Promotion
The process can be neurohumoral (natural), i.e., an increase in the size of the uterus occurs as a result of increased work of the endocrine glands during pregnancy, lactation or during menstruation.
Experts call this condition working; it does not require any medical care and does not cause the woman any particular inconvenience. Pathology is when uterine hypertrophy is provoked by any abnormal processes in the organ itself or by traumatic injuries. For example, the uterus can increase in size due to fibroids, endometriosis, a malignant tumor, and this can also be a consequence of surgical interventions, abortions, complicated childbirth, etc.
On a note:
Often the cause of uterine hypertrophy can be excessive secretion of hormones that stimulate the enlargement of the organ both as a whole and in its individual parts.
Adenoid hypertrophy
Adenoid hypertrophy (adenoid vegetations/growths, hypertrophy of the nasopharyngeal/pharyngeal tonsil) is a persistent enlargement of the nasopharyngeal tonsil. It can occur either alone or in combination with hypertrophy of the palatine tonsils.
The nasopharyngeal tonsil (adenoids) is a collection of lymphoepithelial tissue in the upper part of the nasopharynx. The result of its increase is called adenoid hypertrophy, and with inflammation the process is called adenoiditis. In combination with the palatine, tubal and lingual tonsils, they form the Pirogov-Waldeyer ring, located at the entrance to the upper respiratory tract and digestive tract and acting as a local immune barrier.
Adenoid hypertrophy is most often observed between the ages of 2 and 6 years, but can also occur at a later age.
There are several classifications of adenoids according to the degree of enlargement. The most popular division is based on the doctor’s subjective assessment of the size of the tonsil during examination:
- I degree – the lumen of the choanae is blocked by 1/3
- II degree – the lumen of the choanae is blocked by 1/3–2/3
- III degree – the choanae are completely closed by adenoids.
However, just assessing the size of the tonsil is not enough; a careful history taking is an important criterion.
Causes
Hypertrophy of grade I-II adenoids at the age of 2 to 6 years is often a physiological process that is associated with the fact that the child begins to actively contact the outside world and the amygdala is subjected to constant antigenic stimulation.
Severe (or pathological) hypertrophy, which leads to the symptoms described below, can occur due to an infectious or non-infectious cause. The infectious diseases include the following:
- viral (adenovirus, Coxsackie virus, cytomegalovirus, Epstein-Barr virus, herpes simplex virus, parainfluenza virus, rhinovirus, etc.)
- bacterial (Streptococcus, Haemophilus influenzae, Moraxella catarrhalis, Staphylococcus aureus, Neisseria, Mycoplasma pneumoniae, Fusobacterium, Peptostreptococcus, Prevotella, etc.)
Non-infectious causes include gastroesophageal reflux, allergies, and prolonged exposure to cigarette smoke.
Symptoms
Symptoms of hypertrophy occur due to an increase in the size of the tonsil and/or the presence of chronic inflammatory changes in it (chronic adenoiditis). Although these processes are often related (prolonged inflammation leads to hypertrophy), it must be borne in mind that this does not always happen. For example, with adenoid vegetations of the 1st degree, there may be pronounced signs of chronic inflammation, and with vegetations of the 3rd degree there may be no clinical manifestations at all. With physiological hypertrophy, the process occurs without any symptoms or with minor manifestations.
With severe hypertrophy, the child has difficulty breathing through his nose, and therefore he breathes through his mouth, snores during sleep, sometimes with pauses in breathing (obstructive sleep apnea syndrome). Impaired ventilation of the nasal cavity leads to mucous discharge and frequent rhinosinusitis. Characterized by sneezing and bad breath. Large adenoids put pressure on the soft palate, phonation and articulation are impaired in children, children have a nasal sound. If the adenoids block the auditory tubes, this can manifest as recurrent acute otitis media, hearing loss associated with the accumulation of fluid in the middle ear (exudative otitis media). Over time, due to oxygen starvation, the child becomes lethargic, apathetic, and irritable. Memory deterioration, decreased attention, and learning difficulties occur. Sleeping with your mouth open for a long time leads to disruption of the development of the facial skeleton. The hard palate becomes high and narrow, the lower jaw becomes narrow and elongated, the bite is disturbed, which may require orthodontic treatment in the future. An experienced otolaryngologist will immediately recognize the typical “adenoid” appearance of a child.
There are acute and chronic inflammation of the mucous membrane of the nasopharyngeal tonsil:
- Acute adenoiditis is an acute inflammation caused by a viral or bacterial infection. It manifests itself as a cold: fever, difficulty in nasal breathing, serous or purulent discharge from the nose. The duration of the disease varies and does not exceed one month. In the presence of adenoid hypertrophy, phenomena characteristic of hypertrophy are added to the listed manifestations.
- Chronic adenoiditis is a chronic polyetiological disease, which is based on the disruption of local immune processes and the formation of stable microbial biofilms. It manifests itself as difficulty in nasal breathing, serous or purulent discharge from the nose, mucus running down the throat and coughing. The causes of the disease are the same as those of hypertrophy. There is no consensus on the duration of adenoiditis at which it can be considered chronic. According to various sources, at least 12 weeks must pass from the onset of the disease. This condition can occur independently and over time lead to adenoid hypertrophy.
Diagnostics
The gold standard for diagnosis in global practice and the standard of our hospital is an endoscopic examination of the nasal cavity. We carry out this study with a thin flexible fiberscope under local anesthesia.
Lateral x-rays are a common way to assess hypertrophy but can be misleading. For example, an X-ray may show a picture of grade III adenoids due to enlargement of the tubal tonsils (they are located on the sides of the nasopharyngeal tonsil), due to swelling of the mucous membrane or accumulation of thick mucus. This method is used only if endoscopy of the nasopharynx is impossible.
It is important to carry out differential diagnosis with diseases that may have similar clinical manifestations (juvenile angiofibroma, choanal atresia, polyposis, enlargement of the posterior ends of the inferior turbinates, hypertrophy of the tubal tonsils).
Treatment
Physiological hypertrophy without any manifestations or with minimal symptoms does not require treatment, but endoscopic examination is necessary for differential diagnosis.
Treatment may be conservative. It includes eliminating factors that can cause hypertrophy: allergies, gastroesophageal reflux, passive smoking. Accordingly, in some cases a multidisciplinary approach of an otolaryngologist, allergist, gastroenterologist and pediatrician is necessary. A positive effect can be had by changing the climate to a more favorable one, for example, sea climate. Topical corticosteroids have been used as an adjunctive treatment in children over the age of three, with some studies showing some benefit, but overall evidence on the effectiveness of these drugs is mixed. In acute and exacerbation of chronic adenoiditis in the case of a bacterial infection, treatment is carried out with antibiotics. Irrigation of the nasopharynx with saline solution is also used in treatment. The use of various oils, mucolytics, local antibacterial drugs, silver solutions, herbal remedies, and physiotherapy currently do not have a high-quality evidence base.
In some cases, surgical treatment cannot be avoided. Not every child needs surgery and it requires specific indications. When determining them, doctors at Ilyinskaya Hospital follow the principles of evidence-based medicine, relying on global experience. Indications include failure of conservative treatment, recurrent infections, acute otitis media and sinusitis, and an “adenoid” face. Parents should understand that long-term refusal to undergo surgery if indicated, or an attempt to “wait out” or “outgrow” adenoid hypertrophy can lead to complications, the treatment of which in the future will require a lot of time and can negatively affect the development of the child. According to the recommendations of the American Academy of Otolaryngology and Head and Neck Surgery, adenotomy should be performed in the following cases:
- In children under 4 years of age with symptoms of difficulty in nasal breathing, signs of chronic adenoiditis, as well as exudative otitis media
- In children 4 years of age and older with exudative otitis media
- If you have confirmed obstructive sleep apnea (periodic sudden decrease or cessation of breathing during sleep)
In the presence of pathology of the palatine tonsils, surgery for their resection/removal is often performed simultaneously (adenotonsillotomy/adenotonsillectomy), and in case of exudative otitis media, shunting is performed at the same time.
Adenotomy has undergone significant changes over time. Previously, this operation was performed under local anesthesia using a special knife (adenotome) under the control of a small mirror, and sometimes blindly. During the operation, the child experienced stress, and the surgeon may not have fully performed the operation. Nowadays, this approach is practically not used, but it is preserved in some clinics.
Symptoms
Depending on the cause of hypertrophy, a certain clinical picture will be observed, but the general signs of an enlarged uterus are:
- nagging pain in the lumbar region;
- aching pain in the lower abdomen;
- heavy bleeding during menstruation;
- intermenstrual bleeding;
- frequent bloating, flatulence, constipation;
- frequent urination, urinary incontinence;
- weight gain for no apparent reason;
- pain during intercourse.
Such manifestations may not be observed in all women, so often an enlarged uterus is diagnosed only during a gynecological examination or during an ultrasound of the pelvic organs for another reason.
Diagnosis and treatment of uterine hypertrophy at the Best Clinic SMC
A standard examination by a gynecologist only gives an assumption about changes in the size of the uterus. The doctor receives more accurate information based on the results of ultrasound and hysteroscopy. Having determined the cause of hypertrophy, the doctor may leave the patient under observation if there are no serious indications for treatment, or prescribe additional research methods.
Conservative therapy or surgical intervention - the choice depends on the cause, the woman’s age, her individual characteristics, concomitant diseases, the presence of children or the desire to have them, and many other factors.
At the Best Clinic SMC, doctors very carefully approach each case individually, because not only the woman’s health, but also her entire future life depends on the correct diagnosis and the correct treatment. Applying all their knowledge and using modern methods and technologies, Best Clinic gynecologists and obstetricians try to preserve the only important female function - the ability to conceive, bear and give birth to a healthy baby.
Gynecological appointments at the Best Clinic SMC are carried out only by appointment due to the high workload of doctors. Therefore, take advantage of the opportunity provided on our website and make an appointment in advance with the clinic’s leading specialists by calling the numbers listed on the website or using the appointment form provided.
Molecular mechanisms of cardiac hypertrophy: Part 1. Physiological hypertrophy
Cardiac hypertrophy is a compensatory reaction that occurs in response to an increase in the load on the myocardium. Physiological hypertrophy leads to increased myocardial function: increased ejection fraction, increased contractile activity.
There are two types of cardiac hypertrophy: physiological and pathological, which differ in molecular mechanisms, cardiac phenotype and prognosis for life. The latter is the most important in clinical practice, since physiological hypertrophy helps to “push” blood through at higher loads, while pathological hypertrophy is associated with unfavorable conditions, including myocardial infarction, arrhythmias, and in the long term can lead to death. It should also be noted that these hypertrophy variants are “competitive antagonists”; There is a constant “struggle” between various signaling systems, the result of which will be the development of either physiological or pathological hypertrophy. [1].
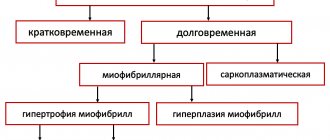
With physiological hypertrophy, there is a slight increase in heart weight (by 10–20%), an increase in cardiomyocytes (CMC) and their proliferation, while the ejection fraction is preserved or increased. There are no sclerotic or necrotic changes in the tissue, and most importantly, physiological hypertrophy is completely reversible (with the exception of postnatal hypertrophy) [1].
Mechanisms of physiological hypertrophy
The initial stimuli leading to hypertrophy do not differ in the case of physiological or pathological variants (for example, physical activity or arterial hypertension). But the reactions triggered in this case are radically different. For the development of physiological hypertrophy, the following is required: an increase in cell size, increased mitochondrial function and energy production, angiogenesis proportional to cell growth, the work of antioxidant systems, regulation of proliferation and regeneration of cardiomyocytes [1].
Physiological hypertrophy of the heart is observed from the birth of a child until he grows up (also called postnatal hypertrophy), with increased physical activity, as well as during pregnancy [2].
Mechanosensors
Mechanotransduction is a fundamental process, the essence of which is the transformation of mechanical signals into biochemical ones. There are sensory systems in the heart that perceive mechanical signals (changes in pressure, volume, etc.) and activate signaling systems responsible for physiological hypertrophy [2].
Ion channels with transient receptor potential (TRP) are one such system. TRPs are a superfamily of transmembrane proteins that function as nonselective ion channels. Among them there are 7 subfamilies; these receptors differ in localization and their role in various cellular processes [3].
In addition to TRP, various integrins exhibit mechanosensitivity. These are transmembrane proteins that can interact with the extracellular matrix (and its various components: fibronectin, laminin, collagen). In response to an extracellular agent, integrins can transmit information into the cell through special integrin-associated protein complexes. This may be focal adhesion kinase (FAK - focal adhesion kinase), integrin-linked kinase (ILK - integrin-linked kinase). The latter, in turn, activate various signaling pathways—standard protein kinase C or PI3K/Act (see below) [2].
We should dwell in somewhat more detail on the integrin-associated protein specific to muscle tissue—melusin. It interacts with β1-integrins, promoting the development of concentric hypertrophy while maintaining cardiac contractility. An increase in melusin synthesis initiates physiological hypertrophy and prevents its development along a pathological path. The opposite is also true: a lack of melusin leads to the development of dilated cardiomyopathy [1].
And, of course, one cannot fail to mention the role of the Z-line as a mechanoreceptor. This part of the cardiomyocyte also responds to stretch by transmitting a signal to many different proteins: teletonin, myopalladin, ankyrins, obscurin... Ultimately, all these processes turn out to be essential for the development of physiological hypertrophy, while defects in some of these proteins lead to cardiomyopathies [2] .
Insulin and insulin-like growth factor 1
These substances regulate many different cellular processes in the heart: proliferation, differentiation, cell growth, metabolism, contractility and apoptosis. Insulin resistance is common in heart failure. IGF1 (insulin-like growth factor 1) is structurally identical to insulin; this agent is synthesized in the liver in response to GH, but can also be formed in other organs (including the heart) [1].
Insulin is known to bind to the insulin receptor, tyrosine kinase, which phosphorylates insulin receptor substrates 1 and 2 (IRS1, 2-insulin receptor substrate). IRSs in turn activate the PI3K/Act signaling pathway. This signaling pathway may be familiar to the reader as a potential target for antitumor therapy. PI3K/Act is involved in the regulation of the cell cycle, and in our case ensures the growth of cardiomyocytes in response to increased load on the myocardium [2], [5].
IGF1, in turn, binds to a specific receptor IGF1R (IGF1 receptor) with subsequent activation of a number of signaling pathways: RAS–RAF–MEK–MAPK, PLC–IP3R3 and others. This also results in physiological hypertrophy of cardiomyocytes.
A little more detail should be taken on the PI3K/Act pathway (it is noteworthy that this signaling pathway is activated during physical activity). PI3K is an enzyme kinase that catalyzes phosphoinositol 3,4,5-trisphosphate. Activation of one of its catalytic subunits—p110α—initiates the processes of physiological hypertrophy and prevents pathological hypertrophy (as we remember, these processes compete with each other). ACT1 is another kinase that is activated by phosphoinositol 3,4,5-phosphate and results in changes in cell metabolism. This is achieved by inhibiting a number of enzymes: glycogen synthase kinase 3β (this kinase inhibits translation initiation factors), fox-O3 (a factor that inhibits the general metabolism of proteins in the cell) and others. As a result, active protein synthesis occurs and the cell increases in size [1].
Triiodothyronine
T3 is triiodothyronine, a thyroid hormone that influences both postnatal cardiac hypertrophy and exercise-induced hypertrophy (although the latter is less reliable) [2].
Immediately after birth, the concentration of T3 in the blood increases significantly. The hormone binds to TRα and TRβ receptors (thyroid hormone receptors), which leads to a “switch” of transcription of the MYH7 gene to MYH6 (these genes encode sequences of myosin heavy chains). MYH7 encodes the β-heavy chains of myosin, MYH6 encodes the α-isoform [1]. This is extremely important, since the α-isoform has greater ATPase activity and, as a result, a cell with active MYH6 is able to contract more strongly [2], [6].
The interaction of T3 with retinoic acid receptors (nuclear receptors for steroid and thyroid hormones, approx.) leads to an increase in the expression of calcium ATPase-2 (SERCA2 - sarcoplasmatic/endoplasmatic reticular calcium ATPase-2), inhibiting MYH7 and the expression of phospholamban (a protein in cardiomyocytes that suppresses SERCA2) [2].
SERCA2 is the evolutionarily most ancient isoform of mammalian calcium ATPases. There are three such isoforms (with subtypes). SERCA2 has three subtypes: a, b and c; for the heart, SERCA2a is most specific (97.5%), there is also a small amount of SERCA2b (2.5%). The function of this protein is to control cytosolic Ca2+ and, as a consequence, to regulate the contractile function of the entire cardiomyocyte [7].
Phospholamban, whose work is inhibited by T3, is a protein regulator of SERCA2 (more precisely, the most studied of these regulators). In its active - dephosphorylated - state, phospholamban suppresses SERCA2 and leads to a decrease in the concentration of Ca2+ in the cytoplasm, which, in turn, reduces the contractility of the cardiomyocyte [2], [7].
In addition, T3 also activates the transcription of β1-adrenergic receptors, sodium and calcium channel proteins, cardiac troponin I, sodium/calcium pump proteins, and adenylate cyclase types V and VI [1], [2]. The hormone also increases the content of TRα1, which activates the already known PI3K [2].
The end result of the work of triiodothyronine in cardiomyocytes is increased myocardial contractile activity and cardioprotection [1].
Nitric oxide (NO)
Physical activity stimulates β3-adrenergic receptors in endothelial cells; in response, endothelial NO synthase (NOS3) is activated, which synthesizes nitric oxide itself, a known vasoactive substance. After this, the following happens:
NO activates soluble guanylate cyclase → cGMP content increases → cGMP-dependent protein kinase G (PKG) is activated → PKG phosphorylates two regulatory proteins (RGS2 and RGS4), which inhibit G-coupled receptors responsible for the development of pathological hypertrophy.
This scheme is needed so that hypertrophy develops according to the physiological variant. A defect in either β3-adrenergic receptors, or NOS3, or the RGS2 and RGS4 proteins leads to a break in the entire chain of cardioprotection, which may well result in myocardial infarction [1].
Angiogenesis
Cells need sufficient blood supply to function normally. If vascular density increases in proportion to the growth of cardiomyocytes, physiological hypertrophy develops. But when cells grow faster than the capillary network can allow, chronic hypoxia occurs.
One of the most important factors determining angiogenesis is vascular endothelial growth factor (VEGF). VEGF may also be familiar to the reader - inhibitors of this factor are used in the treatment of various types of cancer. However, such drugs have significant cardiotoxicity and can lead to various cardiomyopathies (which indirectly indicates the importance of VEGF for myocardial function). Among other things, changes in VEGF levels are also associated with peripartum (postpartum) cardiomyopathy [1], [2].
At the beginning of the article, NFAT signaling molecules were mentioned (in the “mechanosensors” section). VEGF, influencing endothelial cells, acts precisely through the NFAT system, causing enhanced angiogenesis. Thus, these nuclear factors, in addition to their anti-apoptotic role, also affect the blood supply to cardiomyocytes [8].
In addition to VEGF, platelet-derived growth factor (PDGD) also plays an important role. The mechanisms by which PDGD influences angiogenesis are not known for certain; however, a defect in this factor in experiments on mice leads to heart failure and deterioration of blood supply [1].
It is also impossible not to mention another important substance. Hypoxia-inducible factor 1α (HIF1α - hypoxia-inducible factor) is a major transcription factor that ensures a constant supply of oxygen to cells by regulating angiogenesis, vascular modification and regulation of glucose metabolism. In the case of pathological hypertrophy, the p53 antigen (known for its association with tumor development) activates the ubiquitination and proteasomal degradation of HIF1α [1]. Normally, HIF1α activates various signaling molecules (including VEGF) and promotes enhanced angiogenesis.
Neuregulin-1
Neuregulins (1-4) are members of the epidermal growth factor family. In the cardiovascular system, neuregulin-1 is mainly present. The signaling pathway of this factor (and its receptors) plays an important role in the adaptation of the myocardium to the load and the formation of the heart muscle.
Neuregulin-1 acts on the ErbB family kinase (a group of molecules structurally similar to epidermal growth factor; proteins of this family are often considered as potential tumor markers, approx.). Neuregulin-1 activates ErbB2 and ErbB4, which leads to stimulation of the already mentioned PI3K system. The result of this interaction is the proliferation of cardiomyocytes, which prevents ischemic lesions [1].
MicroRNAs and RNA-binding proteins
MicroRNAs (miRNAs) are expressed in the heart during physiological hypertrophy induced by aerobic exercise. For example, miRNA-222 in mice inhibits four targets potentially responsible for decompensation and the development of heart failure: p-27 (encodes a cell cycle inhibitor), Hipk1 and Hipk2 (encodes protein kinases), Hmbox1 (encodes a transcription inhibitor). In other words, activation of miRNA-222 promotes the growth and proliferation of cardiomyocytes [1].
Also, any form of hypertrophy requires de novo protein synthesis. And here, eukaryotic translation factor 4F (elF4F) and the mammalian target of rapamycin mTORC1 are involved - together they trigger increased protein synthesis. In addition, in response to a physiological (and pathological) stimulus in myocardial cells, the poly-A sequence elongates and the expression of specific polyadenylate-binding protein 1 (PABPC1 - polyadenylate-binding protein 1) occurs, which binds to elF4F and thus promotes accumulation in cells various construction (and other) proteins. Increased expression of PABPC1 in cardiomyocytes is necessary for physiological hypertrophy (nothing is known yet about the role of the protein in its pathological version) [1].
Sources:
- M. Nakamura and J. Sadoshima, 'Mechanisms of physiological and pathological cardiac hypertrophy', Nat. Rev. Cardiol., vol. 15, no. 7, pp. 387–407, 2021.
- M. Maillet, J. H. Van Berlo, and J. D. Molkentin, 'Molecular basis of physiological heart growth: Fundamental concepts and new players', Nat. Rev. Mol. Cell Biol., vol. 14, no. 1, pp. 38–48, 2013.
- B. Nilius and G. Owsianik, 'The transient receptor potential family of ion channels', Genome Biol., vol. 12, no. 3, 2011.
- W. T. Pu, Q. Ma, and S. Izumo, 'NFAT transcription factors are critical survival factors that inhibit cardiomyocyte apoptosis during phenylephrine stimulation in vitro', Circ. Res., vol. 92, no. 7, pp. 725–731, 2003.
- M. Osaki, M. Oshimura, and H. Ito, 'PI3K-Akt pathway: Its functions and alterations in human cancer', Apoptosis, vol. 9, no. 6, pp. 667–676, 2004.
- E. M. McNally, R. Kraft, M. Bravo-Zehnder, D. A. Taylor, and L. A. Leinwand, 'Full-length rat alpha and beta cardiac myosin heavy chain sequences', J. Mol. Biol., vol. 210, no. 3, pp. 665–671, 1989.
- P. Vangheluwe, L. Raeymaekers, L. Dode, and F. Wuytack, 'Modulating sarco(endo)plasmic reticulum Ca2+ ATPase 2 (SERCA2) activity: Cell biological implications', Cell Calcium, vol. 38, no. 3-4 SPEC. ISS., pp. 291–302, 2005.
- T. A. Zaichuk, E. H. Shroff, R. Emmanuel, S. Filleur, T. Nelius, and O. V. Volpert, 'Nuclear factor of activated T cells balances angiogenesis activation and inhibition', J. Exp. Med., vol. 199, no. 11, pp. 1513–1522, 2004.