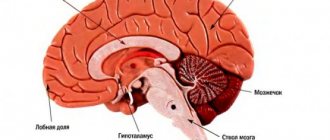
A stroke, formerly called apoplexy or cerebral stroke, is an acute disorder of cerebral circulation. Blood does not enter the brain cells, they stop receiving oxygen and begin to die.
During a stroke, a person's abilities that are controlled by the affected area of the brain, including, for example, memory and muscle control, are lost.
A stroke can happen to anyone, anywhere, at any time, which is why it is important to be educated about the signs of a stroke and know what to do.
Causes of stroke
A stroke can be caused by several reasons. The most important risk factors for this condition are smoking, a sedentary lifestyle, alcohol abuse, obesity, and genetic predisposition (cases of strokes in close relatives can be considered an indirect sign). The cause may vary depending on the type of stroke. The two most common types are described below:
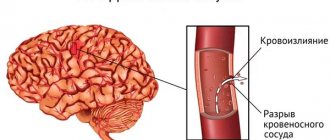
A hemorrhagic stroke occurs due to internal bleeding when a blood vessel inside the brain ruptures. Other names for hemorrhagic stroke are cerebral hemorrhage or intracranial hemorrhage. Among the causes of hemorrhagic stroke are:
- inactive lifestyle;
- increased stress levels;
- alcohol abuse;
- obesity;
- smoking.
Hemorrhagic stroke can also be caused by abnormal formation of blood vessels or rupture of dilated vessels (a complication of cerebral venous insufficiency).
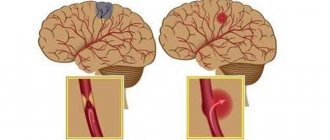
Ischemic stroke occurs due to circulatory problems. A typical case is a blood clot blocking the lumen of a vessel. The cause of ischemic stroke can be:
- diabetes;
- excessive alcohol consumption;
- obesity;
- high cholesterol (causing cerebral atherosclerosis);
- smoking;
- arterial hypertension.
Atrial fibrillation, a type of irregular heartbeat, is another cause of ischemic stroke.
Prevention
Regardless of age, it is important to monitor your blood pressure levels. If there are deviations from the norm, you should contact a specialist. The doctor will prescribe the necessary treatment and give recommendations regarding physical activity, nutrition and rest. Sometimes, during an examination, a young person may be diagnosed with atrial fibrillation or irregular heartbeats. This disease leads to blood stagnation, which in turn can result in a stroke. Any problems with blood circulation require consulting a doctor. Only timely assistance will save you from much more serious problems in the future.
Quitting smoking, controlling alcohol consumption, deciding to exercise, a properly selected diet, and autogenic training are the best prevention of ischemic stroke that a young person can do on his own.
Stroke is a real and serious threat to life. Therefore, from a young age it is necessary to pay attention to prevention, and also consult a doctor if warning signs appear.
Symptoms of a stroke
The first signs of a stroke are:
- violation of speech coherence. It is difficult to understand what the person is saying;
- the appearance of facial asymmetry. Facial expressions are disturbed; as a rule, one side suffers. If you ask a person in this state to smile, the smile will come out crooked;
- severe weakness of the limbs. As a rule, the limbs on one side are affected (right arm and right leg or left arm and left leg). If a person is asked to raise both arms at the same time, one of them will lag noticeably or will not be able to move at all;
- blurred vision. In this case, one pupil will be dilated more than the second, and chaotic movement of the eyeballs is possible. The patient may feel tightness in the eyes and complain of double vision.
How can you tell when someone is having a stroke?
As a mnemonic rule, you can associate the signs of a stroke with the word IMPACT:
"U" - smile
“D” – hand movement
“A” – articulation
“R” - “solution”: if these signs are undeniable, you need to call an ambulance.
Other manifestations of a stroke may include:
- confusion of consciousness. A person who has suffered from a stroke does not understand well what is being said to him;
- dizziness;
- Strong headache
The relevance of the problem of ischemic stroke (IS) in young people (18-45 years old) is primarily determined by the difference in its causes from those in older age groups, the recognition of which requires special laboratory and instrumental studies and is often associated with considerable difficulties. The relevance of the problem is also indicated by the high frequency of cryptogenic stroke (15–40%), i.e., stroke of unknown etiology [1–5]. In addition, the reasons for I.I. have changed in recent years. Thus, advances in the treatment of rheumatism achieved in the last century have led to the fact that cardiogenic embolism of the cerebral arteries, caused by rheumatic damage to the heart valves, which previously was the main cause of IS at a young age, is now uncommon. And diseases previously considered rare or unknown (dissection, antiphospholipid syndrome - APS) are being diagnosed more and more often. Finally, socio-economic factors associated with the young age of patients, as well as the tendency towards an increase in IS at a young age, are of no small importance [6].
The study of the causes of IS in young patients at the Scientific Center for Neurology (hereinafter referred to as the Center) has been carried out over the past 30 years. This review presents an analysis of the causes of IS in more than 600 patients examined over the past 13 years.
The most common cause of IS, according to the present study, is dissection of the arteries supplying blood to the brain (28%). Less common, with almost equal frequency, are cardiogenic embolisms (12%), APS (11%), coagulopathies of unspecified origin, including seronegative (negative antibodies to phospholipids - aPL) Sneddon syndrome (6%), isolated cerebral arteritis (5%) . Other causes, including moya-moya disease/syndrome, polycythemia, genetically determined arteriopathy, mitochondrial diseases, account for 3%. The incidence of arterial hypertension and atherosclerosis, the main causes of IS in older age categories, is low and amounts to 7 and 3%, respectively. Cryptogenic stroke was diagnosed in 25% of patients. Almost half of them had clinical manifestations typical of dissection, but the lack of instrumental confirmation of the latter in the acute period of stroke did not allow them to be classified as strokes with an established etiology [1].
Dissection (dissection) of the wall of the arteries supplying the brain is the most common cause of IS in young people. Clinical diagnosis of this pathology and its active study became possible only in the second half of the 90s of the twentieth century after the introduction of magnetic resonance imaging (MRI) into the clinic, which made it possible to widely carry out non-invasive angiography, identifying signs characteristic of dissection. Dissection is the penetration of blood from the lumen into the arterial wall through an intimal tear with its subsequent spread between the layers of the wall and prolapse towards the intima, which leads to narrowing or even occlusion of the arterial lumen [7]. The latter is one of the causes of IS through the mechanism of cerebrovascular insufficiency, most typical for dissection of the internal carotid artery (ICA) [8]. Another mechanism for the development of stroke during dissection is arterio-arterial embolism due to parietal thrombi formed at the site of intimal rupture, or thrombosed elements of intramural hematoma (IMH) penetrating into the bloodstream during a secondary intimal rupture. This mechanism is more common during vertebral artery (VA) dissection [9]. The preferential spread of IMH towards the outer shell of the artery (edentia) can lead to the formation of a dissecting aneurysm, which can also become a source of arterio-arterial embolism.
The cause of intimal rupture, according to studies carried out in the laboratory of pathological anatomy of the Center, is its dysplastic changes (fibromuscular dysplasia), represented by fiber disintegration, thinning, and in some places the absence of an internal elastic membrane, subintimal fibrosis, incorrect orientation of myocytes of the middle layer, and areas of intimal calcification. Under these conditions, the addition of various provoking factors that cause tension in the artery or its additional “relaxation” leads to intimal tearing and the development of dissection [10, 11]. The cause of dysplastic changes in the arterial wall remains unclear. Genetic studies searching for mutations in the collagen and elastin gene gave negative results [12, 13]. A genome-wide study (GWAS) showed that the G allelic variant of the oligonucleotide substitution rs9349379 in the intronic part of the PHACTR 1
(gene for phosphatase and actin regulator 1) is associated with a lower risk of developing dissection, while the A allelic variant of the same gene is associated with fibromuscular dysplasia [14]. This is consistent with the morphological data of the present study, which showed that fibromuscular dysplasia is the main reason for dissection. It was suggested that mitochondrial pathology plays a role in its development, and this was confirmed by histological and histochemical studies of muscle biopsies obtained from patients with dissection, as well as electron microscopic studies of arteries in skin biopsies [15, 16].
Dissection develops both in the carotid and PA systems. The extracranial part is more often affected than the intracranial part, including its large branches (middle, anterior, posterior cerebral arteries, basilar artery) [7]. Dissection of the ICA occurs more often than VA, and in the first case, men are more likely to suffer, and in the second, women [17–20]. The average age of patients in the Russian population when stroke develops is 37 years. Stroke caused by dissection, as a rule, develops in young people who previously considered themselves practically healthy, who do not have atherosclerosis, diabetes mellitus, or clinical signs of thrombophilia. Before the development of dissection, approximately ½ of the patients suffered from headaches, almost 1/3 had arterial hypotension, and ¼ had an increase in blood pressure, usually moderate [7, 17].
A characteristic sign of a stroke due to ICA/VA dissection is that it is preceded by such provoking factors as head contusions (usually mild, without signs of concussion), physical activity and tension, bending, turning, throwing back the head, including during fitness activities, forced, uncomfortable head position, manual therapy, alcohol intake, contraceptives, common infections suffered in the previous 3 weeks [7, 17]. Another characteristic and diagnostically important clinical feature of a stroke caused by dissection is the presence of preceding neck pain and headache in the projection of the dissected artery. Its pathophysiological basis is irritation of the sensitive receptors of the arterial wall by the hematoma developing in it. Functional recovery after a stroke is determined by the location and size of the cerebral infarction. In general, with extracranial dissection, good restoration of impaired functions is observed, and this is more typical for dissection of the VA than the ICA [17]. Recurrence of dissection occurs in less than 10% of cases [7, 21]. Mortality in stroke caused by dissection is 1-3% and is usually observed with intracranial dissection with the development of extensive cerebral infarctions [7, 10, 11, 22].
For many years, digital subtraction angiography (an invasive technique) was considered the gold standard in diagnosing dissection, while in recent decades priority has been given to neuroimaging studies, primarily magnetic resonance angiography (MRA) and MRI of the neck arteries [23]. MRA allows us to identify changes in the lumen of the arteries that are typical for dissection. These include prolonged uniform stenosis (the “string” symptom) or uneven prolonged stenosis (the “rosary or string of beads” symptom). In the case of ICA occlusion caused by IMG, pre-occlusive cone-shaped narrowing of the artery, characteristic of dissection, is revealed - “candle flame” symptoms. Other angiographic features pathognomonic for dissection include dissecting aneurysm and double lumen artery (true and false) (Fig. 1).
Rice. 1. Angiographic signs of dissection of the extracranial sections of the internal carotid and vertebral arteries. a) 3DTOF MRA. Prolonged uniform stenosis (arrows) of the cervical part of the left ICA; b) 3DTOF MRA. Prolonged uneven narrowing of the lumen of the right VA (long arrows). Hematomas in the walls of the VA, spreading towards the adventitia (short thin arrows); c) CT angiography. Dissection of the left ICA. Cone-shaped pre-occlusive narrowing of the artery is a “candle flame” symptom (long arrow), the common carotid artery (short arrows) is patent; d) dissecting aneurysm of the left ICA (arrow), associated with the main blood flow. The lumen of the artery before and after the aneurysm is narrowed due to the presence of blood in its wall; e) double lumen (true and false) of the left VA (arrow).
MRI of the neck arteries, unlike MRA, can detect IMH. The most informative mode is T1_f/s VI (f/s - “fat suppression”, suppression of the signal from adipose tissue). From the end of the 1st week and over the next approximately 2.5 months, IMG in the T1_f/s mode in the axial projection looks like a hyperintense crescent-shaped formation, narrowing the lumen of the artery, and in the case of occlusion, completely filling it. In the coronal projection, the IMH looks like a formation that follows the contours of the vessel and covers the narrowed lumen like a muff, and in case of occlusion, completely occupies it. The outer diameter of the artery is increased due to the presence of IMG in its wall (Fig. 2). After 2.5-3 months from the development of dissection, the IMG is also not visualized due to its “resorption”, accompanied by restoration of patency in the artery, or, if occlusion persists, the signal from the organized IMG becomes isointense, and therefore it is not differentiated from surrounding fabrics.
Rice. 2. MRI of the neck arteries, T1 f- mode. a) axial projection. Hematoma in the wall of the extracranial part of the left ICA (thick arrow), the outer diameter of the artery is increased. The lumen of the artery is narrowed and eccentrically located (thin arrow); b) coronal projection. Hematoma in the wall of the left ICA, muff-like covering the narrowed lumen of the artery (arrows).
Repeated neuroimaging study during dissection showed that all stenoses of the ICA and VA caused by IMG completely or partially regressed after 3 months. With initial occlusion, complete or partial recanalization is observed only in half of the cases [7, 9, 17, 24, 25]. In this regard, a single study in the acute period of IS can lead to an erroneous diagnosis of thrombosis or atherosclerotic stenosis of unknown origin, resulting in inadequate treatment. A single study in the late stages of a stroke, when patency in the artery has been restored, leads to an erroneous diagnosis of a stroke of unknown origin.
The treatment of IS caused by dissection has not been definitively determined, since there are no randomized placebo-controlled studies performed on a large number of patients [26–29]. In the acute period, anticoagulants or antiplatelet agents are usually used to prevent arterio-arterial embolism and maintain IMG in a liquefied state, facilitating its resolution. It should be borne in mind that the prescription of large doses of anticoagulants is unsafe, as it can lead to an increase in IMH and the resulting stenosis/occlusion of the arterial lumen, and as a result, an increase in the ischemic zone. Stroke outcomes do not differ when treated with anticoagulants and antiplatelet agents [7, 27, 29, 30]. It should be emphasized that the use of anticoagulants and antiplatelet agents is indicated for approximately 3 months, during which the organization of IMH occurs. Their further use for the purpose of secondary prevention of stroke is inappropriate, since its cause during dissection is not hypercoagulation, but weakness of the arterial wall.
Thrombolytic therapy
. There are no indications for thrombolysis, as well as randomized controlled trials to evaluate its effectiveness [30]. Successful implementation of both intravenous and intra-arterial thrombolysis has been described [31–33]. Intra-arterial thrombolysis can be combined with stenting [33]. The incidence of complications of thrombolysis in patients with dissection is apparently the same as with thrombolysis in patients with IS of other origins [29, 30]. The mortality rate for thrombolysis in patients with dissection is 8.1% [34]. The rate of favorable stroke outcomes during dissection did not differ between patients with and without thrombolysis, and the rate of intracerebral hemorrhage (asymptomatic in all cases) was higher with thrombolysis (5.9% versus 0.6%) [35].
Along with drug treatment in the acute period of dissection, especially during the 1st month, when the risk of recurrence of dissection is increased, it is necessary to limit movements in the cervical spine, for which wearing a cervical orthosis is recommended. In addition, it is necessary to avoid physical tension and straining, which can lead to an increase in dissection. The diet should be rich in protein and vitamins. Estrogen-containing contraceptives are contraindicated for women.
Surgical and endovascular treatment
. Indications for them based on randomized controlled trials have not been developed, although there are indications in the literature about such an approach to treatment [36]. J. Robertson and A. Koyfman [30], in a review on cervical artery dissection, note that this type of treatment can be discussed if conservative treatment is ineffective. Our experience indicates that stenting is inappropriate in the acute period of dissection, which leads to stenosis of the artery lumen, since as IMG is organized, all stenoses completely or significantly regress. Attempts to remove IMG, which is usually mistakenly interpreted as a thrombus during angiography, lead to reocclusion and an increase in focal neurological symptoms. The issue of surgical and endovascular treatment should be discussed in cases of severe residual stenosis of the artery, especially if repeated cerebrovascular accidents (CVA) develop in its basin, as well as in the formation of a dissecting aneurysm, which is dangerous as a source of arterio-arterial embolism and the development of recurrent stroke [37] .
Antiphospholipid syndrome
The share of APS among other causes of IS at a young age is 11% [1]. APS is an autoimmune condition characterized by the development of arterial and/or venous thrombosis, obstetric pathology (repeated spontaneous abortions, intrauterine fetal death, late pregnancy complications: preeclampsia, fetal growth retardation) in the presence of aPL (lupus anticoagulant, antibodies to cardiolipin, antibodies to β- 2-glycoprotein 1) [38].
The mechanisms of action of aPL are diverse and include activation of the endothelium, monocytes, platelets, which leads to the release of tissue factor that activates the extrinsic blood coagulation pathway; inhibition of the fibrinolytic system and the natural anticoagulant protein C system; increased platelet aggregation; activation of the complement system; release of pro-inflammatory cytokines; development of intimal hyperplasia leading to stenosis/occlusion of the arterial lumen [39–42]. Arterial thrombosis in APS most often develops in the arteries of the brain, leading to ischemic cerebrovascular accidents. The average age of patients with the first IS was 33 years [43–46]. Women are more likely to get sick (up to 80%), and strokes can develop during pregnancy, the postpartum period, or while taking contraceptives. Strokes in APS are caused by thrombosis of intracerebral rather than extracranial arteries. They develop in different vascular territories, since they are associated with hypercoagulation, and not with local changes in any one vessel, and have a tendency to relapse in the absence of secondary prevention with anticoagulants. An important diagnostic value at the clinical stage is that in approximately three-quarters of patients the development of stroke is preceded by systemic manifestations of APS (miscarriage in women, peripheral venous thrombosis) [43].
Within the framework of APS, Sneddon syndrome is separately identified,
characterized by a combination of two main symptoms - cerebrovascular disorders and widespread livedo [43, 47, 48]. The syndrome is named after the English dermatologist who first described it in 1965, who emphasized the nosological independence of the disease and its difference from diffuse connective tissue diseases (systemic lupus erythematosus, periarteritis nodosa, etc.) [49]. Livedo is a fairly persistent, bluish, branching spot on the skin, caused by impaired blood circulation in it, including due to a disorder of the innervation of the arteries of the dermis [50]. In the late 80s of the twentieth century, for the first time in the world, it was shown that, along with cerebrovascular disorders and livedo, some patients also have other systemic and neurological manifestations. These include: cardiac pathology (damage to the heart valves, coronary heart disease), peripheral venous thrombosis, thrombocytopenia, kidney changes (mild proteinuria, changes in urinary sediment), intrauterine fetal death or spontaneous abortions, headaches, epileptic seizures, etc. In general the spectrum of clinical manifestations of Sneddon syndrome turned out to be similar to those of APS, which initiated the study of antibodies to cardiolipin in our patients. The latter were found in 6 of 17 examined patients [48, 51]. In the same year (1988), the question of the identity of Sneddon syndrome and APS was raised by American researchers S. Levine et al. [52] based on observation of 1 patient. Despite the clinical similarity of Sneddon syndrome and APS, aPL is not detected in all patients, but in approximately half of the cases, while patients without aPL are considered as patients with seronegative APS [43]. The clinical manifestations of Sneddon syndrome in patients with or without aPL are similar, and the approach to the treatment and prevention of recurrent strokes is the same [53]. Cerebrovascular disorders in Sneddon syndrome, compared with those in APS without livedo, are more severe: cerebrovascular accidents occur more often, vascular encephalopathy gradually develops with cognitive impairment of the cortical type up to severe dementia, as well as pseudobulbar and subcortical syndromes [43, 54]. Treatment and secondary prevention of strokes consist of the constant use of indirect anticoagulants—vitamin K inhibitors (warfarin), often in combination with antiplatelet agents (small doses of aspirin) [41, 43]. There are no sufficient data on the effectiveness of the new generation of anticoagulants—direct inhibitors of thrombin (dabigitran) and coagulation factor Xa (rivoroxaban, apixaban, endoxaban) in APS occurring with arterial thrombosis [41, 42]. Little experience with the use of these drugs in APS indicates that they are less effective than warfarin in preventing recurrent cerebrovascular accidents. Plaquenil is used to correct the immunopathological process [41–43].
Cardiogenic embolism
The share of cardiogenic embolism among other causes of IS in young people is, according to our data, 12% [1]. The main cause of cardiogenic embolism in young people is damage to the heart valves (congenital, acquired, artificial valves), less often - cardiomyopathy, aneurysm of the interatrial septum, myocardial infarction, heart tumors. The main cause of cardioembolism in older age groups is atrial fibrillation; it is rare in young patients. A stroke usually develops acutely while the patient is actively awake. In almost ¼ of cases there is a loss of consciousness of varying degrees of severity. Due to spontaneous lysis of the thrombus, occlusion of intracranial arteries is not always detected and the frequency of its detection depends on the timing of the study of the arteries. Identification of the cardiogenic cause of stroke is directly related to echocardiography.
Vasculitis
The frequency of vasculitis as a cause of IS at a young age is not known with certainty, since they are often not diagnosed. Vasculitis is extremely heterogeneous in its clinical manifestations, the size and type of affected vessels, as well as their morphological changes. According to the international unified nomenclature of vasculitis, there are vasculitis affecting large, medium, small vessels, as well as vasculitis in systemic diseases and vasculitis, the etiology of which is not specified, but their association with hepatitis, cancer, and medication is assumed [55]. Almost all of the listed vasculitis can be the cause of I.I. This applies to Takayasu disease and giant cell arteritis; eosinophilic granulomatosis with polyangiitis; Behcet's disease, which is characterized by damage to the venous system of the brain; systemic lupus erythematosus; scleroderma, etc. A separate classification section consists of vasculitis of one organ, within which primary vasculitis of the central nervous system is distinguished. Its criteria, developed in 1988 by American rheumatologists L. Calabrese and J. Mallek [56], include: 1) neurological or mental defect not explained by other reasons; 2) classic angiographic signs of vasculitis (alternating areas of dilation and narrowing of the arteries) or histologically proven vasculitis of the central nervous system; 3) absence of signs of systemic vasculitis or any other diseases that could cause or mimic the angiographic picture of vasculitis (reversible vasoconstrictor cerebral syndrome) or its morphological manifestations.
Primary cerebral vasculitis is a little-known and little-studied cause of IS at a young age; only a few publications are devoted to it. The largest number of observations (163), published in 2015, belongs to the Mayo Clinic (USA) [57]. Initially, it was believed that primary cerebral vasculitis was characterized by damage to small and medium-sized vessels. However, in the early 2000s, it was proposed to distinguish 2 subtypes of cerebral vasculitis: vasculitis with damage to large/medium arteries (intracranial ICA, proximal parts of the middle cerebral, anterior cerebral and posterior cerebral arteries) and small arteries (
intracerebral arteries) [58—60]. The feasibility of isolation is determined by the difference in their clinical manifestations, course, diagnostic and therapeutic approaches. The development of neuroimaging methods for verifying inflammatory changes in the artery wall, namely, high-resolution MRI with slice thickness of 3 mm or less in T1_db_fs mode (suppression of signal from adipose tissue) before and after the administration of a contrast agent, played a decisive role in the study of vasculitis of large cerebral arteries. The accumulation of the latter in the arterial wall is considered a sign of vasculitis [61-63].
According to our studies, primary vasculitis affecting large intracranial arteries was more common in men (76%), the average age of patients was 33.7±11.0 years (19-50 years). The characteristic features of vasculitis are: the most common lesion of the ICA, isolated (86%) or in combination with lesions of the VA (9%); one-sidedness of the lesion (91%); as well as a single-phase, non-progressive course in most cases. As a rule, NMCs developed in individuals who considered themselves practically healthy and had no clinical signs of thrombophilia. In almost 1/3 of cases, stroke was preceded by transient cerebrovascular accidents. Focal neurological symptoms could develop both during the period of active wakefulness of the patient and during sleep. Headache in the acute period was observed in less than half of the patients. MRA or ultrasound examination revealed occlusive-stenotic lesions of the intracranial arteries, which was mistakenly regarded as thrombosis or atherosclerotic stenosis. The source of the erroneous interpretation is the identification of thrombosis and occlusion of the arterial lumen, although the latter includes a broader pathology and can be associated not only with thrombosis, but also with IMH or inflammatory changes in the arterial wall. In addition, the young age of patients and their lack of signs of atherosclerosis and thrombophilia are underestimated.
Verification of vasculitis of the intracranial part of the ICA, VA and their large branches consists of performing MRI in the T1_db_fs mode before and after the administration of a contrast agent. Neuroimaging markers of inflammation are thickening of the arterial wall and accumulation of contrast agent in it (Fig. 3). Stroke with vasculitis of large arteries develops by the mechanism of cerebrovascular insufficiency (in the presence of occlusion or severe stenosis) or by the mechanism of arterio-arterial embolism, the source of which is parietal thrombi formed on the “inflamed wall”.
Rice. 3. MRI, T1_db_fs mode. Arteritis of the right ICA. a) the wall of the petrous part of the right ICA is thickened (arrow); b) accumulation of contrast agent in the wall of the petrous part of the right ICA - a sign of vasculitis (arrow).
The cause of cerebral vasculitis involving large arteries remains unknown. Predominant damage to the intracranial part of the ICA and the proximal part of the middle and anterior cerebral artery, which have rich trigeminal innervation, one-sidedness of the lesion, as well as similarity with non-progressive (monophasic) vasculitis of the central nervous system in children, in the genesis of which in some cases the Varicella
zoster
, persisting in trigeminal ganglion after chickenpox [64, 65], allow us to consider the triggering role of this virus in the development of vasculitis of large intracranial arteries in adults.
Treatment has not been definitively determined. In the acute period of stroke, anticoagulants, short courses of corticosteroids, and, if indicated, acyclovir are used. As vasculitis progresses, immunosuppressants are indicated [64–66].
Hereditary thrombophilias and hyperhomocysteinemia
Great importance is attached in the domestic literature to hereditary thrombophilias as a cause of I.I. According to foreign researchers, hereditary thrombophilias, primarily caused by the 1691G→A mutation in the blood coagulation factor V gene (Leiden mutation), the 20210G→A mutation in the prothrombin gene and the 677 C→T polymorphism in the 5,10-methylenetetrahydrofolate reductase (MTHFR) gene ), are associated with venous thrombosis. They can occur in the veins and sinuses of the brain, leading to the development of venous infarction. In contrast, their role in the genesis of arterial thrombosis, including cerebral, is controversial. These mutations are more common in IS than in the general population and are usually considered an additional risk factor for stroke rather than its sole independent cause [67–70]. The results of our studies of mutations in the prothrombin gene, coagulation factor V (Leiden mutation) and MTHFR in arterial IS at a young age are consistent with this. On the one hand, they showed the absence of an association of these mutations with stroke of unknown origin (cryptogenic), on the other hand, the presence of an association between mutations in the prothrombin gene and the blood coagulation factor V gene with strokes caused by APS and cardiogenic embolism. The latter suggested that these mutations increased the thrombogenic potential in APS and cardiogenic embolism [71]. The study of mutations associated with thrombophilia in young patients with IS is indicated if they have a history of thrombosis and hereditary burden. Domestic neurologists and hematologists often attach causal significance to thrombophilic mutations in IS caused by occlusion of extra- and large intracranial arteries. This is unfounded from a pathophysiological point of view, since thrombosis during hypercoagulation always develops at the level of the microvasculature. It is impossible to assume that thrombophilia can lead to thrombosis of a large artery when its wall is intact and there is a high blood flow velocity in it, since thrombosis primarily develops in small-caliber vessels, in which the blood flow velocity is low. In addition, occlusion of extracranial and large intracranial arteries in young patients is usually caused not by thrombosis, but by dissection or vasculitis, the development of which is not associated with hereditary thrombophilias. Thus, mutations in these thrombophilia genes occur in arterial strokes of various origins and are an additional risk factor for its development, and not the cause.
Moderate hyperhomocysteinemia is quite widespread in the population and is a risk factor for IS of various origins due to endothelial damage and increased prothrombotic tendency [72-74]. In contrast, severe hyperhomocysteinemia (more than 80-100 μg/ml), associated with homocystinuria, is very rare and can lead to the early development of atherosclerosis and thrombosis, acting in these cases as the leading cause of IS [75, 76].
Other, less common causes of IS at a young age include moya-moya disease/syndrome, hereditary microangiopathies (cerebral autosomal dominant or recessive arteriopathy with subcortical infarctions and leukoencephalopathy, hereditary endotheliopathy with retinopathy, nephropathy and stroke, Fabry disease), post-radiation arteriopathy, mitochondrial diseases, spasm of cerebral arteries, drug use and others. Their diagnosis requires intracranial angiography, genetic and special biochemical studies.
In conclusion, it should be emphasized once again that the causes of IS at a young age differ significantly from those in older age groups. Their identification directly depends on the availability of new laboratory and instrumental diagnostic methods in the clinic and the experience/qualification of doctors who determine the research program. Despite great strides made in uncovering the causes of IS in young people, the percentage of cryptogenic stroke remains high and justifies further research aimed at uncovering the unknown causes of IS in young patients.
The authors declare no conflict of interest.