Share information with your Facebook friends
VK
Atypical cells are those cells that, under the influence of various unfavorable factors, have transformed from an ordinary normal cell of the human body into an uncharacteristic structure, changing their size and shape. The body, in turn, loses control over such cells, so there is a risk of degeneration of atypical cells, which can lead to the formation of cancer.
How is it transmitted?
EBV enters the body primarily through airborne droplets, but can be transmitted through blood through organ transplantation or plasma transfusion.
Contact-household transmission through various objects, unwashed hands, kissing and sexual contact, as well as from mother to child during childbirth, is also not excluded.
Anyone can become infected with infectious mononucleosis, regardless of place of residence and time of year. The high-risk group includes young people 14-18 years of age of both sexes.
For people over 40 years of age, the disease is not typical, since in the vast majority of cases, specific immunity is formed by about 32 years of age. The only exceptions are HIV-infected patients, in whom lymphoblastosis, which occurs in a latent form, can worsen.
In children, the clinical picture of EBV resembles acute respiratory infections. Babies under one year old rarely get sick due to their innate immunity.
The incubation period lasts 5-45 days. The release of the virus into the environment by an infected carrier lasts from 6 months to 1.5 years.
About 20% of people have specific antibodies to infectious mononucleosis and its traces on the mucous membranes of the oropharynx.
What causes the appearance of atypical lymphocytes?
When a person is sick and his body needs protection, the immune system must produce a sufficient number of lymphocytes so that together they can cope with the aggressor. Unfortunately, sometimes there are not enough immune resources for this. Cells are produced, but cannot fully mature and prepare to meet a pathogen or allergen. So it turns out that the number of such unusual lymphocytes in the blood increases. They perform the necessary functions, but their “appearance” is not very complete.
As soon as the patient gets better, the blood picture immediately, in just a few days, improves.
Sometimes high lymphocytes in the blood and the appearance of atypical forms are observed not only with ARVI and allergies, but also in more serious situations. Whooping cough, syphilis, tuberculosis, lymphocytic leukemia, brucellosis, toxoplasmosis, and serum sickness can lead to the appearance of large numbers of them.
Development mechanism
The causative agent of infectious mononucleosis in children is a DNA virus from the herpesvirus family. It differs in that it does not kill cells; on the contrary, it activates their growth by cloning in B lymphocytes.
EBV is inhaled and settles on the mucous membranes of the throat, causing local inflammation of moderate intensity. From here it penetrates into nearby lymph nodes and causes lymphadenitis.
Penetrating into the systemic bloodstream, EBV invades B-lymphocytes and begins to actively clone into them, triggering a cascade of immune reactions and pathological changes in cells.
Since the infection is active in immune cells, and this activity largely depends on the person’s immune status, the disease is classified as AIDS-associated.
The EBV virus remains in the body for life and can worsen with a general decrease in immune defense. The number of such exacerbations is not limited.
What to do if there are elevated levels of atypical lymphocytes in the blood?
There is only one option: to be treated by taking therapy prescribed by the doctor. This, depending on the nature of the disease, can be a variety of medications and techniques. In addition, in consultation with your doctor, you can take Transfer Factor.
The presence of atypical lymphocytes indicates that the human immune system is working under strain. This tool allows her to help. It is created on the basis of information molecules extracted from cow colostrum and egg yolks. Molecules “train” lymphocytes to function properly, which accelerates their formation, maturation and improves performance. Regular use of the drug helps to quickly restore health and return normal blood counts.
Classification
According to the nature of the course, infectious mononucleosis is of two types:
- Typical, with characteristic symptoms and the presence of mononuclear cells in the blood.
- Atypical, occurring in a latent or low-symptomatic form.
Taking into account the duration of EBV, it is classified into 5 types, including:
- acute – symptoms persist for up to 3 months;
- protracted – from 3 months to six months;
- recurrent - symptoms appear again a month or less after recovery;
- chronic – the disease lasts more than six months.
Based on the severity of the course, there are mild, moderate and severe forms of EBV, as well as complicated and uncomplicated.
In addition, against the background of infectious mononucleosis, chronic diseases can worsen, and therefore EBV is distinguished with and without exacerbation of chronic diseases.
Symptoms
During the incubation period, symptoms may be absent or limited to weakness, malaise, and mild catarrhal manifestations. In such cases, the clinical picture gradually becomes more vivid: weakness intensifies, body temperature rises to 37.5-38°, the nose is stuffy, the throat is sore and sore.
If the disease begins acutely, chills and fever occur, the person sweats profusely, experiences aches throughout the body, and has a headache and throat. The feverish state may last for several days or weeks.
After 6-7 days, the peak of the disease occurs with the development of all typical signs:
- lymph nodes enlarge;
- tonsils become inflamed;
- the liver and spleen become larger, increasing in size;
- symptoms of general intoxication increase - fever, fever, weakness;
- the tonsils become very red and covered with a loose coating of a yellowish tint.
Any group of lymph nodes can be affected, but the most vulnerable are the submandibular and occipital ones. They become denser, but have little or no pain and remain mobile.
On the part of the hepatobiliary system, not only an enlargement of the liver and spleen, but also darkening of the urine, yellowing of the eye membranes and skin, and indigestion may be observed.
A number of patients develop a rash of various localizations, in which there is no burning or itching.
Symptoms of Epstein-Barr mononucleosis in children
The acute phase of the disease lasts about 2-3 weeks, then gradually subsides and recovery begins. The temperature normalizes, the throat stops hurting, the liver and spleen return to their original sizes.
Sometimes a slight fever can last for several weeks, along with enlarged lymph nodes.
The duration of chronic recurrent mononucleosis increases to a year or more.
It is worth noting that in adulthood, patients more often complain of symptoms associated with liver dysfunction - digestive problems and jaundice. Signs of tonsillitis and inflammation of the lymph nodes are usually mild.
What are atypical lymphocytes and what do they look like:
As is known, when irritated by antigens, as well as during certain diseases (viral diseases, allergies), lymphocyte counts increase. Along with this, some of them change their properties and appearance.
Atypical lymphocytes acquire the following features
They get big. Typically, macrophages are the largest leukocytes, and lymphocytes rarely “grow” to more than 12 microns. When they become atypical, their size can be 30 microns or more.
A normal lymphocyte has a shape close to round. Changed cell shapes acquire an irregular, polygonal shape, and their borders may look “ragged.”
The nucleus of an ordinary cell is round or slightly elongated. In an atypical lymphocyte it may remain this way, but it is often elongated, with “eaten away” contours, constrictions or clefts. It happens to be reduced.
If a blood smear, in which lymphocyte indicators are studied, is stained with standard dyes (hematoxylin and eosin are used for this), then the atypical cells will acquire a brighter color than the “normal” ones. Their nucleus is purple, and the cytoplasm is gray, dark blue or light blue. If atypical lymphocytes appear in a patient infected with cytomegalovirus or Epstein-Barr virus, they are called Downey cells. The fact is that in 1923, an American hematologist with the same name was one of the first to see them under a microscope and describe their properties. Some lymphocytes with atypical properties are also called Reader cells, or amitotic lymphocytes. Their peculiarity is that they have a kidney-shaped shape and nuclei with jagged contours or a constriction in the middle, dividing them into lobules. Also among atypical lymphocytes, Botkin-Klein-Gumprecht cells are distinguished. Three scientists at once described specific cells that occur in lymphadenosis. Actually, these are not living cells, but their remains, which circulate in the blood for some time. Because they are barely visible, these lymphocytes are also called shadow cells.
Diagnostics
A preliminary diagnosis is made based on examination data. First of all, enlarged lymph nodes, liver and spleen are detected.
During the diagnostic examination, the following may be prescribed:
- blood tests (general, biochemistry);
- PCR tests;
- blood tests for antibodies;
- Ultrasound of lymph nodes, liver, spleen, abdominal organs;
- electrocardiogram, if there is a heart murmur and/or heart rhythm disturbance;
- X-ray of the paranasal sinuses with the development of sinusitis;
- X-ray or CT scan of the chest if pneumonia is suspected;
- spinal cord puncture in case of complications on the central nervous system;
- coagulogram if platelet levels are low. Thrombocytopenia is detected in the results of a general blood test.
Thus, the basis of diagnosis is the study of blood composition. General analysis for EBV shows a moderate increase in leukocytes with a dominance of lymphocytes and monocytes. The level of neutrophils is slightly reduced.
The leukocyte formula shifts to the left: new immature neutrophils circulate in the blood, which in healthy people are found only in the bone marrow; they do not exist in the bloodstream.
Specific large cells, called mononuclear cells, also appear in the systemic circulation. To diagnose mononucleosis, up to 10-12% of them are sufficient, although often the number of mononuclear cells reaches 80% or more of the total number of white blood components.
It is characteristic that in the first days of infection, mononuclear cells are not always detected, which, however, does not exclude the main diagnosis. These cells can be formed over 2-3 weeks and remain in the blood even after recovery.
PCR tests have recently been practically not used due to the irrationality and labor-intensive nature of the study.
As for antibodies, immunoglobulins to EBV can be detected almost immediately after infection. At the peak of the disease, absolutely all patients have them and disappear only after complete recovery.
It is these antibodies that are the key diagnostic criterion for infectious mononucleosis. In people who have had this disease, type G globulins remain in the bloodstream for life.
Infectious mononucleosis of the oropharynx
Mandatory HIV testing
All patients with diagnosed or suspected mononucleosis are tested for HIV infection three times, since it is also characterized by the circulation of atypical mononuclear cells in the blood.
The first test is taken in the acute period of the disease, the second - after 3 months, the third - after another 3 months.
Epstein-Barr viral infection in children: modern approaches to diagnosis and treatment
Epstein-Barr virus infection (EBVI) is one of the most common human infectious diseases. Antibodies (Abs) to the Epstein-Barr virus (EBV) are detected in 60% of children in the first two years of life and in 80–100% of adults [3, 13]. The incidence of acute form of EBVI (EBVI) in different regions of the world ranges from 40 to 80 cases per 100 thousand population [2]. The chronic form of EBVI (CEBVI) develops in 15–25% of individuals after EBVI [1, 5, 15]. The role of EBV in the development of malignant neoplasms, autoimmune diseases and chronic fatigue syndrome has been established [3, 5, 14, 15]. All this indicates the relevance of the problem of EBVI.
EBV, discovered in 1964 by M. Epstein and Y. Barr, belongs to the γ-herpes viruses [3]. EBV has 3 antigens: capsid (VCA), early (EA) and nuclear (EBNA). The uniqueness of the pathological process in EBVI is determined by the ability of EBV to transform B-lymphocytes, lifelong persistence in the human body, induction of a secondary immunodeficiency state (IDS), autoimmune reactions, and malignant tumors [1, 3, 5, 12].
The source of EBV infection is patients with manifest and asymptomatic forms. 70–90% of people who have had EEBVI shed the virus in the next 1–18 months. Routes of transmission of EBV: airborne, household contact, parenteral, sexual, vertical. OEBVI is characterized by epidemic rises once every 6–7 years, and is more often registered at the age of 1 to 5 years, in organized groups [4, 7, 9].
The entrance gate for EBV is the mucous membrane of the upper respiratory tract: the virus penetrates the lymphoid tissue, infects B-lymphocytes, polyclonal activation of B-lymphocytes develops, dissemination of the pathogen within B-lymphocytes, the synthesis of antibodies (Ab) in response to antigenic stimulation is reduced. EBV primarily affects lymphoid organs (tonsils, liver, spleen).
The next stage is the formation of a clone of sensitized cytotoxic CD8 cells, the sequential synthesis of Abs to the VCA, EA and EBNA antigens of the virus. Due to disruption of the immune response and functional activity of innate resistance factors (neutrophils, macrophages, NK cells, interferon system), secondary IDS is formed [2–4, 12].
The immune status of 109 patients with OEBVI aged 5 to 14 years in our work revealed signs of activation of the T-cell component of the immune system - an increase in the number of T-lymphocytes (CD3), cytotoxic T-lymphocytes (CD8), cells with markers of late activation (HLA- DR); polyclonal activation of B lymphocytes - an increase in the number of CD20 cells, immunoglobulins (Ig) IgA, IgM, IgG, circulating immune complexes (CIC). Signs of suppression of the immune system were found: normal levels of T-helper cells (CD4), a decrease in the immunoregulatory index CD4/CD8, the number of natural killer NK cells (CD16), and an increase in the readiness of immunocompetent cells for apoptosis (CD95). Activation of oxygen-dependent metabolism of neutrophils and a reduction in its adaptive capabilities were observed.
In a third of the examined children (33.9%), EEBVI occurred in the form of a mixed infection with cytomegalovirus (CMV), herpes simplex viruses types 1 and 2 (HSV-1, HSV-2). During bacteriological examination of oropharyngeal smears, Streptococcus (S.) viridans was isolated in 41.3% of patients, Candida albicans in 11.9%, Staphilococcus (Staph.) epidermidis in 8.2%, S. pyogenes, in 2.7% - Klebsiella (Kl.) pneumoniae, in 41.3% - an association of bacteria. 43.1% of patients had serological markers of an active form of chlamydial infection, and 30.3% had mycoplasmosis.
The following outcomes of EBVI are possible: latent infection, EBVI, IDS, cancer, autoimmune diseases, chronic fatigue syndrome [5, 8, 10, 11]. The transition to CHEBVI is associated with a complex of unfavorable factors in the ante-, intra- and postnatal periods, disruption of neuroimmune-endocrine regulation, and genetic predisposition.
Our examination of 60 children aged 5 to 14 years with CEBVI showed that in this group 86.7% of mothers had a burdened obstetric history; In 83.3% of children, perinatal and postnatal pathologies of the central nervous system, ENT organs, etc. were detected.
The immune status of patients with CHEBVI revealed an increase in the content of interleukin-1 antagonist (IL-1RA), insufficient activation of immunocompetent cells (decrease in HLA-DR) and an increase in their readiness for apoptosis (increase in CD95). There was a disturbance in the functional activity of type 1 T helper cells (Th1) (decreased levels of interferon γ (IFN γ)); decrease in the total pool of T cells (CD3), the number of lymphocytes with receptors for IL-2 (CD25) and NK cells (CD16); the content of cytotoxic CD8 lymphocytes was increased. The persistence of EBV replication markers for a long time in this group indicated a violation of virus elimination; At the same time, an increase in the functional activity of Th2, polyclonal activation of B-lymphocytes (CD20), an increase in the content of IgA, IgM, IgG, CEC, a decrease in the level of neutrophil chemotactic factor (IL-8), and a change in their metabolism were noted.
Immune status disorders led to the activation of opportunistic microflora, viral and fungal infections. In the microbial spectrum of the oropharyngeal mucosa of patients with CHEBVI, S. Viridans (30%), Candida albicans (28.3%), Staph. Epidermidis (25%), S. Pyogenes (20%), Kl. Pneumoniae (8.4%), bacterial association (41.7%); 28.3% had serological markers of the active form of chlamydia, 26.7% had mycoplasmosis. In 90% of patients, the disease occurred in the form of a mixed infection involving herpes viruses: EBV + CMV, EBV + HSV-1, HSV-2.
Classification . There is no generally accepted classification of the disease; We recommend using the working classification of EBVI that we have developed.
- By period of occurrence: congenital, acquired.
- Form: typical (infectious mononucleosis), atypical: erased, asymptomatic, visceral.
- By severity: light, medium, heavy.
- According to the course: acute, protracted, chronic.
- By phase: active, inactive.
- Complications: hepatitis, splenic rupture, meningoencephalitis, polyradiculoneuropathy, myocarditis, sinusitis, otitis, hemolytic anemia, thrombocytopenia, neutropenia, pancreatitis, etc.
- Mixed infection.
Examples of diagnosis:
- Main: Acquired EBVI, typical severe form (infectious mononucleosis), acute course, active phase. Osl.: Acute hepatitis.
- Main: Acquired EBVI, visceral form (meningoencephalitis, hepatitis, nephritis), severe chronic course, active phase. Osl.: acute hepatic-renal failure. Associated with: Respiratory chlamydia (rhinopharyngitis, bronchitis, pneumonia).
The clinical picture of acute EBVI was first described by N. F. Filatov (1885) and E. Pfeiffer (1889). The incubation period lasts from 4 days to 7 weeks. A complete symptom complex is formed by 4–10 days of illness [4, 7].
We examined 109 children with OEBVI. In most patients, the disease begins acutely, with an increase in body temperature and the appearance of symptoms of intoxication; less often, a gradual onset is observed: for several days there is malaise, weakness, lethargy, and loss of appetite. Body temperature is subfebrile or normal. By days 2–4 of illness, the temperature reaches 39–40 °C; fever and symptoms of intoxication may persist for 2–3 weeks or more.
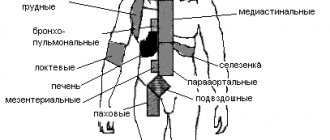
Generalized lymphadenopathy is a pathognomonic symptom of EBVI and from the first days of the disease manifests itself in the form of systemic damage to 5–6 groups of lymph nodes (LNs), with a predominant increase of up to 1–3 cm in diameter of the anterior and posterior cervical, submandibular LNs. LNs are slightly painful on palpation, are not fused to each other and the surrounding tissues, and are arranged in the form of a “chain” or “package”; visible when turning the head, giving the neck a “scalloped” outline. Sometimes there is a pastiness of the soft tissues over the enlarged lymph nodes.
Tonsillitis is the most common and early symptom of OEBVI, accompanied by enlargement of the tonsils to degree II-III. The lacunar pattern is emphasized due to infiltration of tonsil tissue or smoothed due to lymphostasis. On the tonsils there are plaques of yellowish-white or dirty gray color in the form of islands and stripes. They come from gaps, have a rough surface (reminiscent of lace), are easily removed without bleeding, rubbed, and do not sink in water. There is a discrepancy between the size of plaque and the degree of enlargement of regional lymph nodes. With the fibrinous-necrotic nature of the plaques, if they spread beyond the tonsils, a differential diagnosis with diphtheria is necessary. Plaques on the tonsils usually disappear after 5–10 days.
Signs of adenoiditis are found in the vast majority of patients. There is nasal congestion, difficulty in nasal breathing, snoring with an open mouth, especially during sleep. The patient’s face takes on an “adenoid” appearance: puffiness, pastiness of the eyelids, bridge of the nose, breathing through an open mouth, dry lips.
Hepatomegaly can be detected from the first days of the disease, but is more often detected in the second week. Normalization of liver size occurs within six months. In 15–20% of patients, hepatitis develops as a complication.
Splenomegaly is a late symptom and occurs in most patients. Normalization of the size of the spleen occurs within 1–3 weeks.
Exanthema with OEBVI appears on the 3rd–14th days of the disease, has a polymorphic character - spotted, papular, maculopapular, roseolous, punctate, hemorrhagic. There is no specific localization. The rash lasts for 4–10 days, sometimes leaving pigmentation. In children treated with ampicillin or amoxicillin, the rash appears more often (90–100%).
Hematological changes include leukocytosis (10–30 x 109/l), neutropenia with a band shift to the left, an increase in the number of lymphocytes, monocytes, and atypical mononuclear cells up to 50–80%, an increase in ESR up to 20–30 mm/hour. A characteristic hematological sign is atypical mononuclear cells in an amount of 10–50%: they appear by the end of the first week of the disease and persist for 1–3 weeks.
Chronic EBVI is the outcome of EBVI or develops as a primary chronic form [2, 5, 8, 10, 11, 15]. We examined 60 children with CHEBVI, the clinical picture of which included chronic mononucleosis-like syndrome and multiple organ pathology. All patients were found to have lymphoproliferative syndrome (generalized lymphadenopathy, hypertrophy of the palatine and pharyngeal tonsils, enlarged liver and spleen) and signs of chronic intoxication (prolonged low-grade fever, weakness, loss of appetite, etc.). Due to the development of IDS, acute infections of the respiratory tract and ENT organs were observed with exacerbations up to 6–11 times a year: nasopharyngitis (28.3%), pharyngotonsillitis (91.7%), adenoiditis (56.7%), otitis (11. 7%), sinusitis (20%), laryngotracheitis (18.3%), bronchitis (38.3%), pneumonia (25%). Noteworthy was the high frequency of multiple organ pathologies caused by long-term replication of EBV, secondary IDS, and autoimmune reactions (CNS pathology; chronic gastritis, biliary dyskinesia; cardiac syndrome, arthralgia).
In recent years, congenital EBVI has been described. It has been established that its risk with primary EBVI during pregnancy is 67%, with reactivation - 22%. The clinical picture of congenital EBVI is similar to that of CMVI.
The role of EBV in the development of cancer and paraneoplastic processes has been established - Burkett's lymphoma, nasopharyngeal carcinoma, lymphogranulomatosis, tumors of the stomach, intestines, salivary glands, uterus, leukoplakia of the tongue and oral mucosa, as well as a number of autoimmune diseases - systemic lupus erythematosus, rheumatoid arthritis, syndrome Sjögren, lymphoid interstitial pneumonitis, chronic hepatitis, uveitis, etc. [3, 5, 14, 15]. EBV, along with human herpes viruses types 6 and 7, is the etiological factor of chronic fatigue syndrome and the most common cause (15%) of the development of prolonged fever of unknown origin.
Diagnosis of EBVI is based on taking into account risk groups, leading clinical syndromes and laboratory data [8–11]. Risk groups in the mother include a burdened medical history, markers of herpes viral infections, etc., in the child - perinatal damage to the central nervous system, allergic phenotype, IDS, markers of herpes viral infections, etc. The leading clinical syndromes of EBVI are mononucleosis-like, general infectious syndromes, exanthema, syndrome of multiple organ pathology.
The mandatory standard for diagnosing EBVI includes a clinical blood test, a general urine test, a biochemical blood test, a bacteriological examination of the mucus of the oropharynx and nose, serological markers of EBV, other herpes viruses, chlamydia, mycoplasmas, ultrasound of the abdominal organs, consultation with an ENT doctor, if indicated. - radiography of the paranasal sinuses, chest organs, ECG. Additional diagnostic standard (in a specialized treatment and prophylactic institution): markers of EBV, other herpes viruses, chlamydia, mycoplasmas using polymerase chain reaction (PCR), second-level immunogram, consultation with an immunologist, if indicated - coagulogram, morphological picture of sternal puncture, consultation with a hematologist , oncologist.
The method of enzyme-linked immunosorbent assay (ELISA) is used to determine Abs to EBV antigens, which allows for laboratory diagnosis of EBV and determining the period of the infectious process.
IgM class antibodies to VCA appear simultaneously with the clinical manifestations of EBV, persist for 2–3 months, and are re-synthesized during EBV reactivation. Long-term persistence of high titers of these Abs is characteristic of CHEBVI, EBV-induced tumors, autoimmune diseases, and IDS.
IgG class antibodies to EA reach a high titer at 3–4 weeks of OEBVI and disappear after 2–6 months. They appear during reactivation and are absent in the atypical form of EBVI. High titers of Abs of this class are detected in cases of CHEBVI, EBV-induced oncological and autoimmune diseases, and IDS.
IgG antibodies to EBNA appear 1–6 months after the primary infection. Then their titer decreases and persists throughout life. When EBVI is reactivated, their titer increases again.
The study of IgG class Ab avidity (the strength of antigen binding to Ab) is of great importance. During primary infection, Abs with low avidity (avidity index (AI) less than 30%) are first synthesized. The late stage of primary infection is characterized by Abs with medium avidity (IA - 30–49%). High-avidity Abs (IA - more than 50%) are formed 1–7 months after EBV infection.
Serological markers of the active phase of EBVI are IgM Abs to VCA and IgG Abs to EA, low and medium avidity IgG Abs to markers of the inactive phase, IgG Abs to EBNA.
The material for PCR is blood, cerebrospinal fluid, saliva, smears from the oropharyngeal mucosa, organ biopsies, etc. The sensitivity of PCR for EBVI (70–75%) is lower than for other herpesvirus infections (95–100%). This is due to the appearance of EBV in biological fluids only during immune-mediated lysis of infected B lymphocytes.
Treatment. The principles of treatment for EBVI are complex, the use of etiotropic drugs, continuity, duration and continuity of treatment measures at the stages “hospital → clinic → rehabilitation center”, monitoring of clinical and laboratory parameters.
Based on the experience of treating 169 children with EBVI, we have developed a standard of treatment for this disease.
Basic therapy: protective regime; therapeutic nutrition; antiviral drugs: virocidal drugs - inosine pranobex (Isoprinosine), abnormal nucleosides (Valtrex, Acyclovir), Arbidol; IFN preparations - recombinant IFN α-2β (Viferon), Kipferon, Reaferon-ES-Lipint, interferons for intramuscular administration (Reaferon-EC, Realdiron, Intron A, Roferon A, etc.); IFN inducers - Amiksin, ultra-low doses of antibodies to γ-IFN (Anaferon), Cycloferon, Neovir. According to indications: local antibacterial drugs (Bioparox, Lizobakt, Stopangin, etc.); systemic antibacterial drugs (cephalosporins, macrolides, carbapenems); immunoglobulins for intravenous administration (Immunovenin, Gabriglobin, Intraglobin, Pentaglobin, etc.); vitamin and mineral complexes - Multi-tabs, Vibovit, Sanasol, Kinder Biovital gel, etc.
Intensification of basic therapy according to indications:
Immunocorrective therapy under the control of an immunogram - immunomodulators (Polyoxidonium, Likopid, Ribomunil, IRS-19, Imudon, Derinat, etc.), cytokines (Roncoleukin, Leukinferon); probiotics (Bifiform, Acipol, etc.); metabolic rehabilitation drugs (Actovegin, Solcoseryl, Elcar, etc.); enterosorbents (Smecta, Filtrum, Enterosgel, Polyphepan, etc.); second generation antihistamines (Claritin, Zyrtec, Fenistil, etc.); hepatoprotectors (Hofitol, Galstena, etc.); glucocorticosteroids (prednisolone, dexamethasone); protease inhibitors (Kontrikal, Gordox); neuro- and angioprotectors (Encephabol, Gliatilin, Instenon, etc.); “cardiotropic” drugs (Riboxin, Cocarboxylase, Cytochrome C, etc.); homeopathic and antihomotoxic remedies (Ocillococcinum, Aflubin, Lymphomyosot, Tonzilla compositum, etc.); non-drug methods (laser therapy, magnetic therapy, acupuncture, massage, physical therapy, etc.)
Symptomatic therapy.
For fever - antipyretic drugs (paracetamol, ibuprofen, etc.); if there is difficulty in nasal breathing - nasal medications (Isofra, Polydexa, Nazivin, Vibrocil, Adrianol, etc.); for a dry cough - antitussive drugs (Glauvent, Libexin), for a wet cough - expectorants and mucolytic drugs (AmbroHEXAL, bromhexine, acetylcysteine, etc.).
| Rice. 1. Scheme of complex therapy for Epstein-Barr viral infection in children |
For several years, for the treatment of EBVI, we have successfully used a combination stepwise etiotropic therapy regimen, which includes inosine pranobex (Isoprinosine) and recombinant interferon α-2β (Viferon) (Fig. 1, 2). Inosine pranobex (Isoprinosine) suppresses the synthesis of viral proteins and inhibits the replication of a wide range of DNA and RNA viruses, including EBV [3]. The drug has immunocorrective activity - modulates the immune response according to the cellular type, stimulates the production of Abs, cytokines, IFN, increases the functional activity of macrophages, neutrophils and NK cells; protects affected cells from post-viral decrease in protein synthesis. Inosine pranobex (Isoprinosine) was prescribed at 50–100 mg/kg/day orally in 3–4 divided doses. Three courses of treatment were carried out for 10 days with an interval of 10 days. Recombinant IFN α-2β (Viferon) inhibits viral replication by activating endonuclease and destroying viral messenger RNA [6]. The drug modulates the immune response, promotes the differentiation of B-lymphocytes, stimulates the production of cytokines, and increases the functional activity of macrophages, neutrophils and NK cells. The natural antioxidants it contains (vitamins E and C) stabilize cell membranes. The drug was prescribed according to a prolonged regimen (V.V. Malinovskoy et al., 2006) [6].
The effectiveness of etiotropic therapy for OEBVI was assessed in two groups of patients. Patients of the 1st group (52 people) received inosine pranobex (Isoprinosine) in combination with recombinant IFN α-2β (Viferon), patients of the 2nd group (57 children) received monotherapy with recombinant IFN α-2β (Viferon). Clinical and serological parameters before the start of treatment and after 3 months of therapy are presented in Table. 1. Over time, patients in both groups showed a significant decrease in symptoms such as generalized lymphadenopathy, tonsillitis, adenoiditis, hepatomegaly and splenomegaly. However, against the background of combination therapy, the positive dynamics of clinical indicators were more significant; acute respiratory infections (ARI) only in 19.2% of patients in group 1 and in 40.3% of patients in group 2 (p < 0.05). During combination therapy, a more rapid disappearance of serological replication markers was observed.
| Rice. 2. Mechanisms of etiopathogenetic action of the combination of inosine pranobex (Isoprinosine) and recombinant interferon α-βb (Viferon) in Epstein-Barr viral infection in children |
Combination therapy for OEBVI contributed to the modulation of the immune response by cell type (increase in CD3-, CD4-, CD8-, CD16- and HLA-DRT lymphocytes). The readiness of immunocompetent cells for apoptosis (CD95) decreased. There was stimulation of IgA production, switching of Ab synthesis from IgM to IgG, a decrease in CEC content, and improved neutrophil metabolic rates.
The effectiveness of etiotropic therapy was studied in 60 patients with CHEBVI. Patients of group 1 (30 children) received inosine pranobex (Isoprinosine) and recombinant IFN α-2β (Viferon), group 2 (30 people) received monotherapy with recombinant IFN α-2β (Viferon). Regardless of the treatment regimen, 3 months after the start of therapy, there was a significant decrease in the frequency of generalized lymphadenopathy, hypertrophy of the palatine and pharyngeal tonsils, splenomegaly, intoxication, infectious and vegetative-visceral syndromes (Table 2). The combination of inosine pranobex (Isoprinosine) with recombinant IFN α-2β (Viferon) contributed to more significant dynamics of clinical parameters. The number of ARI episodes decreased from 6–11 (7.9 ± 1.1) to 4–6 (5.2 ± 1.2) per year during monotherapy with recombinant IFN α-2β (Viferon), and to 2–4 ( 2.5 ± 1.4) per year during combination therapy (p < 0.05). In both groups, the frequency of EBV replication decreased, but with the combined use of antiviral drugs, this effect was more pronounced.
The use of a combination of inosine pranobex (Isoprinosine) and recombinant IFN α-2β (Viferon) in patients with CEBVI led to more pronounced positive dynamics of immune status indicators (decrease in IL-1RA content, normalization of the expression of activation markers of immunocompetent cells (HLA-DR) and apoptosis receptors ( CD95); increased functional activity of Th1 (increased IFN-γ), restoration of the number of T-lymphocytes and NK-cells, higher content of CD8-lymphocytes than with monotherapy. There was no complete normalization of the expression of the receptor for IL-2 (CD25). during combination antiviral therapy, the functional activity of Th2 decreased (normalization of IL-4 levels). The number of B cells at the end of treatment was normal. An increase in the level of IgA and a switch in Ab synthesis from the IgM class to IgG were recorded; the content of CEC decreased. Indicators of neutrophil metabolism improved The content of neutrophil chemotactic factor (IL-8) did not reach the norm, but was higher than with Viferon monotherapy.
There were no side effects when using inosine pranobex (Isoprinosine) and recombinant IFN α-2β (Viferon).
The results of the study indicate potentiation of the effects of the combination of inosine pranobex (Isoprinosine) with recombinant IFN a-2b (Viferon) in patients with EBVI.
Potentiation of the antiviral, immunomodulatory and cytoprotective effects of these drugs leads to more significant positive dynamics in the manifestations of clinical symptoms of EBVI than with monotherapy, and to the disappearance of serological markers of the activity of the infectious process. It should be noted the high efficiency and safety of combination therapy using inosine pranobex (Isoprinosine) and recombinant IFN α-2β (Viferon).
Rehabilitation. The child is observed by a local doctor and an infectious disease specialist, and is removed from the register 6–12 months after the disappearance of clinical and laboratory indicators of the activity of the infectious process. The frequency of inspections is 1 time per month. According to indications, a consultation with an ENT doctor, immunologist, hematologist, oncologist, etc. is recommended. Laboratory and instrumental studies of patients include: clinical blood test once a month for 3 months, then once every 3 months, more often if indicated; serological markers of EBV using ELISA once every three months, more often if indicated; PCR of blood, oropharyngeal smears once every 3 months, more often if indicated; immunogram - once every 3–6 months; biochemical and instrumental studies - according to indications.
Rehabilitation therapy includes: protective regime, nutritional therapy, antiviral drugs according to prolonged regimens. Under the control of the immunogram, immunocorrection is carried out. According to indications, local antibacterial drugs, courses of vitamin-mineral complexes, pro- and prebiotics, metabolic rehabilitation drugs, enterosorbents, antihistamines, hepato-, neuro- and angioprotectors, cardiotropic drugs, enzymes, homeopathic remedies, and non-drug treatment methods are prescribed.
Thus, EBVI is characterized by a wide distribution, a long course with periodic reactivation of the infectious process in some patients, the possibility of developing complications and adverse outcomes (oncological diseases, autoimmune pathology). The formation of secondary IDS plays an important role in EBVI. The leading clinical syndromes of EBVI are acute and chronic mononucleosis-like syndromes, intoxication, infectious, cerebral, gastrointestinal, cardiac and arthralgic syndromes. Diagnosis of EBVI is based on analysis of risk groups, identification of leading clinical syndromes and laboratory testing. Treatment of EBVI is complex and includes etiotropic drugs (virostatic drugs, interferon and its inducers), drugs for pathogenetic, immunomodulatory, and symptomatic therapy. The combined prolonged use of inosine pranobex (Isoprinosine) and recombinant IFN α-2β (Viferon), potentiating their immunocorrective and cytoprotective effects, significantly increases the effectiveness of treatment. Patients with EBVI need long-term rehabilitation with mandatory monitoring of clinical and laboratory indicators of the activity of the infectious process.
For questions regarding literature, please contact the editor.
E. N. Simovanyan , Doctor of Medical Sciences, Professor V. B. Denisenko , Candidate of Medical Sciences L. F. Bovtalo , Candidate of Medical Sciences A. V. Grigoryan Rostov State Medical University, Rostov-on-Don