Torasemide
The frequency of the side effects listed below was determined according to the following (World Health Organization classification): very common (more than 10%); often (more than 1% and less than 10%); uncommon (more than 0.1% and less than 1%); rare (more than 0.01% and less than 0.1%); very rarely (less than 0.01%), including isolated reports; frequency unknown (cannot be estimated from available data).
From the blood and lymphatic system
Frequency unknown
- thrombocytopenia, leukopenia, agranulocytosis, aplastic or hemolytic anemia.
Metabolism and nutrition
Infrequently
- hypercholesterolemia, hypertriglyceridemia.
Frequency unknown
- decreased glucose tolerance (possible manifestation of latent diabetes mellitus).
From the nervous system
Often -
dizziness, headache, drowsiness;
infrequently -
muscle cramps of the lower extremities;
frequency is unknown
- confusion, fainting, paresthesia in the extremities (feeling of numbness, “crawling” and tingling).
From the side of the organ of vision
Frequency unknown -
visual impairment.
Hearing and labyrinth disorders
Frequency not known? 1a —
Hearing impairment, tinnitus and hearing loss (usually reversible) usually occur in patients with renal failure or hypoproteinemia (nephrotic syndrome).
From the cardiovascular system
Infrequently -
extrasystole, tachycardia, increased heartbeat, facial flushing;
frequency not known -
excessive arterial hypotension, orthostatic hypotension, collapse, deep vein thrombosis, thromboembolism, hypovolemia.
From the respiratory system
Infrequently -
nosebleeds.
From the digestive system
Often
- diarrhea;
uncommon
- abdominal pain, flatulence, polydipsia;
frequency is unknown -
dry mouth, nausea, vomiting, loss of appetite, pancreatitis, dyspeptic symptoms, intrahepatic cholestasis.
From the kidneys and urinary tract
Often -
increased frequency of urination, polyuria, nocturia;
infrequently -
frequent urge to urinate;
frequency unknown
- oliguria, urinary retention (in patients with urinary tract obstruction), interstitial nephritis, hematuria, increased concentrations of urea and creatinine in the blood.
From the genital organs
Frequency unknown
- violation of potency.
General and administration site disorders
Infrequently
- asthenia (exhaustion), thirst, weakness, increased fatigue, hyperactivity, nervousness.
Laboratory indicators
Infrequently -
increase in platelet count;
frequency unknown -
hyperglycemia, hyperuricemia, decrease in the number of red blood cells, leukocytes and platelets, a slight increase in the activity of alkaline phosphatase in the blood, increased activity of some liver enzymes (for example, gamma-glutamyl transferase).
From the skin and subcutaneous tissues
Frequency unknown
- skin itching, rash, urticaria, erythema multiforme, exfoliative dermatitis, purpura, vasculitis, photosensitivity.
From the musculoskeletal system
Frequency unknown
- muscle weakness.
From the side of water-electrolyte and acid-base balance
Frequency unknown
- hyponatremia, hypochloremia, hypokalemia, hypomagnesemia, hypocalcemia, metabolic alkalosis. Symptoms indicating the development of electrolyte and acid-base disorders may include headache, confusion, convulsions, tetany, muscle weakness, cardiac arrhythmias and dyspeptic disorders; hypovolemia and dehydration (more often in elderly patients), which can lead to hemoconcentration with a tendency to develop thrombosis.
If any of the side effects indicated in the instructions get worse ,
or you notice any other side effects not listed in the instructions, tell your doctor.
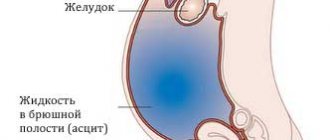
Edema-ascitic syndrome is the most common complication of liver cirrhosis. Its appearance indicates progression of the disease and is associated with a 50% mortality rate over the next 5 years [1–3]. The key drugs for the treatment of edematous-ascitic syndrome include loop diuretics, which improve the quality of life of patients and prevent the development of spontaneous bacterial peritonitis and hepatorenal syndrome. Conservative treatment includes an aldosterone antagonist, spironolactone, and loop diuretics—furosemide and torsemide [4–6]. This combination makes it possible to achieve regression of edematous-ascitic syndrome in approximately 60–75% of patients, especially in the early stages of treatment. For some patients, diuretic therapy may be ineffective due to loss of sensitivity to diuretic therapy with the formation of refractory ascites, in the presence of which the one-year survival rate is only 25% [7]. The use of diuretics in recommended doses limits such adverse events as hypotension, electrolyte disturbances, deterioration of renal function and azotemia, and an increase in hepatic encephalopathy [1, 4]. Resistance to diuretics and side effects of the traditional regimen provided the impetus for the search for new treatment algorithms for patients with edematous-ascitic syndrome. As an alternative, torasemide SR has proven itself, the clinical advantages of which include a greater diuretic effect and a lower risk of developing electrolyte disturbances [1, 8–10]. Important features of the drug (unlike furosemide) are its blocking effect on aldosterone receptors and the absence of the rebound phenomenon, which is associated with sodium and water retention. This makes the use of torasemide SR justified in liver cirrhosis. Torsemide has proven its effectiveness in both patients with chronic heart failure [11–13] and patients with liver cirrhosis. Compared with furosemide, therapy with torasemide SR was accompanied by a decrease in the number of hospitalizations and a decrease in mortality in patients with edematous-ascitic syndrome in cirrhosis of the liver [14]. In recent years, sustained-release torsemide (SR) has become available. Possessing all the properties of torasemide, the prolonged form (Britomar) provides a gradual release of the drug, which allows reducing fluctuations in the concentration of torasemide in the blood plasma [15, 16]. High and predictable bioavailability and a long half-life determine the stability of natriuresis and diuretic effect of torasemide 3V, minimize electrolyte disturbances, and improve the quality of life of patients [17–19]. The use of torasemide ZV in patients with edematous-ascitic syndrome against the background of liver cirrhosis has not been sufficiently studied.
The purpose of the study was to evaluate the effectiveness and safety of the use of torasemide ZV (Britomar) in patients with edematous-ascitic syndrome against the background of decompensated liver cirrhosis.
Material and methods
The study included 42 patients (including 20 women) with liver cirrhosis and edematous-ascitic syndrome. The average age was 57.4±11.5 years. Exclusion criteria were age <18 years, hepatorenal syndrome, hyponatremia <125 mmol/L, hypokalemia <3.5 mmol/L, hyperkalemia >5.5 mmol/L, worsening hepatic encephalopathy or hepatic coma, hypersensitivity to extended-release torsemide , anuria, sinoatrial and atrioventricular blockade of II–III degrees, arterial hypotension (BP <90/60 mm Hg). The study did not include patients with severe acute alcoholic hepatitis.
All patients followed a diet limiting table salt to 3 g/day, abstained from drinking alcohol and received standard therapy for liver cirrhosis: transfusion of albumin, fresh frozen plasma, lactulose, ciprofloxacin, propranolol.
During randomization, patients were divided into 2 groups. The main group received torasemide ZV (n=20), the control group received furosemide (n=22). All patients received spironolactone. The drugs were taken orally in tablet form for an average of 3 weeks. The initial doses of diuretics (torasemide ZV 10 mg, furosemide 40 mg, spironolactone 100 mg/day) were increased by 2 times every 3 days in case of body weight loss of less than 300 g/day. The limitation was a decrease in body weight of more than 500 g/day in patients without peripheral edema [1, 5, 6].
All patients underwent a clinical assessment, laboratory examination, which included a complete blood count, biochemical parameters of blood serum (total protein, albumin, creatinine, electrolytes, alkaline phosphatase, bilirubin, alanine aminotransferase - ALT, aspartate aminotransferase - AST, γ-glutamyl transpeptidase, coagulation system parameters, general urine analysis, daily urinary sodium excretion. In addition, instrumental studies were carried out, including electrocardiography, chest radiography, ultrasound examination of the liver, esophagogastroduodenoscopy. Ultrasound elastometry of the liver using the Fibroscan 502 Touch device (Echosens, France) was performed in all patients after reduction of ascites. The alcoholic genesis of liver cirrhosis was confirmed by the presence of signs of chronic alcohol intoxication. All patients underwent a serological study of markers of viral hepatitis. In patients under 35 years of age, ceruloplasmin was studied. To exclude hemochromatosis, serum iron and ferritin were studied.
The effectiveness and safety of therapy was assessed daily using the following indicators: daily diuresis, body weight, abdominal circumference, edema, blood pressure (BP), heart rate. The level of electrolytes and creatinine in the blood was determined every 3 days, and daily natriuresis was determined before and after diuretic therapy.
Statistical processing of the obtained data was carried out using the Statistica 7.0 program. To assess the statistical significance of differences between groups, nonparametric Mann–Whitney and Wilcoxon tests were used. Correlation analysis was carried out using Spearman statistics. All data are presented as means ± SD. Differences were considered statistically significant at p<0.05.
results
According to the main clinical and demographic indicators, the study groups were comparable (Table 1). In all patients, liver cirrhosis was of alcoholic origin; in 31%, markers of viral hepatitis were detected; in 71%, liver cirrhosis corresponded to Child–Pugh class C. In 14.2% of them, the diagnosis of liver cirrhosis was established for the first time. In all patients, the course of liver cirrhosis was complicated by the development of ascites; in 86%, ascites had grade III severity; 43% had previously taken diuretic therapy. No patient required laparocentesis either earlier or during the study. All of them had swelling of the lower extremities, jaundice, signs of hepatic encephalopathy, and a decrease in the synthetic function of the liver.
The liver density in the torasemide SV group averaged 56±8 kPa, in the furosemide group – 60±10 kPa. By the end of the study, the average dose of torasemide pollutants was 8±2 mg/day, furosemide 60.0±35 mg/day. At the same time, in the furosemide group, the dose of furosemide was increased in 11 (50%) patients, and 2 times in 7. In the torasemide 3 group, no dose increase was required. The groups were comparable in terms of concomitant therapy. Against the background of diuretic therapy, a decrease in edema syndrome was noted in both groups: in the torasemide ZV group, 2 (10%) people remained pasty in the legs and feet; in the furosemide group – in 6 (27%).
All patients experienced regression of ascites to grade I. A more pronounced diuretic effect was observed in the torasemide ZV group.
In the torasemide group, diuresis increased on average by 865±660 ml, and in the furosemide group – by 400±300 ml (p=0.018). At the same time, no statistically significant differences in the dynamics of body weight were detected between the groups: in the torasemide ZV group, body weight decreased by 8±4 kg, in the furosemide group - by 5.8±3.8 kg (p=0.06).
In the long-acting torsemide group, there was a statistically significant increase in daily urinary sodium excretion (93±63.0 mmol/day compared to the furosemide group 51±20 mmol/day; p=0.012). There were no statistically significant differences in the levels of potassium and sodium in the blood plasma in the two groups.
During treatment, renal function was stable, creatinine levels did not increase.
The effect of diuretic therapy on blood pressure, electrolytes and creatinine levels is presented in Table. 2. By the end of the study, all patients showed a statistically significant decrease in the average Child–Pugh score (p = 0.003) and a decrease in the severity of liver cirrhosis (p = 0.003). However, no statistically significant differences were found between the groups (Figure).
Thus, when comparing torasemide ZV with furosemide in the study group, a significantly greater diuretic and saluretic effect was obtained. At the same time, no statistically significant differences in body weight loss and regression of edema syndrome were identified.
Discussion
For the treatment of edematous-ascitic syndrome in patients with decompensated liver cirrhosis, immediate-release torasemide has been most studied. A number of studies have demonstrated that the pharmacokinetics of torasemide IV, in particular bioavailability, are comparable to those of immediate-release torasemide [20]. In liver cirrhosis, an increase in bioavailability (2.5 times) and half-life of torsemide (up to 4.8 hours) was noted [21].
However, an increase in accumulation with long-term use of the drug is not expected, because in such patients, about 80% of the drug dose was excreted in the urine per day (unchanged and in the form of metabolites), i.e. no dose adjustment is required for liver cirrhosis [22].
In the treatment of edematous-ascitic syndrome in patients with liver cirrhosis, it is possible to use torasemide both as monotherapy and in combination with spironolactone [23]. Torsemide therapy is indicated both for decompensation of ascites and for its prevention. Torsemide does not cause hypokalemia even with long-term use [24].
To evaluate the effectiveness of diuretic therapy in patients with liver cirrhosis and ascites, a number of comparative studies of torasemide and furosemide were conducted. A randomized study of 28 patients with ascites treated with spironolactone (200 mg/day) compared the results of 6 weeks of therapy with torasemide (20 mg/day) and furosemide (40 mg/day). Both drugs had comparable effects on body weight, diuresis, and excretion of uric acid, sodium, and chloride, but excretion of potassium, calcium, inorganic phosphate, and magnesium was lower in the torsemide group [25].
In a double-blind crossover study, the results of oral administration of furosemide (80 mg) and torasemide (20 mg) were compared in 14 patients. Torsemide was superior to furosemide in diuretic and natriuretic activity.
Five patients had a weak response to furosemide, while torasemide caused a significant increase in natriuresis and diuresis [26].
The positive effect of torasemide on diuresis and saluresis was shown in a study involving 124 patients with liver cirrhosis: 61 people received torasemide and 63 received furosemide. Doses were selected individually, taking into account the gradation of ascites and response to diuretic therapy. In the torsemide group, daily urinary sodium excretion significantly increased compared with that in the furosemide group (p < 0.05) [10].
An increase in natriuresis (p=0.012) and diuresis (p=0.018) was also observed in our study when using torasemide ZV. A group of 46 patients with liver cirrhosis complicated by ascites (randomized trial) were treated with torasemide 20 mg/day or furosemide 40 mg/day in combination with spironolactone 200 mg/day. If it was not possible to achieve a weight loss of 300 g/day, the doses of diuretics were increased every 3 days to 40, 120 and 400 mg/day, respectively. Torasemide ZV caused a more pronounced increase in diuresis than furosemide, although in general the treatment results in the 2 groups were comparable. Increases in diuretic doses were required in 2 patients in the torasemide 3V group and in 9 patients in the furosemide group (p<0.05) [15].
In our study, when comparing torasemide ZV with furosemide, no statistically significant differences in the dynamics of body weight were detected (p = 0.06). The lack of significant differences between the average body weight of the experimental group and the comparison group may be due to the targeted dosed weight reduction in both groups according to the recommended regimens. It is worth noting that an increase in diuretic doses was required only in the furosemide group.
Torsemide did not affect plasma blood pressure, creatinine and electrolyte levels when using the drug at a dose of 20 mg/day compared with furosemide at a dose of 50 mg/day [22, 27]. The duration of diuretic therapy was 7 days. In our study, patients took torasemide ZV for an average of 3 weeks. In the study group, there was a trend towards lower blood pressure levels before inclusion in the study. However, Britomar had no effect on blood pressure levels. There were no changes in the levels of potassium, sodium and creatinine in the blood.
One study showed that during therapy with torasemide, compared with furosemide, treatment-resistant ascites develops significantly less often (16.4 and 38.1%; p<0.05). The duration of therapy was 15 days [9]. However, only patients with grade III ascites were included. More than 50% of patients had recurrent ascites that could not be corrected with diuretic therapy on an outpatient basis. A decrease in the synthetic function of the liver (hypoalbuminemia) was corrected by transfusion of fresh frozen plasma only. In our study, loss of sensitivity to diuretics was not detected in any of the groups. This may be due to concomitant therapy. Thus, the main method of correcting hypoproteinemia was the transfusion of albumin, rather than fresh frozen plasma. In addition, the severity of ascites was less pronounced. The study involved patients with stage II ascites, 42% had not previously received diuretic therapy, and in 14.2% liver cirrhosis debuted with edematous-ascitic syndrome.
When planning the study, we assumed that the use of torasemide ZV would reduce the length of hospital stay due to a more rapid regression of the edematous-ascitic syndrome. There were no significant differences in the length of stay of patients in the hospital. The dose ratio of torasemide and furosemide is 10–20 and 40 mg/day [21, 28]. In most studies, the starting dose of immediate-release torasemide was 20 mg/day [9, 23–27]. In our study, approximately 50% of patients had not previously received diuretic therapy, had relatively preserved natriuresis and a trend towards lower blood pressure. In this regard, the initial dose of torasemide pollutant was 10 mg/day. During the therapy, positive dynamics were observed in the form of increased diuresis and natriuresis without the development of hypotension, electrolyte disturbances, and a stable creatinine level.
Given the calm safety profile of torasemide ZV, it is likely that a more rapid clinical effect will be observed with increasing doses.
Thus, torasemide ZV (Britomar) can serve as an alternative to furosemide in the treatment of edematous-ascitic syndrome in patients with decompensated liver cirrhosis.
Torasemide tab 5mg 60 pcs
Pharmacological group:
Diuretic.
Pharmacodynamics:
Torsemide is a “loop” diuretic. The main mechanism of action of the drug is due to the reversible binding of torasemide to the sodium/chlorine/potassium ion cotransporter located in the apical membrane of the thick segment of the ascending limb of the loop of Henle. As a result, the reabsorption of sodium ions is reduced or completely inhibited and the osmotic pressure of intracellular fluid and water reabsorption are reduced. Blocks aldosterone receptors in the myocardium, reduces fibrosis and improves diastolic myocardial function.
Torasemide causes hypokalemia to a lesser extent than furosemide, but it is more active and its action lasts longer.
The maximum diuretic effect develops 2-3 hours after taking the drug orally.
Torsemide can be used for a long time.
Pharmacokinetics:
After oral administration, torasemide is quickly and almost completely absorbed from the gastrointestinal tract. The maximum concentration (Cmax) of torasemide in plasma is achieved 1-2 hours after taking the drug orally after a meal. Bioavailability is 80-90% with minor individual variations.
Distribution Plasma protein binding - more than 99%. The volume of distribution (Vd) is 16 l. In patients with liver cirrhosis, Vd doubles.
Metabolism Metabolized in the liver with the participation of isoenzymes of the cytochrome P450 system.
As a result of successive reactions of oxidation, hydroxylation or ring hydroxylation, 3 metabolites are formed (M1, M3 and M5), the binding of which to plasma proteins is 86%, 95% and 97%, respectively.
Elimination The half-life (T1/2) of torasemide and its metabolites is 3-4 hours and does not change in chronic renal failure.
Total clearance is 40 ml/min, renal clearance is 10 ml/min.
On average, about 83% of the dose taken is excreted by the kidneys: unchanged (24%) and in the form of predominantly inactive metabolites (M1 - 12%, M3 - 3%, M5 - 41%).
Pharmacokinetics in special clinical cases Features of the pharmacokinetics of torasemide in certain groups of patients Use for liver dysfunction The drug is contraindicated in hepatic coma and precomatose state. In case of liver failure, the concentration of torasemide in the blood plasma increases due to a decrease in the metabolism of the drug in the liver.
In patients with cardiac or liver failure, T1/2 of torasemide and the M5 metabolite is slightly increased, drug accumulation is unlikely.
In patients with liver cirrhosis, Vd, T1/2 and renal clearance are increased, but the overall clearance remains unchanged.
Chronic heart failure In patients with chronic heart failure in the stage of decompensation, hepatic and renal clearance of the drug is reduced. In such patients, the total clearance of torasemide is 50% less than in healthy volunteers, and T1/2 and total bioavailability are correspondingly higher.
Use for impaired renal function In patients with renal failure, the renal clearance of torasemide is markedly reduced, but this does not affect the total clearance. The diuretic effect in renal failure can be achieved by use in high doses. In renal failure, T1/2 of torasemide does not change, T1/2 of metabolites M3 and M5 increases. Torsemide and its metabolites are slightly eliminated by hemodialysis and hemofiltration.
The drug is contraindicated in renal failure with increasing azotemia.
Use in children Contraindication: under 18 years of age (efficacy and safety have not been established).
Use in elderly patients Elderly patients do not require special dose selection.
The pharmacokinetic profile of torasemide in elderly patients is similar to that in younger patients, with the exception that there is a decrease in renal clearance of the drug due to the characteristic age-related decline in renal function in elderly patients. The overall clearance and T1/2 do not change.
Dependence on gender and race The influence of ethnicity and race on the pharmacokinetics of torasemide has not been studied.