left-handed;
- double-sided
On a note! As a rule, hypoplasia is a congenital disease. But it also happens that pathology develops later due to the influence of certain factors. Thus, arterial hypoplasia can be divided into congenital and acquired. Most often, the pathology affects the right artery - it occurs in every tenth person, according to statistics. This is due to the fact that the length of the right artery is usually slightly longer than the left.
Hypoplasia of the right vertebral artery
Medical Internet conferences
O.A. Fomkin - Saratov State Medical University named after. IN AND. Razumovsky Ministry of Health of Russia, assistant at the Department of Human Anatomy, Candidate of Medical Sciences; V.N. Nikolenko - State Budgetary Educational Institution of Higher Professional Education First Moscow State Medical University named after. THEM. Sechenov Ministry of Health of Russia, Vice-Rector for Scientific and Innovation Activities, Professor of the Department of Human Anatomy, Director of the Research Institute of Molecular Medicine, Professor, Doctor of Medical Sciences; Yu.A. Gladilin – State Budgetary Educational Institution of Higher Professional Education Saratov State University named after. IN AND. Razumovsky Ministry of Health of Russia, Associate Professor of the Department of Human Anatomy, Doctor of Medical Sciences.
Introduction. Variability as a general biological phenomenon does not lose its relevance and deserves the attention of many scientists [1, 2]. Variability shows the plasticity of living systems and is associated with the implementation of the adaptive strategy of a natural population. The study of variability makes it possible to judge the interaction of the genotype with environmental factors during ontogenesis [3].
In response to the demands of clinical medicine, at present, detailed information is required not so much on the typical structure or average anatomical norm of an organ, but on the entire spectrum of its individual, typical and combined variability [2]. This also applies to the arterial vessels of the brain.
The subject of this study is the posterior communicating artery (PCA). As a branch of the cerebral part of the internal carotid artery, it participates in the formation of the arterial (Circle of Willis) circle of the brain. It has been proven that blood through this artery can flow in both directions [4]. In this regard, the PCA plays an important role in the implementation of compensatory collateral blood flow between the systems of the internal carotid and vertebral arteries.
The structure of the posterior communicating arteries varies greatly. Compared to other cerebral arteries, they have a small diameter and almost a pinpoint lumen [5-7].
Purpose of the study: to determine the variants of the posterior communicating artery (PCA) of adults depending on the individual and combined variability of its morphometric parameters.
Materials and methods . The study material was PCA obtained from autopsies of 115 corpses of people aged 21 to 84 years who died for reasons not related to acute or chronic cerebrovascular pathology. A total of 230 artery samples were examined. To study the morphology of the artery, transverse millimeter sections were made using a razor. Then the sections were placed in a Petri dish with saline solution and the outer diameter and wall thickness of the artery were measured under a microscope with an accuracy of 0.01 mm. The diameter of the artery lumen is presented in the study as the difference between the outer diameter and twice the thickness of the artery wall.
The obtained data were processed by the variation-statistical method using the Statistica-6 application package and Microsoft Exsel Windows-XP. To check the presence of a normal distribution, the Kolmogorov-Smirnov test was used. The distribution of parameters in the studied sample did not differ from normal. In this regard, for all parameters, the minimum and maximum values, the arithmetic mean (M), the error of the arithmetic mean (m), the standard deviation (s), and the coefficient of variation (Cv) were determined. To assess the reliability of differences between series, a parametric test (Student's test) was used. In this case, the differences were considered significant at a 95% probability threshold (p < 0.05). When studying individual variability, like most researchers dealing with the range of anatomical norms, we took the variation interval M ± σ as the average value of a trait. Since statistically significant gender differences in the length, wall thickness and lumen diameter of the PCA were found [6], variations in these parameters were calculated separately for men and women.
Results. The average values of the morphometric parameters of the PCA (230 samples), excluding gender, age and cerebral hemisphere, were: 1) length 12.26±0.19 mm (A=5.30-20.10 mm; s=2.89 mm; Сv=23.6%); 2) outer diameter – 1.33±0.02 mm (A=0.80-2.10 mm; s=0.26 mm; Cv=19.2%); 3) wall thickness – 0.23±0.01 mm (A=0.12-0.40 mm; s=0.06 mm; Cv=26.2%); 4) lumen diameter – 0.88±0.02 mm (A=0.46-1.46 mm; s=0.25 mm; Cv=28.0%).
Significant variability in the morphometric parameters of the PCA made it possible to identify groups of variants of their values (Table 1).
According to the length of the PCA, they were divided into: short - length less than 9.89 mm in men and less than 8.80 mm in women; medium in length - with a length from 9.90 to 15.72 mm for men and from 8.81 to 11.51 for women; long – with a length of more than 15.73 mm in men and more than 11.52 mm in women. The average age of subjects with short PCAs was 1.2 times greater than that of people with long arteries – 51.3±2.9 years and 43.0±2.6 years, respectively (p=0.039). The quantitative ratio of men and women in the group of subjects with short arteries is 39.5 and 60.5%; in the group of subjects with long arteries – 71.9 and 28.1%.
Based on the size of the outer diameter, SSAs are: thin - diameter less than 1.06 mm; medium diameter (medium wide) - the diameter ranges from 1.07 to 1.59 mm and wide - with a diameter of more than 1.60 mm. Subjects with wide PCA were on average 1.2 times older than people with thin arteries - 55.0±3.0 years and 45.5±2.1 years, respectively (p=0.011). The quantitative ratio of men and women in the group of subjects with thin arteries is 59.0 and 41.0%; in the group of subjects with wide arteries, the representation of men and women is equal – 50% each.
Based on the wall thickness, the PCA was divided into: thin-walled - wall thickness less than 0.17 mm in men and less than 0.14 mm in women; medium in thickness - with a wall thickness from 0.18 to 0.30 mm for men and from 0.15 to 0.25 mm for women; thick-walled - with a wall thickness of more than 0.31 mm in men and more than 0.26 mm in women. The average age of subjects with thick-walled PCAs was 1.7 times greater than that of people with thin-walled arteries - 69.2±2.3 years and 40.7±2.5 years, respectively (p=1·10-6). The quantitative ratio of men and women in the group of subjects with thin-walled arteries was 25.7 and 74.3%; in the group of those studied with thick-walled arteries – 78.9 and 21.1%.
Depending on the size of the lumen diameter, we have identified PSA: with a narrow lumen - the lumen diameter is less than 0.58 mm in men and less than 0.68 mm in women; with an average lumen - the diameter of the lumen varies from 0.59 to 1.07 mm in men and from 0.69 to 1.19 mm in women; with a wide lumen - the lumen diameter exceeds 1.08 mm in men and 1.20 mm in women. The age of subjects with a narrow and wide lumen of the PCA did not differ significantly - 51.2 ± 2.5 years and 45.6 ± 3.1 years, respectively (p = 0.163). The quantitative ratio of men and women in the group of subjects with a narrow lumen of the PCA was 75.6 and 24.4%; in the group of those studied with a wide lumen of the PCA – 43.6% and 56.4%.
It was noted that approximately 71.9% of all thin ACAs had a narrow lumen, and 28.2% had an average lumen. At the same time, thin PCAs had thin wall thickness in 23.1% of cases, thick wall thickness in 5.2% of cases, and medium wall thickness in 71.8% of cases (Table 2).
Medium-wide arteries in 87.1% of cases were characterized by an average lumen diameter; in 7.7% of cases, such PCAs had a narrow, and in 5.2%, a wide lumen diameter. Medium-wide arteries, as a rule, had an average wall thickness (67.1% of observations); thin- and thick-walled PCA were also found in this group – in 12.9 and 20% of cases, respectively.
Wide PCAs had a wide lumen diameter in 86.1% of cases and an average lumen diameter in 13.9% of cases. Moreover, in 13.9% of cases they were thin- or thick-walled, and in the remaining 72.2% they were characterized by an average wall thickness.
The combined variability of the morphometric parameters of the PCA made it possible to identify 18 of its types. The most common are medium-wide ACAs with an average wall thickness and an average lumen (43.0%). Rare types (frequency of occurrence less than 1%) include thin PCA (with an average wall thickness and lumen diameter, with a thin wall and a narrow lumen, a thick wall and a narrow lumen, a thick wall and an average lumen); ASA with a medium outer diameter, a thick wall and a wide lumen; wide PCA with a thick wall and medium lumen (Fig. 1). Thin-walled SSAs based on our material never had a wide lumen diameter.
Rice. 1.Frequency of occurrence of types of PSA,% ( OD – outer diameter , TS – wall thickness, DP – lumen diameter ): 1 – average for ND, TS and DP; 2 – thin with medium TS and narrow DP; 3 – wide with medium TC and wide DP; 4 – average in ND, with a thick wall and average DP; 5 – average according to ND with a thin wall and average DP; 6 – thin with a thin wall and medium DP; 7 – average according to ND with a thick wall and narrow DP; 8 – average according to ND, with a thin wall and wide DP; 9 – average for ND, vehicles with narrow DP; 10 – wide with a thin wall and wide DP; 11 – wide with average wall thickness and average DP; 12 – wide with a thick wall and wide opening; 13 – thin with medium TC and medium clearance; 14 – average in ND, with a thick wall and wide DP; 15 – thin with a thin wall and narrow DP; 16 – thin with a thick wall and narrow DP; 17 – thin with a thick wall and medium DP; 18 – wide with a thick wall and medium DP.
Discussion. The vast majority of works concerning PCA are devoted to variants of its development: aplasia, hypoplasia, the presence of a vascular network at the site of the artery, etc. [5, 8, 9]. We presented information on the individual typological variability of the morphometric parameters of the PCA for the first time.
As a result of the study, it was revealed that the morphological parameters of the PCA (length, outer and inner diameter, wall thickness) are characterized by significant individual variability. The coefficient of variation of the studied parameters varies from 19.2% (outer diameter) to 28.0% (lumen diameter). For each of the parameters, we have identified 3 groups of arteries: I – with a value of the attribute less than average (<М-σ); II – with the average value of the trait (M±σ); III – with a trait value greater than average (>M+σ). No clear pattern was found in the predominance of men or women in the extreme groups of variability (groups I and II). Thus, among subjects with short, thin-walled arteries and arteries with a wide lumen, women predominate, and among subjects with long, thin, thick-walled arteries and arteries with a narrow lumen, men predominate. In the group of those studied with wide ACAs, the percentage representation of men and women is the same.
The average age of subjects whose PMA, based on the size of their outer diameter, wall thickness and lumen diameter, belongs to group III, is statistically significantly 1.2-1.7 times greater than that of people with PMA belonging to group I variability. The average age of men and women with short PCA (group I), on the contrary, is 1.2 times greater than that of people with long PCA (group III). The age of subjects with narrow or wide lumen PCA did not differ significantly.
Conclusion. Thus, the analysis of individual variability in the length, outer and inner diameters and wall thickness of the PCA made it possible to identify 3 groups of artery variants for each of the parameters: with an average value of the attribute, with a value of the attribute less and more than the average. The combined variability of the morphometric parameters of the PCA made it possible to identify 18 of its types.
The data obtained will complement and organize the existing information on the dimensional characteristics of the PCA, which is important for a better understanding of the area of neuromorphology under study, and can also be useful in modeling blood flow in the arterial circle of the brain.
Reasons for development
Since hypoplasia is usually a congenital disease, the main reasons that cause it are associated with abnormal pregnancy. The development of pathology can be provoked by:
- injuries and falls of the expectant mother;
- the use of a number of medications that affect the development of the embryo;
- alcohol consumption and smoking by a pregnant woman;
- infectious diseases;
- poisoning;
- hereditary factor.
Smoking during pregnancy
Attention! These factors can trigger the appearance of hypoplasia, but this does not mean that they will necessarily lead to its development. In certain cases, children are born already having hypoplasia, but there are no apparent reasons for its appearance.
Symptoms
Arterial hypoplasia is not as easy to identify as it seems. The disease is usually asymptomatic, and even if some signs are present, they can be mistaken for symptoms of completely different diseases - for example, varicose veins, VSD or osteochondrosis.
On a note! If a person has hypoplasia, but feels well, this means that compensation of blood flow due to other vessels is sufficient to nourish the brain.
Symptoms do not appear immediately
That is why the first symptoms of hypoplasia can appear only as a result of age-related changes. This is due to the deterioration of the entire vascular system and the development of atherosclerosis.
Table. Symptoms of hypoplasia.
| Group | Symptoms |
| Local | When palpating the location of the vertebral artery (between the 1st and 2nd vertebrae of the cervical spine), a person experiences a headache. The pain syndrome resembles lumbago or pulsation. |
| Vertebral | Pain in the back of the head or neck, usually throbbing or shooting, felt especially clearly when turning the head, at night or in the morning after sleep. |
| Signs associated with deterioration of blood supply or nervous system function | Increased blood pressure, hearing and vision disorders, migraines, dizziness, impaired coordination, gait, sensitivity. A person may also find himself disoriented in space; this effect is especially often observed when making sudden movements. Dizziness can lead to fainting. Some patients complain of weakness, sensitivity to weather changes, and problems sleeping. |
Aneurysm of the communicating part of the internal carotid artery
Patient T., 30 years old, upon admission to the department complained of pain in the right eye, in the right frontotemporal region, ptosis of the right upper eyelid, double vision, more when looking to the left.
From the anamnesis it is known: About 2 weeks ago he noted the appearance of a headache in the right side of the head, then the appearance of pain in the right eye. I went to an ophthalmologist and was prescribed eye drops - without effect. An MRI of the brain was performed: no MRI data were obtained for the presence of space-occupying lesions in the brain. Doppler ultrasound of extracranial vessels: Hemodynamically significant disturbances of blood flow at the extracranial level were not registered. Signs of increased peripheral resistance in the carotid region. Asymmetry of blood flow in the VA. Evoked potential study: facial and trigeminal (1 branch on both sides) nerves are not affected. I consulted a neurologist and was prescribed Xefocam, naproxen, milgamma, finlepsin, picamilon, which relieved the pain in the eye, but noted nausea and dizziness. 7 days after the onset of pain, he noted the addition of double vision. I contacted a neurologist at the clinic and was sent for emergency hospitalization at Clinical Hospital No. 1.
During the examination:
Laboratory indicators are unremarkable.
ECG: Sinus rhythm with heart rate 65/min. Normal position of the EOS. Features of intraventricular conduction.
CT scan of the chest: Single calcifications in the right lung. Anterosuperior mediastinal cyst.
MRI of the brain and orbital area, with intravenous contrast: No pathological changes in the brain and orbital area were detected.
Visual evoked potentials (for a checkerboard pattern): a disturbance in the conduction of visual afferentation to the cortex at the prechiasmal level on the right with an axonal type of damage is detected.
Ultrasound of the eyeball (right eye): The ultrasound picture may correspond to the presence of retrobulbar neuritis.
Examination of the thyroid gland and regional lymph nodes: Ultrasound signs of focal changes in both lobes of the thyroid gland.
Color duplex scanning of the extracranial sections of the brachiocephalic arteries: 1. Hemodynamically significant obstacles to blood flow and structural variants of the extracranial sections of the brachiocephalic arteries were not identified.
Examined by an ophthalmologist: Background retinopathy and retinal vascular changes. Lagophthalmos of the right eye.
Thus, the patient was ruled out for a space-occupying lesion, a demyelinating disease, and antibodies to acetylcholine were taken to rule out myasthenia gravis (negative).
I received thioctacid, trental, dexazone intravenously, xefocam, milgamma intramuscularly, and omez orally.
During treatment (12 days), semiptosis on the right side, double vision, more to the left, and heaviness in the right side of the head persist. He was transferred for further treatment to our department.
Life history is not burdened.
On examination: the somatic status is unremarkable.
In neurological status: Conscious, contactable, oriented. Speech is not changed. There are no meningeal signs. CMN: Palpebral fissures DS. Photoreactions: live on the left, almost absent on the right. The movements of the eyeballs on the left are full, on the right they are limited upward and to the left. Diplopia when looking to the left and up. Ptosis of the right upper eyelid. There are no sensory disturbances on the face. The face is symmetrical. There is no nystagmus. Hearing is not impaired. The pharyngeal reflex is preserved. Swallowing and phonation are not impaired. Tongue in the midline. Motor sphere: No paresis. Tendon reflexes are alive, S=D. No pathological foot signs are detected. Stable in the Romberg position. PNP performs satisfactorily on both sides. Sensitivity is not impaired. There are no symptoms of tension.
Considering the presence of paresis of the oculomotor nerve on the right and severe pain locally in the right frontotemporal region, additional examination plans include MR angiography and MR venography to exclude cerebral aneurysm and cavernous sinus thrombosis.
MR angiography: a picture of a small saccular aneurysm in the area where the right posterior communicating artery originates from the middle cerebral artery. Extracranial bends of both ICAs. Pronounced asymmetry of the transverse sinuses.
Consulted by a vascular surgeon. Angiography of the brachiocephalic arteries was performed: in the communicating section of the right internal carotid artery, a saccular aneurysm 6.5x4.2 mm, irregular in shape, with a wide neck was visualized. The posterior communicating artery arises from the neck of the aneurysm.
For further treatment, he was transferred to the acute stroke department for endovascular intervention. Endovascular embolization of the posterior communicating artery aneurysm using coils was performed.
The postoperative period was uneventful. Discharge on the 6th day. The cephalgic syndrome regressed, oculomotor disturbances significantly decreased, palpebral fissures D=S, photoreactions: lively on the left, slightly reduced on the right, movements of the eyeballs in full, diplopia when looking up, semiptosis on the right.
At the control CT scan of the brain after 3 months: Endovascular embolization of the aneurysm of the posterior communicating artery using coils dated November 21, 2018. No pathological changes in the substance of the brain were detected. In the neurological status, oculomotor disorders and ptosis completely regressed.
When examined after 6 months, the patient was in a neurological status without oculomotor or focal disorders. During control angiography: the ICA is passable, without significant narrowing. Condition after endovascular embolization of an aneurysm of the communicating part of the ICA. Notes the complete absence of headaches, which often bothered the patient for several years. The patient is fully able to work and has returned to his profession.
Differences between right-sided and left-sided hypoplasia
There are no serious differences in both symptoms and the nature of the disease between right- and left-sided hypoplasia. The main difference is that each artery supplies a specific part of the brain with blood. Thus, the patient may have different complications and consequences of the disease.
With hypoplasia on the right side, the main trouble is concomitant diseases, in which this pathology is a kind of catalyst. For example, this could be atherosclerosis, which itself can narrow blood vessels and thereby lead to additional problems with blood circulation.
Atherosclerosis
On a note! With right-sided hypoplasia, patients more often than in other cases complain of severe sensitivity to weather changes.
With hypoplasia on the left side, symptoms may appear even longer than with the right-sided form of the pathology. The most important sign of the development of the disease is pain in the neck. But if there are no other symptoms, then such pain syndrome is usually considered a sign of other diseases and it is extremely difficult to make a diagnosis. And fluctuations in blood pressure in this form are secondary.
How does hypoplasia of the left vertebral artery occur?
As a result of numerous epidemiological studies, the main factors leading to the development of cerebrovascular pathology from the initial signs of cerebral circulatory failure to stroke have been established [2-5, 9]. In nature, there are mammals in which the only source of blood supply to the brain is the vertebral arteries (VA): squirrels, hares, guinea pigs. PA pathology, including hypoplasia, under certain conditions can contribute to or be a direct cause of both chronic and acute forms of cerebrovascular accident.
VA hypoplasia is a decrease in the internal diameter of the artery to less than 2 mm. However, there is no uniform agreement regarding the diameter of the vessel, and in some studies, a decrease in the outer diameter of the VA of less than 3 mm was considered a sign of VA hypoplasia, which occurred in 6% of cases when examining 50 healthy people [11]. In another study, among patients with various variants of PA syndrome, according to ultrasound duplex and triplex scanning, hypoplasia of one of the PAs was detected in 23.9% of cases [6]. According to A.V. Komyakhov and M.V. Zhukova [7], in patients with unilateral Kimmerle anomaly, VA hypoplasia was detected in 43% of cases, and in patients with bilateral Kimmerle anomaly - in 73%. The diameter is measured in the V2 segment, since the remaining segments of the VA are less convenient, are at risk of atherosclerotic stenosis due to their non-linear course, and measurement errors are possible in other segments [15, 14]. The contralateral hypoplastic VA is usually called the dominant artery [12, 14].
During a post-mortem X-ray angiographic study of brachycephalic arteries in children of different ages, it was possible to establish that a fairly intensive growth of VA occurs at the ages of 5 to 7 years and from 7 to 9 years. The basilar artery is the most stable segment of the posterior sections of the arterial circle during the analyzed period of growth - from the 1st year of life to 9 years. The posterior cerebral arteries grow stably and evenly without clear spasmodic tendencies. The growth of the posterior communicating arteries stabilizes at the age of 5-9 years. The diameter of the VA at the age of up to 5 years remains approximately stable - 1.1-2.0 mm [10].
In the literature, PA hypoplasia is considered as a manifestation of undifferentiated connective tissue dysplasia, but there is no data on its frequency in people with hereditary connective tissue pathology. Various data on the prevalence of VA hypoplasia, according to the literature, are associated with the imaging methods used. Thus, with magnetic resonance angiography (MRA) using a Siemens 1.5 T tomograph with a bolus intravenous administration of 10 mg of Magnevist and subsequent computer processing, unilateral hypoplasia of the VA with an internal diameter of less than or equal to 2 mm was determined in 106 (35.2%) out of 306 healthy people studied, and 3.4% had bilateral VA hypoplasia [15]. According to ultrasound duplex scanning of the VA on a Sonus 5500 ultrasound scanner, when measured in the C5-C6 intervertebral space, out of 407 healthy people, VA hypoplasia with an internal diameter of less than or equal to 2 mm occurred in 15.8% of cases (7.8% - right VA and 8% - left) [13].
The absence of signs of vertebrobasilar insufficiency among patients with VA hypoplasia allows us to consider it as a variant of normal structure and leads to an underestimation of the role of its influence in the development of stroke. On the other hand, in patients with anterior and posterior circular ischemic stroke, hypoplasia of the ipsilateral VA was determined in 27.1 and 45.6% of cases, respectively, allowing the authors to conclude that hypoplasia of the VA is an independent risk factor for stroke in the territory of the posterior inferior cerebellar artery on the ipsilateral side [15]. N.S. Alekseeva found that VA stenosis of 70% or more is critical for the development of cerebellar infarction [1]. There was also a high incidence of distal atherosclerotic stenosis in the intracranial segment in people with VA hypoplasia compared to people without VA hypoplasia ( p
<0.001) [15]. According to a study by J. Park et al. [15], 89.1% of patients with VA hypoplasia and developing ischemic stroke in the posterior inferior cerebellar artery had atherosclerotic stenosis or occlusion. VA hypoplasia in patients with cerebral atherosclerosis leads to decompensation of cerebral hemodynamics and contributes to the development of ischemic stroke with degrees of carotid artery stenosis lower than in patients without vertebral artery hypoplasia [8].
Material and methods
At the Department of Neurology and Medical Genetics with a Course of Neurosurgery of the Yaroslavl State Medical Academy, 1549 patients with chronic cerebrovascular accidents (CVA) and 87 patients who suffered acute cerebrovascular accidents (ACVA) were examined.
The study of blood vessels was carried out using a Philips En Visor ultrasound scanner, in duplex and triplex scanning modes. In all patients, vascular patency, the state of the vascular wall, blood flow indicators (systolic and diastolic velocity, resistance index), the state of the vascular lumen were determined, the mouth of the VA, the origin from the subclavian artery and the entry of the VA into the spinal canal were examined.
results
Among 1549 patients with CNM, VA pathology was observed in 369 (23.8%) people. VA hypoplasia was diagnosed in 55 (3.5%) patients - 37 (67%) women and 18 (33%) men. The minimum artery diameter was 1.3 mm. Hypoplasia of the VA was determined in patients of different ages with symptoms of vertebrobasilar insufficiency. Under the age of 30 years - in 5 (9.0%) men and 2 (3.6%) women; aged 30 to 50 years - in 6 (10.9%) men and 25 (45.4%) women; over 50 years old - in 7 (12.7%) men and 10 (18.2%) women. Hypoplasia of the right VA was observed more often - 39 (70%) cases and less often - of the left VA - 16 (30%) cases. During the study, blood flow in the main artery in 12 (21%) cases was below normal by 10-30% (normal is more than 40 cm/s); in the remaining 43 (79%) cases, blood flow decreased during a rotation test. In 16 (29%) cases, a combination of VA hypoplasia with pathological deformation of both internal carotid arteries, predominantly S- and/or Z-shaped, was noted.
Analysis of the examination results of 25 patients with VA hypoplasia showed asymmetric venous outflow along the vertebral venous plexus in all cases. In the vast majority of cases, accelerated venous outflow was recorded on the side of VA hypoplasia. In 8 patients it was presented as accelerated polyphase with multiple retrograde reflux, “whistling”, in 5 patients it was predominantly monophasic, accelerated, “blowing”. In 12 people, blood flow in the V2 segment was not accelerated, but significant acceleration was recorded in the V1 segment and direct venous sinus. When performing a test with 2-minute hyperventilation, there was a noticeable acceleration of blood flow through the vertebral venous plexus by 40-50%; after hyperventilation, the blood flow gradually decreased.
Among 87 patients with stroke aged from 17 to 45 years (average age - 35.4 years), VA hypoplasia was observed in 16 (18.4%) patients (6 men and 10 women): in 12 (75%) patients - right PA and in 4 (25%) patients - left PA. Stroke in 10 (62.5%) patients developed in the vertebrobasilar system and in 6 (37.5%) patients - in the carotid system. In the medical history, 3 patients had indications of previous strokes. Among the risk factors, arterial hypertension was present in 5 (31.3%) patients and mitral valve prolapse in 6 (37.5%). It should be noted that in this group, VA hypoplasia was more often recorded in patients with undifferentiated connective tissue dysplasia syndrome (UCTD) than in patients without phenotypic manifestations of this syndrome (27.9 and 6.8%, respectively). However, no dependence on the severity of UCTD syndrome and the frequency of VA hypoplasia was identified. Hypoplasia of the VA in 3 patients was combined with deformation of the internal carotid arteries (ICA) and/or VA, and in 4 patients - with tortuosity of the ICA and/or VA. In 2 patients from this group, simultaneously with VA hypoplasia, atherosclerotic lesions of the common carotid arteries (CCA) with stenoses of 20-30% were detected. Among patients with stroke, taking into account the etiological subtype of ischemic stroke, according to the TOAST criteria, VA hypoplasia was detected in the atherothrombotic variant - in 2 cases, cardioembolic - in 2, lacunar - 2, in 3 cases - in stroke with another known cause, and in 7 patients with stroke of unknown etiology.
Thus, the high frequency of detected angiodysplasias in young people with strokes and their predominance in patients with UCTD syndrome is probably one of the manifestations of this syndrome and can be both the result of a hereditary form of connective tissue damage, and a consequence of acquired genesis as a result of the influence of various unfavorable factors on the fetus during its intrauterine development, which leads to a defect in the formation of the connective tissue framework of the vascular wall. Hypoplasia of the VA, alone or in combination with pathological deformations and/or tortuosity of the ICA, may be one of the risk factors for the development of circulatory disorders in the vertebrobasilar system.
Possible consequences
The danger of hypoplasia lies, first of all, in an increased risk of stroke due to the fact that the blood supply to the brain is deteriorated. If you believe the statistics, about 30% of strokes are associated with circulatory disorders in this area. The disease can cause problems with hearing, vision, and the functioning of the vestibular apparatus. But in general, the consequences of this pathology are completely unpredictable. In any case, the disease worsens the quality of life, but is not fatal in itself.
Stroke and its consequences
In fact, the prognosis in the presence of this disease will depend largely on how underdeveloped the affected artery is, what state the human body is in, the presence of a number of certain pathologies, etc. In general, the prognosis is considered conditionally favorable. But if there are certain risk factors, it is important to take a number of preventive measures. Sometimes you have to do surgery.
Ischemic stroke in the posterior cerebral arteries: problems of diagnosis and treatment
I.A. KHASANOV, E.I. BOGDANOV
Republican Clinical Hospital of the Ministry of Health of the Republic of Tatarstan, Kazan
Kazan State Medical University
Khasanov Ildar Akramovich
doctor of the neurological department for patients with acute cerebrovascular accidents
420064, Kazan, st. Orenburgsky Trakt, 138, tel. (843) 237-35-47, e-mail
In the light of modern data, the article examines the problems of diagnosis and treatment of ischemic strokes in the posterior cerebral arteries (PCA), taking into account the characteristics of their etiology, clinical picture and neuroimaging data. The paired posterior cerebral arteries, formed by the bifurcation of the basilar artery and being its terminal branches, serve as the main sources of blood supply to the upper part of the midbrain, the thalami and the posteroinferior parts of the cerebral hemispheres, including the occipital lobes, the mediobasal parts of the temporal lobes and the inferomedial parts of the vertex. Ischemic strokes in the posterior cerebral artery basin account, according to various sources, from 5-10 to 25% of cases of all ischemic strokes. The most common cause of isolated infarctions in the PCA territory is embolic occlusion of the PCA and its branches, which occurs in approximately 82% of cases. In 9% of cases, thrombosis in situ is detected in the PCA; in another 9% of cases, the cause of stroke is vasoconstriction associated with migraine and coagulopathy. A very rare cause of infarction in this region can also be arterial dissection affecting the PCA. The most common and characteristic signs of infarctions in the PCA region are visual disturbances (homonymous hemianopsia), central paresis of the facial nerve, headache, sensory disturbances, aphasic disorders, hemiparesis and nigility.
Key words:
ischemic stroke, cerebral infarction, posterior cerebral artery, neuroimaging, thrombolytic therapy
I.A. KHASANOV, EI BOGDANOV
Kazan State Medical University
Republican Clinical Hospital of the Ministry of Health of the Republic of Tatarstan, Kazan
Ischemic stroke in a system of posterior cerebral arteries: problems of diagnosis and treatment
In the article on the basis of present knowledge are considered the problems of diagnosis and treatment of ischemic strokes in a system of posterior cerebral arteries (PCA) taking into account their causation, clinical presentation and neuroimaging data. Paired posterior cerebral arteries, which are shaped by basilar artery bifurcation and are its terminal branches, are the main sources of blood supply of the upside of midbrain, thalamus and back and bottom parts of cerebral hemispheres, including occipital lobes, mediobasal branches of temporal lobes and lower medial crown branches. Ischemic strokes in a system of posterior cerebral arteries amount to 5-10% or up to 25% of all ischemic strokes. The most common cause of isolated heart attacks
in a system of PCA is the embolic occlusion of PCA and its branches, which occurs in about 82% of cases. In 9% of cases in PCA is revealed thrombosis, in other 9% of cases the cause of stroke are vasoconstriction associated with migraine, and coagulopathy. A very rarely reason for a heart attack in this system can be artery dissection which affects the PCA. The most frequent and characteristic features of heart attacks in a system of PCA are visual impairments (equilateral hemianopsia), central paresis of facial nerve, headache, sensation disorders, aphatic disorders, hemiparesis and neglect.
Key words:
ischemic stroke, cerebrovascular accident, posterior cerebral artery, neuroimaging, thrombolytic therapy.
Ischemic strokes in the posterior cerebral arteries (PCA) account, according to various sources, from 5-10 to 25% of cases of all ischemic strokes [1-4]. They can be the cause of a number of clinical symptoms, which are not always promptly and adequately recognized by the patients themselves, their relatives and doctors, because an acute gross motor deficit, which is usually associated with a stroke, in this case may be unexpressed or completely absent. A delay in timely diagnosis or incorrect diagnosis casts doubt on the possibility of providing the patient with adequate therapy (primarily thrombolysis), which in turn cannot but affect the outcome of the disease [5]. An important role in making a diagnosis is played by the possibility of using neuroimaging, the correct choice of method and competent interpretation of the results [2]. It seems important to present and analyze the features of the clinical picture, neuroimaging and treatment of ischemic strokes in the posterior cerebral arteries in the light of modern data.
The most common cause of isolated infarctions in the PCA territory is embolic occlusion of the PCA and its branches, which occurs in 82% of cases. At the same time, cardiogenic genesis is observed in 41% of cases, while arterio-arterial embolism from the vertebral and basilar arteries is observed in only 32% of cases. In 10% of patients, the source of the embolism cannot be determined. In 9% of cases, thrombosis in situ is detected in the PCA. Vasoconstriction associated with migraine and coagulopathies are the causes of cerebral infarction in 9% of cases [6]. If isolated infarctions in the PCA territory in most cases are of a cardioembolic nature, then involvement of the brainstem and/or cerebellum in combination with an infarction in the PCA territory is most often associated with atherosclerotic lesions of the vessels of the vertebrobasilar system [7, 8]. A very rare cause of infarction in this region can also be arterial dissection affecting the PCA [9]. Regardless of the cause of the infarction, it usually only partially involves the PCA territory [10, 11].
Paired posterior cerebral arteries, formed by the bifurcation of the basilar artery and being its terminal branches, serve as the main sources of blood supply to the upper part of the midbrain, thalamus and posteroinferior parts of the cerebral hemispheres, including the occipital lobes, mediobasal parts of the temporal lobes and inferomedial parts of the vertex [10, 12, 13].
In the early stages of development of the human body, the posterior cerebral artery is a branch of the internal carotid artery (ICA) and is supplied with blood from the carotid system, while the posterior communicating artery (PCA) plays the role of its proximal segment. Subsequently, blood begins to flow into the posterior cerebral arteries from the main artery, and the PCA, being a branch of the internal carotid artery, becomes the most significant anastomosis between the carotid and vertebrobasilar areas. According to various sources, from 17 to 30% of adults have a fetal (embryonic) type of PCA structure, in which the ICA remains the main source of blood supply to the PCA throughout life. The fetal type of PCA structure is in most cases observed unilaterally, with the opposite PCA usually starting from an asymmetrically located, curved basilar artery. In cases where both posterior cerebral arteries are branches of the internal carotid arteries, as a rule, well-developed large posterior communicating arteries are observed, and the superior segment of the basilar artery is shorter than usual (the basilar artery ends with the two superior cerebellar arteries arising from it). In approximately 8% of cases, both PCAs originate from the same ICA [7, 8, 12, 14, 15].
The PCA joins the PCA approximately 10 mm distal to the bifurcation of the basilar artery. Each PCA can be conditionally divided into 3 parts: the precommunication part, or P1 segment according to Fisher, - the section of the PCA proximal to the place where the PCA flows into it, the postcommunication part, or P2 segment, located distal to the place where the PCA flows into the PCA, and the final (cortical) the part that gives off branches to the corresponding areas of the cerebral cortex [12, 16]. The paramedian mesencephalic, posterior thalamoperforating and medial posterior choroidal arteries depart from the precommunicative part, participating primarily in the blood supply to the ventrolateral nuclei of the thalamus and the medial geniculate body. The left and right posterior thalamoperforating arteries may arise from a common trunk called the artery of Percheron; a similar variant of the structure usually occurs in combination with unilateral hypoplasia of the P1 segment and the fetal structure of the PCA. The branches of the postcommunication part are the peduncular perforator, thalamogeniculate and lateral posterior choroidal arteries, supplying the lateral geniculate body, dorsomedial nuclei and thalamic cushion, part of the midbrain and the lateral wall of the lateral ventricle [2, 12, 17]. The main cortical branches of the PCA are the anterior and posterior temporal, parietotemporal and calcarine arteries [10]. The boundaries of the watershed of the middle and posterior cerebral arteries basins fluctuate significantly. Usually the border of the PCA basin is the Sylvian fissure, but sometimes the middle cerebral artery supplies blood to the outer parts of the occipital lobe up to the occipital pole. At the same time, the PCA always supplies blood to areas of the cerebral cortex in the area of the calcarine sulcus, and the optic radiation in some cases receives blood from the middle cerebral artery; accordingly, homonymous hemianopsia does not always imply a heart attack in the PCA territory [12].
With ischemic strokes in the PCA region, depending on the location of the vessel occlusion, as well as on the state of collateral blood supply, the clinical picture may reveal symptoms of damage to the midbrain, thalamus and cerebral hemispheres. In general, up to 2/3 of all infarctions in the PCA territory are cortical, the thalamus is involved only in 20-30% of cases, and the midbrain in less than 10% of cases [7, 18, 19]. Accordingly, the most common variant of ischemic stroke in the PCA basin is an isolated infarction of the cerebral hemispheres, primarily the occipital lobes; combined damage to the thalamus and cerebral hemispheres is less common, in a small percentage of cases - an isolated infarction of the thalamus and, finally, a combination of damage to the midbrain, thalamus and /or hemispheres is the rarest option [2].
Sometimes there is bilateral damage to areas of the brain supplied by blood from the PCA. This occurs primarily in top of the basilar syndrome, which is an embolic occlusion of the distal basilar artery and is characterized by depression of consciousness, visual disturbances, oculomotor and behavioral disorders, often without motor dysfunction [2].
According to a number of authors, the most common and characteristic signs of infarctions in the PCA are visual disturbances (up to 95% of cases), homonymous hemianopia (66.7% of cases), central paresis of the facial nerve (52% of cases), headache, mainly in the occipital region. areas (50 cases), sensory disorders (40% of cases), aphasic disorders (38% of cases), hemiparesis (18% of cases) and niglect (10% of cases). Patients usually have a combination of symptoms [2, 7, 8, 11].
Homonymous hemianopia occurs on the contralateral side with infarctions in the areas of blood supply to the hemispheric branches of the PCA due to damage to the striate cortex, optic radiation or lateral geniculate body. In the absence of occipital pole involvement, macular vision remains intact. The visual field defect may be limited to only one quadrant. Superior quadrant hemianopsia occurs when there is an infarction of the striate cortex below the calcarine sulcus or inferior part of the optic radiation in the temporo-occipital region. Inferoquadrant hemianopsia is a consequence of damage to the striate cortex above the calcarine sulcus or the superior part of the optic radiation in the parieto-occipital region. Occlusion of the calcarine sulcus may also be associated with pain in the ipsilateral eye. Visual disturbances may also be more complex, especially with bilateral occipital lobe lesions, including visual hallucinations, visual and color agnosia, prosopagnosia (agnosia for familiar faces), blindness denial syndrome (Anton syndrome), visual attention deficits, and optomotor agnosia ( Balint's syndrome). Often, visual impairment is accompanied by afferent disorders in the form of paresthesia, disorders of deep, pain and temperature sensitivity. The latter indicate involvement of the thalamus, parietal lobe or brainstem (due to occlusion of the proximal vertebrobasilar region) [2, 8, 10, 20].
Neuropsychological abnormalities associated with PCA infarctions vary significantly and are present in more than 30% of cases. A stroke in the basin of the callosal branches of the left PCA in right-handed people, affecting the occipital lobe and splenium of the corpus callosum, is manifested by alexia without agraphia, sometimes color, object or photographic anomia. Right hemisphere infarctions in the PCA territory often cause contralateral hemiglect. With extensive infarctions involving the medial parts of the left temporal lobe or bilateral mesotemporal infarctions, amnesia develops. Also, with mono- or bilateral mesotemporal infarction, agitated delirium may develop. Extensive infarcts in the territory of the left posterior temporal artery may clinically manifest as anomia and/or sensory aphasia. Thalamic infarctions in the areas of blood supply to the penetrating branches of the PCA can cause aphasia (if the left pillow is involved), akinetic mutism, global amnesia and Dejerine-Roussy syndrome (disorders of all types of sensitivity, severe dysesthesia and/or thalamic pain and vasomotor disturbances in the contralateral half of the body, combined with usually transient hemiparesis, choreoathetosis and/or ballism). Also, infarctions in the PCA region may be associated with dyscalculia, spatial and temporal disorientation. [6, 12, 21, 22].
Bilateral thalamic infarcts are often associated with deep coma. Thus, occlusion of the Percheron artery causes the development of bilateral infarcts in the intralaminar nuclei of the thalamus, which leads to severe impairment of consciousness [2, 12].
Hemiparesis during infarctions in the PCA region occurs in only 1/5 of patients, is often mild and transient and is usually associated with involvement of the cerebral peduncles in the pathological process [23, 24]. Cases of infarctions in the PCA region have been described, when patients exhibited hemiparesis without involvement of the cerebral peduncles. These patients had damage to the distal parts of the PCA, primarily involving the thalamogeniculate, lateral and medial posterior choroidal arteries [23, 25]. It is assumed that hemiparesis during infarctions in the posterior choroidal arteries may be associated with damage to the corticobulbar and corticospinal tracts, even in the absence of visible damage to the internal capsule or midbrain according to neuroimaging data [23]. There are opinions that the development of hemiparesis is associated with compression of the internal capsule by edematous tissue of the thalamus [12].
Infarctions in the PCA territory mimic infarctions in the carotid system in 17.8% of patients [24], especially with combined lesions of the superficial and deep branches of the PCA, which is observed in approximately 38% of cases [7, 19, 26]. Differential diagnosis can be difficult due to the presence of aphasic disorders, nigella, sensory deficits, and usually mild and transient hemiparesis resulting from the involvement of the pyramidal tracts. In addition, memory impairment and other acute neuropsychological disorders can significantly complicate the examination of such patients [2, 18, 19].
Among other conditions that often clinically mimic infarctions in the PCA, we should highlight some infectious diseases (primarily toxoplasmosis), posterior reversible leukoencephalopathy syndrome, neoplastic lesions, both primary and metastatic, and thalamic infarctions caused by deep cerebral vein thrombosis [2 , 27]. Neuroimaging methods often play a decisive role in making a diagnosis.
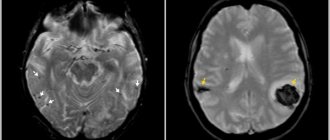
The main requirements for neuroimaging in the acute period of ischemic stroke are the speed of the study and the information content of the data obtained. The main tasks facing the doctor when using these methods are to exclude a non-ischemic cause of the patient’s symptoms, determine the location and size of ischemic foci and the presence of viable brain tissue, determine the condition of the cerebral vessels, identify cerebral edema and displacement of the midline structures, as well as the presence of hemorrhagic impregnation of ischemic foci. These data should help in quickly determining the patient’s treatment tactics—the possibility of intravenous or intra-arterial thrombolysis, mechanical plaque removal, and brain decompression surgery [28, 29].
Computed tomography (CT) usually does not detect ischemic changes in the brain parenchyma during the first few hours after the onset of stroke, the time most important for initiating therapy, and sometimes even later in the disease. Visualization of the posterior regions of the brain is especially difficult due to artifacts caused by the bones of the skull. However, with strokes in the territory of the PCA, as well as with strokes in the territory of the middle cerebral artery, in some cases, CT may show a hyperintense signal from the PCA itself, which is the earliest sign of a stroke in its territory and is detected in 70% of cases within the first 90 minutes from onset of the disease and in 15% of cases within 12 to 24 hours. This sign appears due to visualization of a calcified embolus or atherothrombosis in situ. On a standard CT scan, the slice plane is parallel to the orbitomeatal line (the line connecting the outer corner of the eye with the external auditory canal and then going to the first cervical vertebra). Based on the course of the SMA, its lumen is usually visualized in one section, which makes it easy to identify hyperdense SMA, especially in the presence of atrophic changes in the brain. The course of the PCA is more complex. Typically, its proximal segment ascends laterally around the cerebral peduncles and, reaching the bypass cistern, goes horizontally inward to the temporal lobe, in close proximity to the tentorium cerebellum. The circular part (P1 and P2 segments) ends in the quadrigeminal cistern, where the cortical part of the PCA begins. Only the P2 segment runs parallel to the cut inside the bypass tank and, accordingly, hyperdensity, if present, can most likely be detected in this area. Subsequently, CT signs of ischemic changes appear as areas of hypointensity in the brain parenchyma [2, 3, 30].
Magnetic resonance imaging (MRI) makes it possible to more accurately determine the presence and nature of ischemic changes in the brain during stroke. Diffusion-weighted imaging (DWI) can detect early ischemic changes, often within an hour of symptom onset, and localize and extend lesions more accurately than CT [2]. The combined use of DWI, ADC and FLAIR modes makes it possible to differentiate acute, subacute and chronic ischemic changes in the brain parenchyma, as well as to distinguish cytotoxic brain edema observed in ischemic stroke from vasogenic edema in the syndrome of posterior reversible leukoencephalopathy and hypertensive encephalopathy [2, 27, 31 , 32].
CT angiography (CTA) plays a significant role in the non-invasive diagnosis of steno-occlusive lesions of large extra- and intracranial arteries. This technique makes it possible to identify the degree of stenosis, plaque morphology, as well as the presence of arterial dissection in both vertebrobasilar and carotid vessels. In addition, the anatomical features of collaterals and circulation options of the PCA are assessed [2, 33, 34]. Additional information about vascular anatomy can be obtained using contrast-enhanced MR angiography, which, in combination with CTA, allows for data that previously could only be obtained using classical angiography. In addition, these methods are important in assessing the effectiveness of thrombolytic therapy in the case of arterial recanalization [2].
Currently, thrombolytic therapy for ischemic stroke can be used for damage to the arteries of both the carotid and vertebrobasilar areas. Nevertheless, all currently existing guidelines for thrombolysis are focused primarily on vascular catastrophe in the carotid region, primarily the middle cerebral artery; this is primarily due to the presence in such patients of obvious neurological deficits in the form of severe paresis and sensory disturbances. A typical functional deficit in a patient with a heart attack in the PCA region in the acute period is not always regarded by the doctor as disabling. The assessment of neurological deficit according to the National Institutes of Health Stroke Scale (NIHSS), which is one of the criteria for selecting patients for thrombolytic therapy, usually is not able to fully reflect the severity of the condition of a patient with a vertebrobasilar infarction [7]. There are no recommendations at all regarding an isolated visual field defect in acute infarction in the PCA territory [2]. Therefore, thrombolytic therapy in patients with infarctions in the PCA is not widely used. However, given that hemiparesis in some cases is a significant clinical component of infarctions in the PCA territory, such patients, in the absence of contraindications, are justifiably treated with systemic and/or intra-arterial thrombolysis [35].
When comparing the efficacy and safety profiles of intravenous thrombolysis administered within the first three hours from the onset of symptoms in patients with carotid infarctions and PCA infarctions, no significant difference in safety and treatment outcome was found [7]. At the same time, according to a number of authors, when conducting intravenous thrombolytic therapy for ischemic lesions in the vertebrobasilar region, and in particular the PCA, it is possible to expand the therapeutic window to 6.5-7 hours and even more compared to 4.5 hours for infarctions in the carotid pool [36, 37].
Intra-arterial thrombolysis for occlusion of the middle cerebral artery is recommended within 6 hours from the onset of symptoms, and for occlusion of the basilar artery - no later than 12 hours [28]. However, to date there are no clear recommendations on the time limits for intra-arterial thrombolysis in patients with PCA lesions [15]. N. Meier et al. (2011) described 9 cases of intra-arterial thrombolysis in patients with PCA occlusion within the first 6 hours from the onset of the disease. 3 months after treatment, functional independence (modified Rankin scale 0-2 points) was detected in 67% of patients, which correlates with similar data for the carotid system [15].
An early diagnosis of ischemic stroke in the PCA allows the doctor to promptly determine the patient’s treatment tactics and, in the absence of contraindications, consider the possibility of thrombolytic therapy, which undoubtedly makes the prognosis for the patient more favorable.
LITERATURE
1. Brandt T., Steinke W., Thie A., Pessin MS, Caplan LR Posterior cerebral artery territory infarcts: clinical features, infarct topography, causes and outcome. Multicenter results and a review of the literature // Cerebrovasc. Dis. - 2000. - Vol. 10. - P. 170–182.
2. Finelli P. Neuroimaging in acute Posterior Cerebral Artery Infarction // The Neurologist. - 2008. - Vol. 14. - P. 170-180.
3. Krings T., Noelchen D., Mull M. et al. The hyperdense posterior cerebral artery sign // Stroke. - 2006. - Vol. 37. - P. 399-403.
4. Hill MD Posterior cerebral artery stroke // e-medicine, 2005.
5. Khasanov I.A. Features of infarctions in the basin of the posterior cerebral arteries // Neurological Bulletin. - 2012. - T. XLIV, issue. 3. - pp. 69-74.
6. Caplan L. Posterior Circulation Ischemia: Then, Now, and Tomorrow: The Thomas Willis Lecture-2000 // Stroke. - 2000. - Vol. 31. - P. 2011-2023.
7. Breuer L., Huttner H. B., Jentsch K. et al. Intravenous Thrombolysis in Posterior Cerebral Artery Infarctions // Cerebrovasc Dis. - 2011. - Vol. 31. - P. 448-454.
8. Caplan L., Bogousslavsky J. Posterior cerebral artery syndromes // Cerebrovascular Disease: Pathology, Diagnosis and Management. - 1998. - P. 1028.
9. Caplan L., Estol C., Massaro A. Dissection of the posterior cerebral arteries // Arch Neurol. - 2005. - Vol. 62. - P. 1138-1143.
10. Brazis P. Topical diagnostics in clinical neurology / P. Brazis, D. Masdew, H. Biller - M.: MEDpress-inform, 2009. - 736 p.
11. Caplan L. Posterior Circulation disease: Clinical Findings, Diagnosis and Management / Boston, MA: Butterworth-Heinemann, 1996. - 533 p.
12. Beer M. Topical diagnosis in neurology according to Peter Duus / M. Beer, M. Frotscher. - M.: Practical Medicine, 2009. - 468 p.
13. Tatu L., Moulin T., Bogousslavsky J. et al. Arterial territories of the human brain // Neurology. - 1998. - Vol. 50 - P. 1699-1708.
14. de Monye C, Dippel DW, Siepman TA et al. Is a fetal origin of the posterior cerebral artery a risk factor for TIA or ischemic stroke? A study with 16-multidetector-row CT angiography // J. Neurol. - 2008. - Vol. 255 - P. 239-245.
15. Meier N., Fischer U., Schroth G. Outcome after thrombolysis for acute isolated posterior cerebral artery occlusion // Cerebrovasc. Dis. - 2011. - Vol. 328. - P. 79-88.
16. Phan T., Fong A., Donnan G. et al. Digital map of posterior cerebral artery infarcts associated with posterior cerebral artery trunk and branch occlusion // Stroke. - 2007. - Vol. 38. - P.1805-1811.
17. Chaves CJ Posterior cerebral artery. Stroke syndromes. 2nd edition / Chaves CJ, Caplan LR Cambridge, New York: Cambridge University Press. — 2001. — 747 p.
18. Cals N., Devuyst G., Afsar N. et al. Pure superficial posterior cerebral artery territory infarction in the Lausanne Stroke Registry // J. Neurol. - 2002. - Vol. 249. - P. 855-861.
19. Kumral E., Bayulkem G., Atac C., Alper Y. Spectrum of superficial posterior cerebral artery territory infarcts // Eur. J. Neurol. - 2004. - Vol. 11. - P. 237-246.
20. Ng YS, Stein J, Salles SS et al. Clinical characteristics and rehabilitation outcomes of patients with posterior cerebral artery stroke // Arch. Phys. Med. Rahabil. - 2005. - Vol. 86. - P. 2138-43.
21. Brandt T., Thie A., Caplan L. et al. Infarkte in Versorgungsgebiet der A. cerebri Posterior // Nervenarzt. - 1995. - Vol. 66. - P. 267-274.
22. Savitz SI, Caplan LR Vertebrobasilar disease // N. Engl. J. Med. - 2005. - Vol. 352. - P. 2618-26.
23. Finelli P. Magnetic Resonance Correlate of Hemiparesis in Posterior Cerebral Artery Infarction // Journal of Stroke and Cerebrovascular Disease. - 2008. - Vol. 17. - P. 378-381.
24. Maulaz AB, Bezerra DC, Bogousslavsky J. Posterior cerebral artery infarction from middle cerebral artery infarction // Arch. Neurol. - 2005. - Vol. 62. - P. 938-941.
25. Neau J.-P., Bogousslavsky J. The syndrome of posterior choroidal artery territory infarction // Ann. Neurol. - 1996. - Vol. 39. - P. 779-788.
26. Lee E., Kang DW, Kwon SU, Kim JS Posterior cerebral artery infarction: diffusion-weighted MRI analysis of 205 patients. Cerebrovasc. Dis. - 2009. - Vol. 28. - P. 298-305.
27. Bogdanov E.I., Khasanov I.A., Mamedov Kh.I. and others. Posterior reversible leukoencephalopathy syndrome in patients with preeclampsia and eclampsia // Neurological Journal. - 2011. - No. 5. - P. 35-40.
28. Adams H., Del Zoppo G., Alberts M. et al. Guidelines for the early management of adults with ischemic stroke // Stroke. - 2007. - Vol. 38. - P. 1655-1711.
29. Wahlgren N., Ahmed N., Davalos A. et al. Thrombolysis with alteplase for acute ischemic stroke & the Safe implementation of thrombolysis in stroke - monitoring study (SITS-MOST): an observational study // Lancet. - 2007. - Vol. 369. - P. 275-282.
30. Berge E., Nakstad PH, Sandset PM Large middle cerebral artery infarctions and the hyperdense middle cerebral artery sign in patients with atrial fibrillation // Acta Radiol. - 2001. - Vol. 42. - P. 261-268.
31. Covarrubias DJ, Leutmer PH, Caumpeau NG Posterior reversible leukoencephalopathy syndrome: prognostic utility of quantitative diffusion-weighted MR image // AJNR Am J. Neuroradiol. - 2002. - Vol. 23, N 6. - P. 1038-1048.
32. Garg R. Posterior leukoencephalopathy syndrome // Postgrad. Med. J. - 2001. - Vol. 77, N 903. - P. 24-28.
33. Choi C., Lee D., Lee J. et al. Detection of intracranial atherosclerotic steno-occlusive disease with 3D time-of-flight magnetic resonance angiography with sensitivity encoding at 3T // AJNR Am J. Neuroradiol. - 2007. - Vol. 28. - P. 439-446.
34. Lev M., Farkas J., Rodrigues V. et al. CT angiography in the rapid triage of patients with hyperacute stroke to intraarterial thrombolysis: accuracy in the detection of large vessel thrombus // J Comput Assist Tomogr. - 2001. - Vol. 25. - P. 520-528.
35. Ntaios G., Spengos K., Vemmou AM et al. Long-term outcome in posterior cerebral artery stroke // European Journal of Neurology. - 2011. - P. 156-162.
36. Forster A., Gass A., Kern R. et al. MR Imaging-Guided Intravenous Thrombolysis in Posterior Cerebral Artery Stroke // AJNR Am J. Neuroradiol. - 2011. - Vol. 32. - P. 419-421.
37. Montavont A., Nighoghossian N., Derex. L et al. Intravenous r-TPA in vertebrobasilar acute infarcts // Neurology. - 2004. - Vol. 62. - P. 1854-1856.
Diagnostics
It is not easy to diagnose hypoplasia precisely because of the mild symptoms. The treatment of pathology is carried out by a neurologist, to whom you need to come for an appointment. Typically, treatment occurs after the first signs appear - it is better not to delay, since the brain is clearly experiencing problems with blood supply, otherwise there would be no symptoms.
Diagnostic features
First, the doctor interviews the patient, finds out what he is complaining about, and then examines the patient. Next, you will need to undergo a series of tests and undergo some studies in order to be able to make an accurate diagnosis.
Doctors use the following research methods to confirm the diagnosis.
- Ultrasound of the vessels of the neck and head . Thanks to such a study, it is possible to clarify the diameter of the artery and the intensity of blood flow. If the artery has a diameter of less than 2 mm, then this is already considered a pathology. The normal diameter of the artery of the spinal column is 3.6-3.8 mm.
- Computed tomography of the brain and neck . The study makes it possible to assess the condition of blood vessels through the use of a special contrast agent.
- Angiography . This is a study using x-rays and special contrast agents. Makes it possible to detect anomalies in the structure of blood vessels.
MRI image
Corpus callosum and borderline personality disorder
The CC (corpus callosum) contains 200 to 800 million fibers that are roughly organized in a topographical manner. Small diameter fibers, which are more common in the genu and CC splenium, connect the prefrontal and temporoparietal regions of the brain, whereas large diameter fibers, which are more common in the brain and isthmus of the corpus callosum, connect the visual, auditory, and somatosensory areas. Thus, the CC is involved in the integration of information between the hemispheres, which is necessary for the effective coordination of cognitive processes, emotions and behavior. It has been found that impaired communication between the hemispheres is associated with cognitive, emotional and behavioral disorders, including severe ones, such as suicidal behavior.
Structural changes in the corpus callosum are quite common among psychiatric disorders that are characterized by significant deficits in emotion and impulse regulation, such as those found in bipolar disorder (BD); or attention-deficit/hyperactivity disorder (ADHD). Borderline personality disorder (BPD) shares features with BD.
Structural changes in the corpus callosum (CC), the main area of white matter that functionally connects the brain regions of the two hemispheres, are associated with emotional instability, impulsivity and suicidal behavior in various mental disorders. There was a positive correlation between suicidal behavior in borderline personality disorder and fractional anisotropy (FA) in the CC splenium and genu and a negative correlation between suicidal behavior in borderline personality disorder patients and mean diffusivity (MD) in the CC splenium.
The literature indicates the feasibility of using DTI to study the structural integrity of the CC, a major region of white matter that functionally connects brain regions in the two hemispheres. Structural changes in the genu and splenium of the CC appear to explain suicidal behavior in a variety of disorders, presumably due to general rather than disordered deficits in emotion regulation and impulse control. However, patients prone to suicidal behavior not only demonstrate deficits in emotion regulation and impulse control, but also deficits in problem solving.
How to live with hypoplasia?
Step 1. You need to stop eating low-quality or unhealthy foods.
Avoid harmful foods
Step 2. You need to eat only high-quality and natural products. First of all, it is important to remember that fats should be healthy. Otherwise, the circulatory system suffers greatly.
Eat quality foods
Step 3: It is recommended to eat as much fiber as possible. There is a lot of it in broccoli, rice, whole grain bread, etc.
Eat more fiber
Step 4: It is important to drink plenty of pure water or water with antioxidants, such as lemon.
Drink water with antioxidants
Step 5. It is recommended to evenly distribute work and rest time and be sure to introduce physical activity into your life.
Don't forget about physical activity
Athletic belt
Step 6. You should stop smoking.
Quit smoking
Step 7. It is necessary to protect yourself from stress and spend time having fun and in pleasant company as often as possible.