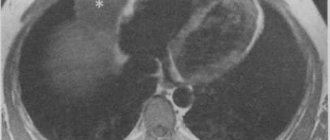
Puncture is the collection of biomaterial by puncturing an organ with a hollow needle. In cardiac surgery, this method is used to puncture the pericardium, a dense membrane around the heart filled with fluid.
The procedure is prescribed for therapeutic purposes and for diagnostics. This is a complex technique that is used only when absolutely necessary.
It gives doctors complete information about the condition of the heart and pericardial region, sometimes even saving the patient’s life. It is better to learn about the specifics of preparation and possible complications in advance.
Description of the procedure
The human heart is placed in a kind of bag, which is called the pericardium. This dense pouch is formed from two layers - inner and outer. The small space between the walls of the heart muscle and the pericardium is filled with fluid. In this part of the body there may be inflammation, purulent processes, and mechanical injuries.
Content:
- Description of the procedure
- Patient preparation
- Features of the event
- Possible complications
- Recovery after puncture
- Important questions
A puncture of the pericardium (pericardiocentesis) is done in order to take part of the fluid for analysis. In some cases, for example, with purulent formations or an increase in the amount of fluid, puncture becomes a therapeutic measure.
The technique is quite complex; the doctor must accurately determine the desired area of the heart and find points for inserting the needle.
To do this, the procedure is carried out under ultrasound or radiography monitoring. The procedure is reminiscent of a mini-operation: the patient is first examined, then anesthetized, and after the procedure is left for observation in a hospital.
But such an operation is much easier to tolerate than a strip opening of the chest. A puncture is prescribed in cases of accumulation of blood, air or fluid in the pericardial cavity, heart injuries, pericarditis (for diagnostic purposes).
As a rule, such manipulation is carried out in case of cardiac tamponade - compression of the organ with liquid or air. If there are such indications, during pericardiocentesis the excess is pumped out, which improves the patient’s well-being, and in emergency cases, saves life. In case of pericarditis, material sampling is needed to determine the cause of the accumulation of exudate or blood.
Larrey pericardiocentesis technique
The doctor puts on a sterile gown, mask and gloves. After treating the skin of the chest and upper abdomen with an antiseptic solution, limit the puncture site with sterile material or use a sterile film. The injection site is numbed with a thin needle. To do this, 2-5 ml of a 1% lidocaine solution is injected into the point located between the left costal arch and the xiphoid process. Attach a ten-millimeter syringe with 1% lidocaine to a long needle measuring 16-18 G or to a long intravenous “catheter on a needle”.
In the first variant of needle insertion, the needle is injected at an angle of 30° to the skin in the frontal plane and directed forward along the axis of the body. The needle should go flush with the costal edge. When using the second option, the needles are directed to the patient's left shoulder. The needle is positioned at a shallower angle relative to the heart. This makes it easier to pass the guidewire and catheter.
After the end of the fluid has reached the edge of the costal arch, 0.5-1.0 ml of lidocaine is carefully injected. Then the needle is advanced 4-5 mm forward. In this case, the syringe plunger is constantly pulled towards itself. Then the cycle is repeated. With this approach in adults, the average distance from the skin to the pericardium is 6-8 cm.
The needle can be inserted under electrocardiogram control. The doctor attaches the chest lead wire of the electrocardiograph or cardioscope to the needle using a clamp. Then turns on the “chest lead” mode on the recording device or connects the distal electrode to the wire of the right hand, and the proximal electrode to the wire of the left hand. The nurse turns on the first lead on the cardioscope or cardiograph. If the needle is in the pericardial cavity, a negative ST wave will appear on the ECG.
The needle can be inserted under ultrasound cardiography control. In this case, it is easier for the doctor to choose the optimal access point. An apical or left parasternal approach is used. Measure the distance to the effusion and mark the direction of the central ultrasound beam. The direction of the needle should be the same.
After receiving the liquid, disconnect the syringe with the remaining lidocaine and make sure that it is not blood that is coming from the needle, but pericardial fluid. Collect 10-15 ml of pericardial effusion into a clean 10-15 ml tube for testing. If the patient's condition is severe due to cardiac tamponade, 50-100 ml of effusion is aspirated through a needle before installing the catheter. A catheter is installed. If the catheter is on the needle, remove the needle. If not, use the standard Seldinger catheterization technique.
After making sure that the liquid is freely aspirated, secure the catheter with a nylon suture or adhesive tape and connect the extension to the drainage container. To avoid acute dilatation of the right ventricle as a result of sudden decompression, fluid from the pericardium is removed in parts, no more than one liter at a time.
Patient preparation
The puncture is performed in emergency situations or planned. Depending on the situation, the patient is prescribed several types of examination.
In urgent cases, the cardiologist decides what tests are needed before intervention. For a planned procedure the following is prescribed:
- echocardiogram;
- chest x-ray;
- blood tests (required for clotting);
- electrocardiogram.
Patients undergoing systematic treatment should inform their doctor about all medications they are taking. 4-6 hours before the procedure, you are prohibited from eating or drinking. Be sure to tell the doctor about all medications that were taken during the last 24 hours.
Principles for diagnosing acute pericarditis
P
Ericarditis is a manifestation or complication of many diseases, including infectious diseases, pneumonia, coronary heart disease (CHD) and non-coronary myocardial diseases, systemic connective tissue diseases, tumors and allergic processes. In some cases, pericarditis may be the main manifestation of the disease itself.
Features of the diagnosis of pericarditis
The development of instrumental diagnostic methods has significantly increased the possibilities of diagnosing pericarditis. Some techniques for physically identifying signs of the disease, which at one time helped to recognize pericarditis, have lost their meaning. Echocardiography (ECHOCG) began to play a particularly important role in verifying changes in the pericardium. Nevertheless, the possibilities of instrumental examination should in no way supplant the classical methods of diagnosing pericarditis - they only usefully complement them, sometimes ahead of the clinical detection of the disease and shifting diagnostic judgments to the desired level. Thus, the unexpected detection of a layer of fluid in the pericardium during echocardiography raises the question of the nature of the effusion, the very presence of which was so difficult to establish just 20 years ago. At the same time, incorrect interpretation of instrumental data not only makes it difficult to establish an etiological diagnosis, but also generates a significant number of errors that negatively affect the course of the disease and the choice of treatment tactics.
Another reason that allows us to pose the problem of diagnosing pericarditis in relation to the present stage of its tasks and opportunities is a change in the structure of heart diseases and the pericardium itself in recent decades, a decrease in the proportion of infectious (especially purulent) pericarditis and a progressive increase in the number of allergic, autoimmune, and oncological lesions of the heart. shirts.
The degree of interest of surgeons has seriously changed, on the one hand, in determining the indications for surgical treatment of patients with diseases of the heart and blood vessels and, on the other hand, in the timely detection of pericarditis after cardiac surgery. Expanding technical capabilities of surgery and increasing the number of cardiac surgical interventions require increasingly reliable and accurate characterization of pericardial diseases.
Primary diagnosis of dry pericarditis
Complaints from patients with dry pericarditis are usually associated with a feeling of dull, monotonous pain to the left of the sternum. Pain
with pericarditis, it has a more gradual onset, is monotonous, lasts for several hours, is not relieved by nitroglycerin, and is temporarily weakened by the use of non-narcotic analgesics. There may be complaints of palpitations, shortness of breath, dry cough, general malaise, chilling, which bring the picture of the disease closer to the symptoms of dry pleurisy. The dependence of pain on breathing, movements, and changes in body position is characteristic. The patient cannot take a deep breath, breathes shallowly and frequently.
Pericardial friction rub is of great diagnostic value.
, which in patients under medical supervision allows even painless forms of pericarditis to be diagnosed. At the height of pain, the friction murmur is gentle, limited in extent, and difficult to distinguish from a short systolic murmur. With an increase in fibrinous deposits on the pericardial layers, the noise becomes rough and is heard over the entire zone of absolute dullness of the heart. It can be two- or three-phase, as it occurs even during atrial systole and in the fast diastole phase. All components of such noise are similar in character and strength; it is compared to the rhythm of a steam locomotive. The friction murmur is always limited to the zone of absolute dullness of the heart or localized in some part of it. A distinctive feature of pericardial murmur is its poor conductivity; it “dies where it was born.”
When, during acute pericarditis, the subepicardial layers of the myocardium are involved in the inflammatory process, this is reflected in ECG changes
. An early sign of acute pericarditis is a concordant rise in the ST segment, covering all standard leads within 1-2 days (the greatest rise is noted in lead II). The ST segment smoothly turns into a high positive T wave. After 1-2 days, the ST interval drops below the isoelectric line, becomes convex upward, then within a few days returns to the isoelectric line, despite the ongoing inflammatory process in the pericardium. The T wave, which is positive and even slightly enlarged in the early stages of pericarditis, then flattens and after 10-15 days becomes negative or biphasic in those leads in which the ST segment dynamics occurred.
Depending on the etiology of dry pericarditis, in some cases a rapid positive dynamics of the process is noted, a friction noise is heard for only a few hours (epistenocardiac), in others the course of pericarditis becomes protracted or recurrent, in others there is a transformation into effusion pericarditis.
Exudative pericarditis
Exudative pericarditis means total involvement of the heart lining in the inflammatory process
. Liquid effusion can accumulate after the stage of dry pericarditis or, bypassing it, with rapidly beginning total pericarditis (allergic) and with primary chronic “cold” (tuberculosis, tumor).
With the slow accumulation of fluid, the volume of the pericardial sac gradually increases, the pericardial pockets are filled, the outer layer of the pericardium is stretched, and intrapericardial pressure sometimes does not increase even with large effusions (up to 2-3 l).
With large effusions, percussion determines the expansion of cardio-pericardial dullness in all directions. The boundaries of dullness change depending on the position of the patient’s body: when he stands up, the zone of dullness in the second and third intercostal spaces decreases by 2-4 cm on each side (shifts medially), and dullness in the lower intercostal spaces expands by the same amount. Therefore, having noted the boundaries of cardiac dullness when the patient is lying on his back, the study is repeated in a standing position. Absolute dullness in the lower parts comes close to the boundaries of relative dullness, and there is a sharp transition to tympanitis over the compressed lung.
Heart sounds, even when a large effusion accumulates in the pericardial sac, often remain clear and well audible, but only medially from the apex beat.
The radiologist may suspect the presence of fluid in the pericardium based on the increase in the size of the “heart” shadow. However, since an increase in the shadow of the heart can also occur as a result of its dilatation, simply establishing an increase in the “heart” shadow is not enough to resolve the issue of fluid accumulation in the pericardium. The difficulty lies in the fact that radiographically, behind the shadow of the fluid-filled pericardial sac, the shadow of the heart itself is not visible.
An early radiological sign of the accumulation of exudate in the cardiac membrane is not so much an increase in size as a change in the silhouette of the “heart” shadow
.
The triangular shape of the shadow occurs with long-term chronic pericardial effusions due to loss of elasticity of the outer layer of the pericardium. The spherical shape of the shadow speaks in favor of a fresher and increasing effusion. A characteristic sign of exudative pericarditis is a weakening of the pulsation of the shadow contour
. The aortic pulsation remains clear. In case of a recurrent course of the process with the formation of adhesions, radiographic examination may reveal jaggedness of the cardiac contours.
The possibilities for early diagnosis of acute pericarditis have increased with the widespread use of echocardiography. The layer of fluid anterior and posterior to the cardiac contour is confidently visualized as an echo-negative space. Often there is also compaction of the pericardial layers and heterogeneous shadows of fibrinous deposits, and with large effusions, characteristic vibrations of the heart inside the stretched pericardial sac, depending on the respiratory phases.
Echocardiographic overdiagnosis of pericardial effusion is observed in the case of left-sided pleural effusion, in persons with a giant left atrium with severe mitral stenosis, when a duplication of the left atrium is formed behind the left ventricle, with pronounced fatty deposits near the heart, and with the location of the lumen of large vessels.
Cardiac tamponade
With the rapid accumulation of effusion in the pericardial cavity, cardiac tamponade develops, tachycardia occurs, and pulse filling decreases.
There is no congestion in the lungs due to an obstruction to the blood supply to the right heart. The presence of congestive rales in the lungs contradicts the diagnosis of cardiac tamponade. The left heart becomes empty as you inhale, and the pulse volume decreases. This phenomenon is called paradoxical pulsus
. The paradoxical nature of the pulse is of decisive diagnostic importance.
Echocardiogram confirms cardiac tamponade
a decrease in the size of its cavities, overflow of the hepatic veins, and sometimes prolapse of the mitral valve leaflets (disappear after unloading puncture). Echocardiographic signs of cardiac tamponade also include bowing of the wall of the right ventricle and its diastolic collapse: the wall of the right ventricle is pressed against the interventricular septum in diastole. On inhalation, an increase in the size of the right ventricle and a decrease in the size of the left ventricle can be detected; on exhalation, the opposite phenomena occur - an increase in the size of the left ventricle and a decrease in the size of the right ventricle occur - an echocardiogram equivalent of paradoxical pulsus.
Doppler ultrasonography allows us to judge the increase in pressure in the right atrium and right ventricle and the filling pressure of the right ventricle (sometimes equal to the left ventricular filling pressure).
However, echocardiographic signs of tamponade are not as informative as clinical symptoms, especially with a negative conclusion. The higher the intrapericardial pressure, the higher the venous pressure, the peripheral and cervical veins swell. The liver enlarges and becomes painful on palpation, especially its left lobe. Since in certain positions the basin of the superior vena cava is partially unloaded, the patient with increasing cardiac tamponade takes a characteristic position in bed. Usually he sits, his torso is tilted forward, his forehead rests on a pillow (Breitman pose), or he freezes in a deep bow position. There are painful attacks of weakness with a small, barely perceptible pulse, and the patient experiences a feeling of fear of death. The skin is covered with cold sticky sweat, cyanosis increases, the extremities are cold, and consciousness is disturbed at times. There are vital indications for pericardial puncture. The faster tamponade develops, the more dangerous the delay; sometimes the count is not days, but hours or minutes.
Pericardial effusion and pericardial effusion
Echocardiography makes it possible to establish initial forms of pericarditis that were previously inaccessible for diagnosis. These small, usually spontaneously resolving effusions should in no way be identified with exudative pericarditis (as is sometimes described in the echocardiogram report): often it is a non-inflammatory effusion (hydropericardium) or an initial form of the catarrhal process. It has become obvious that dry pericarditis is not the initial form of pericarditis. Its development indicates the transition of the inflammatory process from catarrhal to “lobar” with the entry of fibrinogen into the exudate and the loss of fibrin while maintaining effective suction of liquid fractions through the lymphatic vessels.
Echocardiography reveals an increase in the amount of intrapericardial fluid up to 100 and even 500 ml. With a targeted examination of patients with acute myocardial infarction, effusion can be detected in 1/3 of cases during the first week of the disease - much more often than signs of dry epistenocardial pericarditis occur.
To the appearance of hydropericardium
may give general or local reasons. General diseases include diseases that disrupt the oncotic properties of the blood and the permeability of vascular membranes, heart failure, hydremic, cachetic, and arrowroot conditions. They, as a rule, lead to the accumulation of transudate also in other serous cavities and to anasarca. In severe forms of myxedema, effusion almost always forms in the pericardium. Usually it is small. The involvement of the pericardium in the process has been described in ankylosing spondylitis, systemic lupus erythematosus, Reiter's syndrome, and rheumatoid arthritis.
There is usually no pain in the area of the heart and no friction noise with hydropericardium, but sometimes a touch noise similar to a short light friction is heard.
Pericardial puncture
The final diagnostic and highly effective treatment measure in the clinic of effusion pericarditis remains puncture. It allows you to conduct a cytological examination, perform bacteriological, immunological and biochemical tests.
Based on the nature of the contents obtained , hydropericardium, cholesterol pericarditis, chylopericardium are established, and supuration of effusion is detected
(beginning of purulent inflammation).
Indications for pericardial puncture
: cardiac tamponade (vital indications, puncture is performed urgently); purulent nature of the process and prolonged resorption of exudate (therapeutic and diagnostic); effusion pericarditis, the nature of which needs clarification or verification (diagnostic).
Several ways of needle insertion (trocar or catheter with stylet) have been proposed. At present, only two have retained their significance: 1) in the angle between the cartilage of the VII rib and the xiphoid process to the left of it (according to Larrey) or downward from the xiphoid process (according to Marfan); 2) 2-3 cm medially from the left border of absolute dullness in the fifth or sixth intercostal space (according to Kurshman), if the apical impulse is clearly defined medial and above this point.
It is advisable to insert a string through the needle, and then a catheter along it. This allows you not only to completely drain the pericardial cavity and introduce oxygen into it, but also to leave the catheter in the cavity for 72 hours for subsequent manipulations (only for puncture using lower approaches!).
Punctures through the intercostal spaces are strictly contraindicated, no matter how they are performed, even under ultrasound control.
Etiological diagnosis of acute pericarditis
Although the detection of even initial forms of pericarditis has been significantly simplified due to the introduction of instrumental methods for examining patients, their etiological diagnosis remains complex, and in many cases, only speculative.
Nonspecific coccal pericarditis
confidently diagnose purulent effusion, based on the predominance of neutrophils in the effusion, according to bacterial culture. In other cases, the diagnosis is made presumably on the basis of the development of pericarditis in connection with acute pneumonia or as a complication of sepsis, infective endocarditis, or mediastinitis.
Specific bacterial pericarditis
recognized by the general symptom complex of the disease, they are always difficult for etiological diagnosis in cases of isolated damage to the pericardium. Meanwhile, in some cases, of all the serous membranes, one pericardium is affected.
Tuberculous pericarditis
occurs more often in persons with a hyperergic tuberculosis process of another localization or in those who have had tuberculosis in the past. Pain in the heart area is rare. Low-grade fever, night sweats, and dry cough are observed. The course of the disease is long and torpid, intrapericardial effusion can be large without the development of tamponade. Sometimes the effusion persists stably for years, almost without being accompanied by fever and inflammatory changes in the blood (“cold” flow). The myocardium is not involved in the process, and ECG changes do not occur. They attach importance to high tuberculin tests. In later stages, radiologically it is possible to detect areas of calcification.
Pericardial tuberculosis is severe and often unfavorable
– one of the forms of organ tuberculosis: high temperature, leukocytosis, night sweats, rapid accumulation of exudate in the pericardial cavity, loss of body weight. The process, even with active treatment, often leads to constriction after 1.5-2 months and then requires urgent pericardiectomy.
Viral pericarditis
- a complication of a viral infection, although relatively recently the viral origin of a significant number of acute benign pericarditis, currently identified primarily as allergic and autoimmune, was assumed. The viral etiology of pericarditis is assumed when the disease begins with pharyngitis, rhinitis, herpes, focal or interstitial pneumonia, or herpangina, myalgia, pleurisy, serous meningitis (ECHO virus, Coxsackie virus). Coxsackie-III is the most cardiotropic. Myopericarditis caused by this strain is dangerous due to severe myocarditis; pericarditis with this infection always occurs in combination with myocarditis. Infectious mononucleosis, involving the pericardium, is recognized by enlargement of the lymph nodes, liver and spleen, polymorphic roseola rash, leukopenia, and mononuclear blood reaction are characteristic. The course is sometimes recurrent.
Suggest a rheumatic etiology of pericarditis
possible on the basis of concomitant myocarditis, polyarthritis and other clinical manifestations of rheumatism, prolongation of the PQ interval on the ECG, increased hyaluronidase activity of serum, increased content of g-globulins and immunoglobulins, high titer of antistreptolysin.
At a time of high incidence of rheumatism, signs of pericarditis usually appeared at 1-2 weeks of joint attack, and with relapses of polyarthritis - at 3-4 weeks. In the cardiac form of rheumatism, pericarditis develops from the first days of clinical manifestations of the disease. Involvement of the pericardium in rheumatic carditis indicates a high degree of activity of the process and gives grounds to diagnose its stage III (pancarditis). Dry pericarditis occurs in rheumatism three times more often than exudative pericarditis. Large pericardial effusions are an exception; cardiac tamponade almost never develops in adults. The increase in the boundaries of dullness is due not only to the accumulation of effusion, but also to dilation of the heart itself. Effusive pericarditis in rheumatism is an unfavorable prognostic sign, especially if the effusion becomes hemorrhagic.
Rheumatism is one of the common causes of intrapericardial adhesions. The rheumatic etiology of adhesive pericarditis is judged not only by anamnesis, but also by the presence of rheumatic heart disease in the patient.
Allergic pericarditis
characterized by an acute onset with sharp pain in the heart area and a tendency to relapse, occurring some time after exposure to a resolving factor (administration of serum or allergenic medication). They usually occur in the form of myopericarditis with the formation of serofibrinous effusion, skin rashes and other manifestations of a drug-induced disease or allergic condition.
Autoaggressive (alterogenic) pericarditis
associated with various damage to the cardiac membrane: post-infarction, post-commissurotomy, post-pericardotomy.
The most common post-infarction syndrome ( Dressler syndrome
) with clear, sometimes violent manifestations occurs in the 3rd week of acute myocardial infarction, when the highest titer of circulating antibodies to myocardial antigens is detected. Post-infarction syndrome can first form in a wide time range - from 10 days to 2 years after a heart attack, depending on the nature of the course of coronary artery disease and a number of associated factors, and with a re-infarction it often occurs from the first days of acute coronary syndrome.
Postcommissurotomy, postpericardotomy, and posttraumatic syndromes have similar pathogenetic mechanisms and a similar clinical course. All of these variants of autoimmune pericarditis can occur with simultaneous pleurisy and focal pneumonia, with high fever for several days. In case of relapses of alterogenic syndrome, patients may lack its central clinical sign – pericardial friction rub, if obliteration of the cardiac membrane has occurred. In these cases, the activation of the process is indicated by pain, ECG changes and other signs of relapse of post-infarction (or post-pericardotomy) syndrome, including processes of extra-cardiac localization - focal pneumonia, pleurisy, arthritis. Eosinophilia is detected in the blood; the effusion also contains many eosinophils and is sterile. Treatment with corticosteroid drugs is effective (diagnosis ex juvantibus).
Lupus pericarditis
develops more often in young women, occurs in the form of a dry, exudative (usually hemorrhagic) or adhesive process. As a rule, pleurisy and pneumonitis occur simultaneously. Sometimes percarditis occurs earlier than other manifestations of a systemic disease, begins acutely, and is characterized by a persistent relapsing course.
Uremic pericarditis
It can be dry, serofibrinous or hemorrhagic, has few symptoms, and is not accompanied by pain in the heart area. It is identified by the pericardial friction noise, which was considered the “death knell of brights.” Routine hemodialysis removes the gloomy prognostic significance of uremic pericarditis, but it has become a criterion for the urgency of hemodialysis.
Pericarditis may be caused by local radiation trauma.
, in particular, with gamma or radiotherapy on the mediastinal area in doses of 25-40 Gy for tumor processes. The difficult task of differential diagnosis of tumor (relapse) and post-radiation pericarditis arises. The latter is often delayed for 1-5 years after irradiation and takes the form of dry recurrent exudative or constrictive pericarditis.
Pericarditis due to tumors
usually hemorrhagic, but in 50% of cases, at the first puncture, an effusion not stained with blood is detected; it becomes hemorrhagic later. A large amount of effusion accumulates in the cavity. In the exudate of cancerous pericarditis, up to 90% of leukocytes are often lymphocytes, and conglomerates of tumor cells are found. Cytological examination is highly informative.
Features of the event
Manipulations are carried out in several ways; such techniques are named after their inventors. The Larrey and Marfan methods are most often used; they differ only in the needle insertion points. For the patient himself, it practically does not matter what technique will be used. Whatever type of pericardiocentesis is prescribed, the operation will be almost the same for the patient.
The patient changes into sterile hospital clothing or exposes the upper body. Takes a semi-sitting position on the treatment table, sometimes a pillow is placed under the back. Sedatives are injected into the vein, all other manipulations are carried out after 20 minutes. The chest is treated with antiseptic agents.
After determining the insertion point, the doctor processes the instrument. The puncture needle is thin and will deliver an anesthetic for local anesthesia. First, the anesthetic is injected to numb the skin, then a little deeper, all the way to the pericardium.
The process will be carried out under the control of fluoroscopy or echocardiography. The doctor will slowly insert the needle, take samples, and withdraw the instrument. To remove air or liquids, a catheter is inserted into the needle cavity, through which the excess is drained out.
In case of pericarditis, the cavity can be rinsed and an antibiotic and oxygen injected into it. At the end of the operation, the puncture site is treated with an antiseptic and sometimes sealed with Xeol.
The patient then remains under medical supervision for at least 2 hours. If drainage has been installed, the hospital stay is extended for several days. The biomaterials are sent for analysis, and another examination is carried out: a chest x-ray (to ensure the integrity of the organs), a pulse and blood pressure check.
Methods for performing pericardial puncture
Pericardial puncture is not performed in patients suffering from coagulopathy and receiving anticoagulant treatment. A relative contraindication to the procedure is a limited-volume effusion and a platelet count in the blood of less than 50×109/L. Pericardial puncture is not performed for dissecting aortic aneurysm, post-infarction myocardial ruptures, or traumatic hemopericardium. In all these cases, leading cardiac surgeons perform surgery at partner clinics.
Before pericardial puncture, chest X-ray and echocardiography are performed. If the puncture is carried out under the control of electrocardiography or blindly, doctors make sure that at least 2 cm of effusion has accumulated between the layers of the pericardium. The purpose of the procedure is explained to the patient and his consent is obtained.
In order for the pericardial effusion to move to the anterior inferior pericardial sinus, the patient is placed in a semi-sitting position. Peripheral venous access is established, electrocardiogram, blood pressure, pulse and oxygen saturation are monitored (measurement of the amount of oxygen bound to hemoglobin cells in the circulatory system). In case complications develop, everything necessary for resuscitation measures is prepared, including a defibrillator.
Pericardiocentesis kit includes:
- introducer needles;
- expander;
- conductor;
- bent radiopaque catheter;
- multi-purpose tube adapter.
The point for puncture of the pericardium when using the Larrey technique corresponds to the apex of the angle between the left costal arch and the base of the xiphoid process on the left. When performing a Marfan puncture, the doctor punctures at a special point located under the xiphoid process.
Possible complications
During such manipulations there may be complications. The heart or lungs may be damaged by the puncture instrument, this is considered the most serious consequence of the procedure.
This happens when the patient or the doctor himself makes sudden movements during manipulations; insufficient examination also leads to such consequences. In this case, the patient receives emergency care immediately; especially difficult situations require urgent surgical intervention.
Also, when inserting a needle, infection is possible if the instruments or chest have not been treated with an antiseptic.
If the exudate is removed too quickly, the body experiences stress, and the heart does not have time to adapt to the changed pressure. This is fraught with disturbed heart rhythm. It is important to monitor your well-being after pericardiocentesis; you should contact a cardiologist if:
- chills and fever appeared;
- there are chest pains that do not go away;
- causeless cough, shortness of breath, difficulty breathing occurs;
- blood is released from the puncture site;
- severely dizzy, nauseous;
- swelling and redness appeared around the puncture area.
Excess weight, bad habits, and the patient's state of shock increase the risk of complications. If the patient does not warn the doctor about the medications he is taking, blood clotting may be reduced, leading to serious consequences.
Puncture of formations in the lungs under CT navigation
Puncture of formations in the chest is performed according to the following indications:
Lung biopsy
When a mass formation is detected in the lung or mediastinum, a biopsy is often required to clarify the diagnosis and develop a treatment plan.
Modern CT scanners are able to detect lesions smaller than 1 cm that are not visible with conventional radiography. The only possible way to perform a puncture of such a formation is a biopsy under the control of a computed tomograph.
First, a survey tomography is performed, based on the results of which the puncture route is planned. Since the chest contains a large number of vital organs and structures: heart, aorta, pulmonary arteries and veins, superior vena cava, etc.; When calculating the route, it is very important to avoid these structures and place the tip of the needle in the center of the formation.
Next, a biopsy needle is passed along the intended route. Control tomograms are periodically performed to correct the direction of the needle. When a formation is reached, a biopsy is performed.
The accuracy of needle positioning when using CT navigation is 1-2 mm.
Abscess drainage
Lung abscesses are one of the complications of various diseases: pneumonia, bronchiectasis, emphysema, thromboembolism, etc. Despite modern antibiotic therapy, in some cases patients are resistant to treatment. A chronic abscess is formed, delimited by a connective tissue capsule that prevents its spontaneous regression. The contents of the abscess lead to chronic intoxication of the body, dysfunction of the kidneys, heart, and other organs.
A radical method of treating such abscesses is to remove the affected part or the entire lobe of the lung.
Another, less invasive method of treatment is puncture of the abscess under CT navigation (the technique is described in the section “Lung biopsy”). After removing the contents of the abscess and washing its cavity with an antiseptic solution, a drainage tube can be installed.
Radiofrequency ablation of lung tumors
Surgical removal of a primary or metastatic lung tumor is the main radical treatment method. Surgical treatment is impossible in the presence of serious concomitant diseases, as well as in case of multiple bilateral lung lesions. You should also take into account the risk of developing respiratory disorders when performing extensive, especially repeated operations on the lungs.
To treat this category of patients, minimally invasive methods are currently successfully used: radiofrequency ablation, microwave and cryoablation.
An electrode or applicator under CT guidance (see “Lung Biopsy”) is installed in the tumor node and the tumor is exposed to high frequency current or microwave energy or extremely low temperature. The tumor tissue in the ablation zone becomes necrotic and is replaced by connective tissue.
As a result of the operation, minimal damage is caused to healthy lung tissue; repeated interventions are possible in the area of previous ablations.
Telephone for consultations : 8 (985) 146-06-96. Shelesko Andrey Anatolievich
Recovery after puncture
The patient remains in the hospital for observation for some time. After discharge, the doctor gives recommendations for a speedy recovery. For the first time after pericardiocentesis, it is strictly forbidden to lift weights, overexert yourself, or have sex. Smoking and alcohol are excluded if possible altogether, at least for 2 weeks.
When you are discharged, you should consult with your doctor about what medications you can take. A painkiller will be prescribed; you need to drink it in a clearly indicated dosage. At the first sign of complications, you should consult a doctor or call an ambulance.
For quick rehabilitation, a healthy diet is recommended; as a rule, diet No. 15 is prescribed. From time to time you need to go for leisurely walks and breathe fresh air. In winter, be sure to dress warmly; in summer, avoid overheating and sunbathing on the beach. Stressful situations are especially undesirable in case of heart disease and should be avoided as much as possible. For those who are especially sensitive, a cardiologist may recommend sedatives. You can return to an active lifestyle after your doctor approves it.