One of the functions of the heart is contractility - the ability to contract under the influence of impulses and perform the function of a pump. The elementary contractile unit of cardiac muscle fiber (cardiomyocyte) is the sarcomere - a section of myofibril bounded on both sides by dark lines, the so-called Z-lines. The distance between the Z-lines depends on the degree of muscle contraction or stretching and ranges from 1.6 to 2.2 µm. The sarcomere consists of evenly alternating light - anisotropic (A) and dark - isotropic (I) stripes (disks), giving the myocardial fibers a characteristic striated appearance (Fig. 1).
Figure 1. Uniform alternation of anisotropic discs, areas of focal subsegmental contracture damage to cardiomyocytes in TBI with a rapid rate of death. Polarization microscopy. Uv. 640. A-disks are located in the center, formed by thick myosin filaments. I-discs consist of thin filaments containing actin, troponin complex (I, T, C), tropomyosin, titin and are half the width of A-discs [1-6].
The contraction of cardiomyocytes is an energy-dependent process, regulated by calcium, adenosine triphosphate and the balanced content of potassium, sodium, and magnesium in the myocardium [3, 7—12]. The force of contraction of the heart muscle depends on its initial length. The most powerful sarcomere contraction is observed at a length of 2.2 µm. It is at this length that the location of anisotropic and isotropic disks relative to each other is most favorable for their interaction, which determines the maximum inotropic function of the myocardium (Frank-Starling law) [4, 13-15].
Experimental data have been obtained that in cardiac muscle the length of the sarcomere is directly proportional to the length of the muscle. If the sarcomere length increases to 3.65 μm, then the thin filaments completely extend beyond the A-band and the generated voltage drops to zero. As the sarcomere length decreases to 2.0–1.5 μm, the I disks first narrow and then disappear, while the width of the A disks remains constant. At this moment, the Z-lines abut the edges of the A-bands and the generated voltage approaches zero. In such cases, twisting of thin threads and their double bending are possible. At the same time, the sensitivity of the troponin-actin complex to calcium ions and, consequently, the force of muscle contraction decreases [16-18].
It is advisable to assess the degree of sarcomere contraction and its pathomorphological changes using polarization microscopy. In case of death from various causes, the following stereotypical independent types of acute pathology of cardiac muscle fibers are noted: 1) contracture type of damage (segmental and subsegmental contractures); 2) intracellular myocytolysis; 3) clumpy disintegration of myofibrils; 4) cytolysis; 5) relaxation; 6) cracks; 7) dissociation [5, 19-21].
The contracture type of cardiomyocyte damage is characterized by excessive contraction of myofibrils and varying degrees of shortening or disappearance of I-discs. Segmental contractures involve the entire muscle cell, while subsegmental contractures are characterized by a reduction in individual groups of sarcomeres. With segmental contractures of the first degree, a slight convergence of the A-discs occurs, the length of the sarcomeres remains the same or decreases slightly, and the height of the I-discs does not change. The development of degree II contractures is accompanied by shortening of sarcomeres due to a decrease in the height of I-discs. The transverse striation of cardiomyocytes is preserved. With contractures of the third degree, the I-discs completely disappear, the A-discs merge into a continuous luminous conglomerate, and the transverse striation of the cardiac muscle fibers does not differ [5, 19, 21-23].
Lumpy disintegration of myofibrils is characterized by the disintegration of myofibrils into clumps as a result of focal mosaic lysis and contracture of individual groups of sarcomeres. Intracellular myocytolysis is manifested by disaggregation and lysis of myofibrils in a separate area of the cardiomyocyte; perifocally, sections of the muscle fiber retain transverse striations. This polarized microscopic picture is called the “moth-eaten appearance of tissue.” Cytolysis is expressed in the fact that in externally unchanged muscle cells, due to the destruction of the I-disks of myofibrils, the gradual disappearance of transverse striations occurs [5, 19, 21, 22].
Relaxation of cardiomyocytes is characterized by an increase in the height of the I-disc, while the height of the A-disc does not change. Cracks in cardiac muscle fibers are verified by the presence of thin transverse straight or stepped cracks along the cardiac muscle fiber. Dissociation of cardiomyocytes is associated with expansion of Z-bands. Contractures of the third degree, lumpy disintegration and intracellular myocytolysis are considered irreversible. Markers of ventricular fibrillation are areas of cracks and dissociation of cardiomyocytes. Morphological manifestations of asystole have not been identified to date [19, 24-28].
In the premortem period, impaired myocardial contractility is accompanied by a progressive decrease in blood pressure. Despite the variety of causes of death, they are all grouped into 4 types of terminal conditions: cerebral, cardiac, pulmonary and mixed. An imbalance of electrolytes in the myocardium causes its electrical instability. Cessation of cardiac activity occurs from ventricular fibrillation (98% of all cases) or asystole [24, 26-31]. Let's look at some causes of death.
In those who died from an isolated traumatic brain injury (TBI), accompanied by rapid onset of death due to damage to the brain stem, the polarization picture is dominated by a uniform alternation of anisotropic disks in both ventricles of the heart (see Fig. 1)
, which corresponds to the physiological structure of the sarcomere.
In TBI with a short premortem period, focal subsegmental and segmental contractures of grade I cardiomyocytes prevail in the myocardium of the left and right ventricles (Fig. 2).
Figure 2. Contracture damage to cardiomyocytes of the first degree, focal subsegmental contractures, cracks and dissociation of cardiomyocytes in TBI with experience. Polarization microscopy. Uv. 500. These changes reflect the minimal degree of hypercontraction of sarcomeres. Regardless of the rate of death, markers of ventricular fibrillation were detected in all cases, and pericardial fluid parameters (concentrations of glucose, sodium, potassium, calcium and magnesium) indicated no changes in carbohydrate and mineral metabolism in the myocardium [32-34]. Consequently, in those who died due to the cerebral type of terminal state in cases of isolated TBI, regardless of the duration of the premortem period, cardiac arrest is not associated with loss of myocardial contractility. It is caused by a violation of its innervation from the central nervous system (CNS) with the subsequent occurrence of ventricular fibrillation of the heart (ventricular fibrillation of central origin).
In those who died from acute coronary heart disease (CHD), markers of ischemic myocardial damage were detected in the left ventricle of the heart using polarization microscopy. In cases of acute coronary insufficiency (ACF), they represented contracture damage to cardiomyocytes of the third degree (Fig. 3),
Figure 3. Contracture damage to cardiomyocytes of II-III degree, areas of relaxation, cracks and dissociation of cardiomyocytes in acute intestinal tract.
Polarization microscopy. Uv. 500. in acute myocardial infarction in the pre-necrotic stage (AMIDS) - a combination of third degree contractures with areas of intracellular myocytolysis (Fig. 4)
Figure 4. Intracellular myocytolysis of cardiomyocytes in AIMDS. Areas of myofibril dissolution have the appearance of “moth-eaten tissue.” Polarization microscopy. Uv. 640. and clumpy disintegration of myofibrils [34-37]. These changes are irreversible and are associated with the complete disappearance of dark I-disks in the structure of the sarcomere due to the breakdown of the troponin complex. Many scientific works have been devoted to the study of cardiac troponins in ischemic heart disease [38–42], but the relationship between the presence of cardiac troponin I in the pericardial fluid and microscopic changes in the myocardium in acute forms of ischemic heart disease has been established [36].
Additionally, in both ACI and AIMDS, zones of contracture changes of the second degree and relaxation of cardiomyocytes, markers of fibrillation of cardiac muscle fibers were found in the left ventricle of the heart.
There were no markers of ischemic damage in the right ventricle in both forms. Areas of cracks and dissociation of cardiomyocytes predominated, as well as contractures of cardiac muscle fibers of the I-II degree, focal subsegmental contractures and relaxation of sarcomeres [34, 37, 43]. The phenomenon of cardiomyocyte relaxation as a sign accompanying myocardial ischemia has been described only for the left ventricle of the heart [21, 25, 28, 45].
In ACI, the distribution of markers of ischemic damage to the left ventricular myocardium has a “mosaic” character. The ischemic process simultaneously affects at least three of its areas at different depths: in the subepicardial, subendocardial and intramural regions. In those who died from AIMDS, the appearance of ischemic markers is focal and usually limited to one or two adjacent areas of the left ventricle. Transmural damage to the myocardial wall predominated; subendocardial or subepicardial lesions were less common. In the right ventricle, zones of hypercontraction and relaxation of cardiomyocytes were localized in three topographic areas, and were most often found in the subendocardial parts of the myocardium. Less often they involved intramural zones, less often they were observed subepicardially [34, 37, 43].
A study of pericardial fluid in patients with ACI and AIMDS revealed an imbalance of electrolytes with an increase in the content of potassium, calcium and magnesium, and a decrease in sodium concentration. In those who died from acute insufficiency, the disturbance of carbohydrate metabolism was characterized by an increase in glucose content, and in case of acute intestinal dystrophy it decreased. The difference between acute forms of IHD is due to the duration of the ischemic period [33, 34, 44].
So, with ACI and AIMDS, impaired carbohydrate metabolism and electrolyte imbalance cause electrical instability of the myocardium. In the left ventricle of the heart, areas of hypercontraction (second degree contractures), sarcomere disintegration (third degree contractures, zones of intracellular myocytolysis and clumpy disintegration of myofibrils) alternate with areas of relaxation (increase in sarcomere length) of cardiac muscle fibers, which causes an irreversible loss of its contractility. In the right ventricle of the heart, zones of hypercontraction (contractures of cardiac muscle fibers of the I-II degree, focal subsegmental contractures) are also combined with relaxation of the sarcomeres of cardiomyocytes. The consequence of these processes is asynchronous contraction of the myocardium of the left and right ventricles of the heart.
Asynchronous contraction of the myocardium against the background of its electrical instability, according to some authors [46-49], is the cause of ventricular fibrillation through the re-entry mechanism. Taking into account that markers of fibrillation were detected in both ventricles of the heart simultaneously, there is every reason to believe that areas of loss of myocardial contractility, both in areas of ischemic damage and in areas of cardiomyocyte relaxation, are trigger zones for the occurrence of ventricular fibrillation [43].
Thus, in acute forms of coronary artery disease, which is characterized by a cardiac type of terminal condition, cardiac arrest is associated with the disintegration of the sarcomere structures of cardiomyocytes and the irreversible loss of its contractility, which ultimately causes the occurrence of ventricular fibrillation of the heart (ventricular fibrillation of cardiac origin).
In pneumonia, using polarization microscopy, markers of fibrillation were identified - areas of cracks and dissociation of cardiomyocytes, as well as a combination of degree I and II contractures with relaxation of cardiac muscle fibers in the left and right ventricles of the heart. Polarization changes were simultaneously localized in the anterior, lateral and posterior walls of the left ventricle, as well as the anterior, lateral and posterior walls of the right ventricle. More often than others, they involved the subendocardial regions, less often intramural, and were found with the lowest frequency in the subepicardial zones [34].
A study of pericardial fluid revealed disturbances in carbohydrate (decreased glucose concentration) and protein (increased urea content) metabolism, an imbalance of electrolytes (increased magnesium and potassium content, decreased sodium concentration) [33, 34]. These processes are caused by disruption of ventilation, perfusion and filtration processes in the lungs, disruption of airway patency and hemodynamics of the pulmonary circulation. A change in the gas composition of the blood with a decrease in the concentration of oxygen in it causes hypoxia of tissues and organs, disruption of the activity of the respiratory and vasomotor centers of the medulla oblongata. An increase in urea concentration is associated with protein catabolism in the body and its increased synthesis in the liver, and pericardial fluid is an ultrafiltrate of blood and intercellular fluid [29, 31, 40-42, 50].
Thus, in pneumonia, which is characterized by the pulmonary type of terminal condition, the alternation of zones of hypercontraction and relaxation of the sarcomeres of cardiomyocytes (in different topographic areas and at different depths of the wall) causes asynchronous contraction of the myocardium of the left and right ventricles and a subsequent decrease in the contractility of the heart. An imbalance of electrolytes leads to electrical instability of the myocardium, and dysfunction of the respiratory and vasomotor centers of the medulla oblongata causes a disruption of its innervation from the central nervous system. Consequently, cardiac arrest is caused by ventricular fibrillation of mixed origin.
In those who died from strangulation mechanical asphyxia (SMA) due to complete hanging with a loop placed on the anterior surface of the upper third of the neck and a posterolateral location of the node, examination of microslides in polarized light established the presence of widespread subsegmental contractures in the left and right ventricles of the heart (Fig. 5).
Figure 5. Widespread subsegmental contracture damage and zones of cardiomyocyte relaxation during death from strangulation mechanical asphyxia. Polarization microscopy. Uv. 640. In the left ventricle they were most often found in the intramural sections, and in the right ventricle they were most often observed in the subendocardial zones, less often in the intramural and even less often in the subepicardial sections. In both ventricles, widespread subsegmental contractures alternated with zones of relaxation of cardiac muscle fibers and markers of ventricular fibrillation. A study of the pericardial fluid revealed an increase in the content of glucose, potassium, magnesium and calcium, and a decrease in the sodium content [32-34].
It has now been established that in SMA, due to complete hanging, a sharp change in the gas composition of the blood occurs with a decrease in the oxygen concentration in it and the accumulation of carbon dioxide, toxic under-oxidized metabolic products. Activation of the sympathetic-adrenal and pituitary-adrenal systems, sudden hypoxia of the body increases the automaticity of the heart, excitability and contractility of the myocardium of both ventricles, significantly increasing their need for oxygen. Irritation of the receptors of the vagus nerve of the neck inhibits the formation of impulses in the sinoatrial node and reduces the contractility of cardiomyocytes. The result of all these processes is progressive myocardial hypoxia. The toxic effect of carbon dioxide and a decrease in the partial pressure of oxygen in the blood act on the vasomotor center of the medulla oblongata. Death occurs due to a mixed type of terminal condition - cerebral and cardiac [30, 51-53].
Consequently, in the examined cases of death from SMA, areas of hypercontraction and relaxation of the sarcomeres of cardiomyocytes in different topographic areas and at different wall depths cause asynchronous contraction of the myocardium of the left and right ventricles with a subsequent decrease in cardiac contractility. Changes in carbohydrate metabolism and electrolyte imbalance lead to electrical instability of the myocardium. Severe hypoxia contributes to disruption of the respiratory and vasomotor centers of the medulla oblongata and disorganization of its innervation from the central nervous system. In this regard, there is every reason to believe that cardiac arrest is caused by ventricular fibrillation of mixed origin.
Thus, with isolated TBI, cessation of cardiac activity occurs without loss of its contractility due to disruption of innervation from the central nervous system. In acute forms of IHD (ACI and AIMDS), the loss of cardiac contractility is caused by the irreversible disintegration of the contractile structures of the sarcomeres of left ventricular cardiomyocytes, alternating zones of hypercontraction and relaxation of right ventricular cardiomyocytes, followed by asynchronous contraction of the myocardium of both ventricles. In cases of pneumonia and SMA (due to complete hanging with a loop on the anterior surface of the upper third of the neck and a posterolateral location of the node), a decrease in the contractility of the heart is caused by alternating zones of hypercontraction and relaxation of cardiac muscle fibers with the subsequent occurrence of asynchronous contraction of the left and right ventricles of the heart.
The data presented were obtained using an additional research method - polarization microscopy, which must be widely introduced into the practical activities of the forensic medical examination bureau. The research results must be used when addressing issues of forensic medical expert practice.
Basics of cardiac physiology
1.1. Brief outline of the morphology of the heart
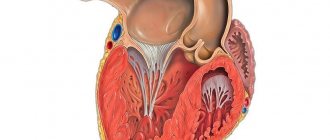
The heart (Fig. 1) is the central organ of the circulatory system. Thanks to the continuous contractile activity of the heart muscle, blood moves through the vessels and, therefore, ensures human life.
The heart is a hollow muscular organ located in the chest, anterior mediastinum, so that its base faces the spine, and its apex is at the level of the fifth left intercostal space downward and inward from the left nipple. Thus, the longitudinal axis of the heart runs obliquely: from the right and from top to bottom and to the left. As a result, the heart is located asymmetrically in the chest: one third is to the right of the midplane of the body, and two thirds is to the left of it. The asymmetry of the position of the heart is also manifested in the fact that the surface facing anteriorly is formed mainly by the wall of the right ventricle and right atrium and only to a small extent by the anterior wall of the left ventricle. In clinical practice, the boundaries of the heart are determined by percussion or fluoroscopy. The weight of the heart of an adult is 0.40–0.46% of body weight (on average about 300 g).
The human heart cavity is divided into four chambers: two atria and two ventricles. The left atrium and ventricle together make up the left, or arterial, heart, pumping arterial blood, and the right atrium and ventricle make up the right, or venous, heart, pumping venous blood. The right and left atria are separated from each other by a septum, as are the right and left ventricles. Between the right atrium and the right ventricle, as well as the left atrium and the left ventricle, there are atrioventricular openings through which blood is directed into the ventricles during their contraction.
Rice. 1.
Mammalian heart:
A
- cross section: 1 - left atrium;
2 - branches of the left pulmonary vein; 3 - parietal layer of pericardium; 4 - pericardial cavity; 5 - mitral valve; 6 - epicardium (visceral layer of pericardium); 7 - myocardium; 8 - endocardium; 9 - left ventricle; 10 - top; 11 - interventricular septum; 12 - right ventricle; 13 - tricuspid valve; 14 - right atrium; b
— internal structure: 1 — pulmonary artery; 2 - pulmonary veins; 3 - left atrium; 4 - left atrioventricular (bicuspid) valve; 5 - aortic valve; 6 - left ventricle; 7 - interventricular septum; 8 - right ventricle; 9 - inferior vena cava; 10 - right atrium; 11 - pulmonary veins; 12 - superior vena cava; 13 - aorta
These openings are equipped with leaflet valves: the right atrioventricular opening is tricuspid, or tricuspid, and the left atrioventricular opening is bicuspid, or mitral. During ventricular relaxation, the leaflet valves are open, whereas during ventricular contraction, these valves close the atrioventricular orifices, which prevents blood from flowing back from the ventricles into the atria.
The aorta departs from the left ventricle, through which blood rushes into the vessels of the systemic circulation, after which it returns through the vena cava (superior and inferior) to the right atrium and then to the right ventricle. In addition, venous blood from the tissues of the heart itself flows into the right atrium (through the coronary sinus of the heart). The pulmonary trunk departs from the right ventricle, through which blood enters the pulmonary circulation, and through four pulmonary veins it returns to the left atrium and left ventricle.
Thus, the movement of blood is carried out through two circulatory circles connected in series in the heart. The amount of blood flowing per unit time through the systemic and pulmonary circulation is normally the same.
The main progressive features in the general course of cardiac evolution in mammals and humans are:
— complete separation of the greater and lesser (pulmonary) circulation;
- more complete unification of the sinus region with its own atrium, which is achieved by reduction, often in the early embryonic period, of both sinus valves;
- a secondary increase in the volume of the sinus region and a change in the inclination of the draining vena cava with the development of myocardial layers at their mouths;
— development in humans during embryogenesis of special formations based on the posteroinferior end of the right sinus valve: the valve of the caudal vena cava (Eustachian), which serves to direct blood flow into the foramen ovale, and the valve of the coronary sinus (tebesian);
- reduction of the left cranial vena cava and formation of the coronary sinus, the mouth of which is covered either by a special valve (large tetrapods) or a special valve (humans);
- more complete retraction of the mouth of the primary pulmonary vein into the left atrium and the formation of its four primary mouths; the formation of three orifices in tetrapods and secondary divergence to the sides of the posterior pulmonary veins in anthropoids with the formation of four trunks;
- concentration inside the heart sac of strong myocardial layers on the collector trunks of the pulmonary veins, forming special cuffs;
- noticeable reduction of the atrial appendages, especially pronounced on the left;
— stabilization of the position, shape and size of the leaflets in the atrioventricular valves: three in the right and two in the left in accordance with the conditions of intracardiac hemodynamics;
- the formation of a high and dilated ascending aorta with a very steep arch and the formation of a specific threshold-like isthmus in humans at the border of the second fracture with the descending aorta. These structural features create special hemodynamic conditions - a kind of dam with increased pressure - to direct the blood flow vertically to the head with a large brain;
- the tendency in humans to shift the mouths of both coronary arteries of the heart from the pockets of the aortic valve higher, directly to the initial part of the aorta itself (freeing them from covering the semilunar valves), which creates conditions for maintaining a high value of coronary blood flow in diastole;
- the formation of a relatively large foramen ovale and relatively weak patency of the ductus arteriosus (with its branch from the very terminal part of the pulmonary artery) in anthropoids. This allows you to quickly switch placental blood circulation to constant;
- the formation in the higher placentals of the oval opening in the valve in the second half of embryonic life of a special, circularly located cardiac muscle, developed especially in anthropoids. This allows the fetus to regulate blood flow through the foramen ovale depending on the phases of atrial contraction. The progressive development of the cardiac muscles before birth thereby, as it were, preliminarily separates functionally both halves during systole;
— the formation at the end of the valve of the oval opening in the second half of embryonic life in large forms of mammals of special elastic network-like formations that help close the oval opening at birth;
- release of the base of the heart from the enclosing cardiac sac with the formation of serous outgrowths in humans, which allows the heart to move more freely.
The heart is surrounded by the pericardium, or pericardium.
, which has two leaves: internal (visceral) and external (parietal).
Between these layers, a slit-like pericardial cavity is formed, lined with mesothelium and containing a small amount of serous fluid (normally about 30–50 ml). This fluid reduces the mutual friction of the pericardial layers during heart contractions. The parietal layer of the pericardium passes into the adventitia of large vessels, and is attached to the sternum anteriorly. The visceral layer of the pericardium forms the outer layer of the heart - the epicardium
.
The inner lining of the heart is the endocardium
— lines the cavities of the heart from the inside. It is formed by connective tissue elements, smooth muscle cells and epithelial tissue (endothelium) covering the surface of the endocardium facing the heart cavity. Folds (duplicates) of the endocardium form the heart valves. Between the right atrium and the right ventricle there is a tricuspid or tricuspid valve, and between the left atrium and the left ventricle there is a bicuspid or mitral valve. In the proximal parts of the aorta and pulmonary trunk there are semilunar valves, each of which consists of three pocket-like folds, directed with their free edges into the lumen of the vessels.
The main mass of the heart is its middle layer - the cardiac muscle, or myocardium.
, formed by coelomic striated muscle tissue. The atrial myocardium consists of two layers: the superficial, formed by circular fibers, which is common to both atria, and the internal, formed by longitudinally located fibers, independent in each atrium. The inner layer of the atria myocardium forms around the mouths of the vena cava and pulmonary veins a kind of sphincters, which, when the atria contract, almost completely block the lumen of these vessels, preventing the reverse flow of blood from the atria into these veins.
In the ventricles, the myocardium is formed by three layers: superficial, middle and deep. The obliquely located fibers descend superficially to the apex of the heart, where they bend inward and pass into the deep longitudinal layer. The derivatives of the latter are papillary (papillary) muscles protruding into the lumen of the ventricles. Tendon filaments (chords) extend from these muscles, which are attached to the atrioventricular valves on the side facing the ventricular cavity. When the ventricular myocardium contracts, the papillary muscles also contract. As a result, the tendon threads are stretched and keep the leaflet valves from bending into the atrium cavity. Insufficiency of this function, for example genetically determined, leads to sagging (prolapse) of the valve leaflets into the atrium cavity during ventricular contraction and disruption of intracardiac hemodynamics.
Located between the superficial and deep, the middle layer of the myocardium is formed by circular fibers, independent for each ventricle. The thickness of the myocardium depends on the load placed on them: the walls of the left chambers of the heart in adults are thicker than the walls of the right, and the walls of the ventricles are thicker than the walls of the atria. The wall of the left ventricle, which pushes blood through the vessels of the systemic circulation, has the greatest thickness (10–15 mm). The thickness of the walls of the right ventricle is 5–8 mm, while the thickness of the walls of the atria is only about 2–3 mm. However, when the heart adapts to increased physical activity, for example in athletes, the myocardial mass and the thickness of the heart walls may increase (working myocardial hypertrophy).
The main tissue component of the myocardium is muscle tissue of the cardiac (coelomic) type. Cardiac muscle fibers are smaller than skeletal muscle fibers. They have a ribbon-like shape (15–20 µm wide with a thickness of about 5 µm) and are divided into individual cells - cardiomyocytes. Up to 35.8% of the mass of cardiomyocytes is made up of mitochondria - organelles of energy metabolism. In addition to cardiomyocytes, the myocardium includes connective tissue fibers. The connective tissue frame of the heart connects muscle fibers with each other, as well as with the endo- and epicardium, influencing the mechanical characteristics of the heart muscle - its extensibility and elasticity.
Along with the myocardium itself, the heart includes two groups of papillary (papillary) muscles that connect the inner surface of the myocardium with the leaflets of the mitral and tricuspid valves. At the beginning of ventricular contraction, the papillary muscles pull the leaflets of the mitral or tricuspid valves down into the ventricular cavity. Holding the ends of the valves leads to the collapse of the basal sections of the valves first and thereby ensures their hermetic closure. Since the papillary muscles are formed by the same muscle tissue as the myocardium, but are anatomically separated from it, they are often used as a model object for studying the biophysical patterns of heart function.
Several morphofunctional types of cardiomyocytes are distinguished in the composition of cardiac muscle tissue:
1. Contractile (typical, working) cardiomyocytes make up 99% of the myocardial mass. They provide the contractile function of the heart and contain a large number of ordered myofibrils and mitochondria, have a developed sarcoplasmic reticulum and a T-tubule system.
Rice. 2.
Longitudinal arrangement and transverse striation of cardiomyocyte myofibrils
Cardiomyocyte myofibrils, like skeletal muscles, are characterized by a pattern of longitudinal arrangement and transverse striations, visible under a microscope using polarized light (Fig. 2).
Under these conditions, there are light isotropic (I), or homogeneous, stripes, dark anisotropic (A), or inhomogeneous, stripes and Z-bands located transversely to them (German zwischenscheibe
- dividing). The classic unit of longitudinal division of each myofibril of cardiomyocytes, as in skeletal muscle, is the sarcomere, which contains two halves of the I-band and one A-band. The boundaries of the sarcomere are Z-bands. Thus, in cardiomyocytes, as in skeletal muscles, the sarcomere is the functional unit of the contractile apparatus. Since the sarcomeres in the myofibril are arranged sequentially, contraction of the sarcomeres causes contraction of the myofibril and its overall shortening.
Myofibrils, consisting of protein filaments - myofilaments - are located in the sarcomere parallel to each other with high order and are surrounded by membranes of the sarcoplasmic reticulum cisterns, as well as mitochondria. There are two types of myofilaments: thick, formed by the protein myosin, and thin, formed by another protein - actin (Fig. 2-1).
The myosin molecule consists of a long tail, a narrowed neck and a thickened head. Each thick filament contains more than 100 myosin molecules, assembled into a bundle, in the middle part of which there are tail particles of the molecules, and at both ends there are heads protruding above the surface of the filament. Each thin filament consists of two linear actin molecules helically twisted together. The grooves between the actin filaments contain linear molecules of the protein tropomyosin (two pairs of molecules per step of the actin filament helix). Near the connections between two consecutive tropomyosin molecules, globular molecules of another protein are attached to actin - troponin, consisting of three subunits: I, T and C. It takes part in the processes of coupling excitation and contraction of the working myocardium.
Rice. 2-1.
The work of the actomyosin complex:
A
— the thin filament consists of three proteins.
Its basis is actin. In the relaxed state, the myosin-sensitive site of the actin molecule is blocked by tropomyosin. When calcium binds to troponin, the latter undergoes a conformational rearrangement, as a result of which the interaction between actin and myosin becomes possible; b
— attachment of the myosin head to actin;
c
- sliding of thin and thick filaments relative to each other.
As a result of hydrolysis of the ATP molecule, ADP and inorganic phosphate Pi are formed; d
- attachment of a new ATP molecule to the myosin head
2. Conducting (atypical, specialized) cardiomyocytes have a poorly developed contractile apparatus and form the conduction system of the heart. Among this type of cardiomyocytes, P cells and Purkinje cells are distinguished:
a) round P- cells
- pale) with light cytoplasm, almost devoid of contractile elements, have the ability to periodically generate electrical impulses, ensuring (normally) the automation of the heart muscle;
b) Purkinje cells have an extended shape with a large diameter and form fibers, carrying out fast, undamped, timely and synchronous conduction of excitation to contractile cardiomyocytes. Automaticity is present in Purkinje cells, but it is less pronounced than in P cells.
Transitional cardiomyocytes, or T-cells
- transitional), located between conductive and contractile cardiomyocytes and have intermediate cytological characteristics. These cells ensure the interaction of other types of cardiomyocytes.
4. Secretory cardiomyocytes are located mainly in the atria and perform an endocrine function. In particular, these cells secrete into the internal environment atrial natriuretic peptide, a hormone involved in the regulation of water-electrolyte balance and blood pressure.
Morphologically, cardiac muscle tissue, unlike skeletal muscle tissue, does not have a symplastic structure, however, individual cardiomyocytes are both structurally and functionally closely connected to each other through intercalary discs, especially well expressed between contractile cardiomyocytes. Mechanical connection is provided by desmosomes and interdigitation located in the area of the intercalary disc, and functional interaction is provided by gap junctions
), or nexuses. In the gap junction zone, which occupies about 10–20% of the area of the intercalary disk, the membranes of neighboring cells are at a very small (about 2–3 nm) distance from each other and are penetrated by channels, which are complex protein complexes (connexons) and are permeable to ions. This structure of intercellular contacts ensures their low electrical resistance and free transmission of an electrical signal from one cell to another (like an electrical synapse). Intercalary discs located at the ends of the cells connect cardiomyocytes end to end, which leads to the formation of muscle fibers, which are also connected to each other through intercalary discs.
Thus, cardiomyocytes are united into a continuous electrical network - a functional syncytium, which distinguishes the myocardium from skeletal muscles. Due to these structural features of the myocardium, the excitation that arises in one cardiomyocyte is transmitted at high speed to other cells and quickly covers the entire myocardium. However, with damaging effects on the heart, for example, under conditions of hypothermia, the permeability of channels in the area of gap junctions sharply decreases, which leads to disturbances in the conduction of excitation in the myocardium. It is also important to note that most of the muscle fibers of the atria and ventricles are attached to fibrous tissue that separates the chambers of the heart and electrically insulates them from each other. As a result, separate sequential contraction of the atria and ventricles is possible.
All myocardial cells are highly differentiated and do not have the ability to divide, therefore, in the postembryonic period of human life, cardiac muscle tissue is not capable of regeneration and the processes of working myocardial hypertrophy develop due to an increase in the size and volume of individual cardiomyocytes, and not their total number (hyperplasia). In the case of necrosis of an area of the myocardium (infarction), for example, with coronary heart disease, the damaged area is replaced by connective tissue, which leads to the formation of a scar. Therefore, the use of stem cells is promising in the treatment of myocardial infarction. These cells, when introduced directly into the myocardium under the influence of cellular growth factors, can transform into cardiomyocytes and thus replenish the lost contractile function of the myocardial area. However, the widespread use of cell technologies in clinical practice requires expensive high-tech equipment and additional clinical studies.