Action potential (AP) is a short-term amplitude change in the resting membrane potential (RMP) that occurs when a living cell is excited. Essentially, this is an electrical discharge - a rapid, short-term change in potential in a small area of the membrane of an excitable cell (neuron or muscle fiber), as a result of which the outer surface of this area becomes negatively charged in relation to neighboring areas of the membrane, while its inner surface becomes positively charged in relation to to adjacent areas of the membrane. The action potential is the physical basis of a nerve or muscle impulse that plays a signaling (regulatory) role.
general characteristics
Action potentials can differ in their parameters depending on the type of cell and even on different parts of the membrane of the same cell. The most typical example of differences is the action potential of the heart muscle and the action potential of most neurons. Nevertheless, the basis of any action potential is the following phenomena:
- “The membrane of a living cell is polarized” - its inner surface is charged negatively in relation to the outer one due to the fact that in the solution at its outer surface there is a larger number of positively charged particles (cations), and at the inner surface there is a larger number of negatively charged particles (anions).
- “A membrane has selective permeability” - its permeability to different particles (atoms or molecules) depends on their size, electrical charge and chemical properties.
- “The membrane of an excitable cell is capable of quickly changing its permeability for a certain type of cations, causing a transition of positive charge from the outside to the inside
The first two properties are characteristic of all living cells. The third is a feature of excitable tissue cells and the reason why their membranes are able to generate and conduct action potentials.
The main mathematical model describing the generation and transmission of action potentials is the Hodgkin-Huxley model.
The repolarization phase of the action potential is due to the diffusion of ions
Hello readers of my project “Biology for Students”! Preparing for exams, tests and state exams, as well as essays and presentations, takes a lot of time if prepared using textbooks. There are three ways to prepare for the exam: using a textbook, using lectures, and searching on the Internet. It takes a long time to prepare using a textbook.
As for lectures, not everyone has good lectures, since not all teachers read them well, and besides, not everyone has time to write them down. And the third option remains - to look for answers to questions on the Internet. It's no secret that currently most students prefer this option.
During the five years of studying at the Faculty of Biotechnology and Biology, preparing for the session took me a lot of time. There are not many biological sites on the RuNet. Notes on economics, history, sociology, political science, and mathematics are very easy to find. And answers to questions on botany, zoology, genetics, biophysics, and biochemistry are much more difficult.
Probably because biology is not the most common specialty. In addition, biological subjects are not general education subjects, unlike, for example, economics and history, which are studied in almost any specialty. In RuNet, I have not found a single site that would provide the necessary content for preparing for exams, tests and state exams in biological disciplines. And I decided to create it.
I would also like to ask you to tell your classmates, friends and acquaintances who are students of biological specialties about this site. This will help the development of this project.
Phases
Five phases of development of PD can be clearly distinguished:
Rising (depolarization)
The occurrence of an action potential (AP) is associated with an increase in the permeability of the membrane for sodium ions (20 times compared to the permeability for K +, and 500 times compared to the initial permeability of Na +) and a subsequent increase in the diffusion of these ions along the concentration gradient into the cell, leads to a change (decrease) in membrane potential.
A decrease in membrane potential leads to an increase in membrane permeability to sodium by opening voltage-gated sodium channels, and an increase in permeability is accompanied by increased diffusion of sodium into the cytoplasm, which causes even more significant depolarization of the membrane. Due to the presence of positive feedback, depolarization of the membrane during excitation occurs with acceleration and the flow of sodium ions into the cell increases all the time. The intensity of the flow of potassium ions directed from the cell outwards in the first moments of excitation remains at the beginning. The increased flow of positively charged sodium ions into the cell first causes the disappearance of excess negative charge on the inner surface of the membrane, and then leads to recharging of the membrane. The influx of sodium ions occurs until the inner surface of the membrane acquires a positive charge sufficient to balance the sodium concentration gradient and stop its further passage into the cell. The sodium occurrence of PD is confirmed by experiments with changes in the external and internal concentrations of this ion. It was shown that a tenfold change in the concentration of sodium ions in the external or internal environment of the cell corresponds to a change in PD of 58 mV. When sodium ions were completely removed from the fluid surrounding the cell, PD did not occur. Thus, it has been established that AP occurs as a result of excess, compared to rest, diffusion of sodium ions from the surrounding fluid into the cell. The period during which the membrane permeability for sodium ions increases when sodium channels open is short (0.5-1 ms), followed by an increase in membrane permeability for potassium ions due to the opening of voltage-dependent potassium channels, and, consequently, increased diffusion these ions out of the cell. The “all or nothing” principle According to the “all-or-nothing” law, the cell membrane of an excitable tissue either does not respond to the stimulus at all, or responds with the maximum force possible for it at the moment. The action of the stimulus usually leads to local depolarization of the membrane. This causes the opening of sodium channels, which are sensitive to changes in potential, and through this increases sodium conductance, which leads to even greater depolarization. The existence of such feedback ensures regenerative (renewable) depolarization of the cell membrane. The magnitude of the action potential depends on the strength of the stimulus, and it occurs only when depolarization exceeds a certain limiting level specific for each cell. This phenomenon is called “all or nothing.” However, if depolarization is 50-75% of the limiting value, then a local response may occur in the cell, the amplitude of which is significantly lower than the amplitude of the action potential. The absence of an action potential at the pidboundary level of depolarization is explained by the fact that sodium permeability does not increase sufficiently to cause regenerative depolarization. The level of depolarization that occurs does not cause the opening of new sodium channels, so sodium conductance quickly decreases, and the resting potential in the cell is again established.
Overshoot
Depolarization of the membrane leads to a reversal of the membrane potential (the MP becomes positive).
In the overshoot phase, the Na + current begins to rapidly decrease, which is associated with the inactivation of voltage-dependent Na + channels (the open state time is milliseconds) and the disappearance of the electrochemical Na + gradient. Refractoriness One of the consequences of the disappearance of the Na + gradient is refractoriness - a temporary inability to respond to a stimulus. If the stimulus occurs immediately after the passage of the action potential, then excitability will not occur either with a stimulus strength at the threshold level or with a significantly stronger stimulus. This state of complete inexcitability is called the absolute refractory period. This is followed by a relative refractory period, when a suprathreshold stimulus can cause an action potential with a significantly lower amplitude than normal. An action potential of the usual amplitude under the action of a threshold stimulus can be evoked only after a few milliseconds after the preliminary action potential. The absolute refractory period limits the maximum frequency of generation of action potentials.
Repolarization
An increase in the potassium ion flow directed outward from the cell leads to a decrease in the membrane potential, which in turn causes a decrease in the permeability of the membrane to sodium ions, which, as indicated, is a function of the membrane potential. Thus, the second stage is characterized by the fact that the flow of potassium ions from the cell outward increases, and the counter flow of sodium ions decreases. This membrane repolarization continues until the resting potential is restored—membrane repolarization. After this, the permeability to potassium ions also drops to its original value. Due to the positively charged potassium ions released into the environment, the outer surface of the membrane again acquires a positive potential relative to the internal one.
Trace depolarization and hyperpolarization
In the final phase, the restoration of the resting membrane potential slows down, and trace reactions are recorded in the form of trace depolarization and hyperpolarization, due to the slow restoration of the initial permeability for K + ions.
POLARIZATION AND DEPOLARIZATION
In cases where charge separation occurs and positive charges are located in one place and negative charges in another, physicists talk about charge polarization. Physicists use the term by analogy with opposite magnetic forces that accumulate at opposite ends, or poles (the name is given because a freely moving magnetized strip points its ends towards the geographic poles) of a strip magnet. In the case under discussion, we have a concentration of positive charges on one side of the membrane and a concentration of negative charges on the other side of the membrane, that is, we can talk about a polarized membrane.
However, in any case where charge separation occurs, an electric potential immediately arises. Potential is a measure of the force that tends to bring separated charges closer together and eliminate polarization. Electric potential is therefore also called electromotive force, which is abbreviated as emf.
Electric potential is called potential precisely because it does not actually move charges, since there is an opposing force that keeps opposite electric charges from approaching each other. This force will exist as long as energy is spent to maintain it (which is what happens in cells). Thus, the force tending to bring charges together has only the ability, or potency, to do so, and such approach occurs only when the energy expended in separating the charges is weakened. Electrical potential is measured in units called volts, after Voltas, the man who created the world's first electric battery.
Physicists have been able to measure the electrical potential that exists between the two sides of the cell membrane. It turned out to be equal to 0.07 volts. We can also say that this potential is 70 millivolts, since a millivolt is equal to one thousandth of a volt. Of course, this is a very small potential compared to 120 volts (120,000 millivolts) of AC power or thousands of volts of power line voltage. But it's still an amazing potential, given the materials a cell has at its disposal to build electrical systems.
Any reason that interrupts the activity of the sodium pump will lead to a sharp equalization of the concentrations of sodium and potassium ions on both sides of the membrane. This, in turn, will automatically lead to equalization of charges. Thus, the membrane will become depolarized. Of course, this happens when the cell is damaged or dies. But there are, however, three types of stimuli that can cause depolarization without causing any harm to the cell (unless, of course, these stimuli are too strong). These lamps include mechanical, chemical and electrical.
Pressure is an example of a mechanical stimulus. Pressure on a section of the membrane causes expansion and (for reasons not yet known) will cause depolarization at that location. High temperature causes the membrane to expand, cold contracts it, and these mechanical changes also cause depolarization.
The same result is achieved by exposing the membrane to certain chemical compounds and to weak electric currents. (In the latter case, the cause of depolarization seems most obvious. After all, why cannot the electrical phenomenon of polarization be changed by an externally applied electrical potential?)
Depolarization that occurs in one place of the membrane serves as a stimulus for depolarization to spread across the membrane. The sodium ion, which rushes into the cell at the place where depolarization has occurred and the action of the sodium pump has ceased, displaces the potassium ion out. Sodium ions are smaller and more mobile than potassium ions. Therefore, more sodium ions enter the cell than potassium ions leave it. As a result, the depolarization curve crosses the zero mark and rises higher. The cell again turns out to be polarized, but with the opposite sign. At some point, the flare acquires an internal positive charge due to the presence of excess sodium ions in it. A small negative charge appears on the outside of the membrane.
Opposite polarization can serve as an electrical stimulus that paralyzes the sodium pump in areas adjacent to the site of the original stimulus. These adjacent areas are polarized, then polarization occurs with the opposite sign and depolarization occurs in more distant areas. Thus, a wave of depolarization sweeps across the entire membrane. In the initial section, polarization with the opposite sign cannot last long. Potassium ions continue to leave the cell, gradually their flow equalizes the flow of incoming sodium ions. The positive charge inside the cell disappears. This disappearance of the reverse potential reactivates to some extent the sodium pump at this location in the membrane. Sodium ions begin to leave the cell, and potassium ions begin to penetrate into it. This section of the membrane enters the repolarization phase. Since these events occur in all areas of membrane depolarization, following the depolarization wave, a repolarization wave sweeps across the membrane.
Between the moments of depolarization and complete repolarization, the membranes do not respond to normal stimuli. This period of time is called the refractory period. It lasts for a very short time, a small fraction of a second. A depolarization wave passing through a certain area of the membrane makes this area immune to excitation. The previous stimulus becomes, in a sense, singular and isolated. How exactly the minute changes in charges involved in depolarization realize such a response is unknown, but the fact remains that the membrane’s response to a stimulus is isolated and single. If a muscle is stimulated in one place with a small electrical discharge, the muscle will contract. But not only the area to which the electrical stimulation was applied will shrink; all muscle fibers will contract. The wave of depolarization travels along the muscle fiber at a speed of 0.5 to 3 meters per second, depending on the length of the fiber, and this speed is sufficient to create the impression that the muscle is contracting as one whole.
This phenomenon of polarization-depolarization-repolarization is inherent in all cells, but in some it is more pronounced. In the process of evolution, cells appeared that benefited from this phenomenon. This specialization can go in two directions. First, and this happens very rarely, organs can develop that are capable of creating high electrical potentials. When stimulated, depolarization is realized not by muscle contraction or other physiological response, but by the appearance of an electrical current. This is not a waste of energy. If the stimulus is an enemy attack, the electrical discharge can injure or kill him.
There are seven species of fish (some of them bony, some of them belong to the cartilaginous order, being relatives of sharks), specialized in this direction. The most picturesque representative is the fish, which is popularly called the “electric eel”, and in science it has a very symbolic name - Electrophorus electricus.
The electric eel is a freshwater inhabitant and is found in the northern part of South America - in the Orinoco, Amazon and its tributaries. Strictly speaking, this fish is not related to eels, but was named for its long tail, which makes up four-fifths of the body of this animal, which is from 6 to 9 feet long. All the normal organs of this fish fit into the front part of the body, which is about 15 to 16 inches long.
More than half of the long tail is occupied by a series of blocks of modified muscles that form an "electric organ". Each of these muscles produces a potential that is no greater than that of a normal muscle. But thousands and thousands of elements of this “battery” are connected in such a way that their potentials add up. A rested electric eel is capable of accumulating a potential of about 600 - 700 volts and discharging it at a rate of 300 times per second. When tired, this rate drops to 50 times per second, but the eel can withstand this rate for a long time. The electric shock is strong enough to kill the small animals on which this fish feeds, or to cause a sensitive injury to a larger animal that suddenly decides to eat the electric eel by mistake.
The electric organ is a magnificent weapon. Perhaps other animals would gladly resort to such an electric shock, but this battery takes up too much space. Imagine how few animals would have strong teeth and claws if they took up half their body weight.
The second type of specialization, which involves the use of electrical phenomena occurring on the cell membrane, is not to increase the potential, but to increase the speed of propagation of the depolarization wave. Cells with elongated processes appear, which are almost exclusively membranous formations. The main function of these cells is to transmit stimuli very quickly from one part of the body to another. It is from such cells that nerves are made - the same nerves with which this chapter began.
NEURON
The seals that we can observe with the naked eye are, of course, not individual cells. These are bundles of nerve fibers, sometimes these bundles contain a lot of fibers, each of which represents a part of a nerve cell. All fibers of the bundle go in the same direction and, for the sake of convenience and space saving, are interconnected, although individual fibers can perform completely different functions. In the same way, separate insulated electrical wires that perform completely different tasks are combined into one electrical cable for convenience. The nerve fiber itself is part of a nerve cell, also called a neuron. It is a Greek derivative of the Latin word for nerve. The Greeks of the Hippocratic era applied this word to nerves in the true sense and to tendons. Now this term refers exclusively to an individual nerve cell. The main part of the neuron - the body - is practically not much different from all other cells of the body. The body contains the nucleus and cytoplasm. The biggest difference between a nerve cell and other cells is the presence of long extensions from the cell body. From most of the surface of the nerve cell body there are projections that branch throughout. These branching projections resemble the crown of a tree and are called dendrites (from the Greek word for tree).
On the surface of the cell body there is one place from which one especially long process emerges, which does not branch along its entire (sometimes huge) length. This process is called an axon. I will explain later why it is called that. It is axons that represent typical nerve fibers of a nerve bundle. Although the axon is microscopically thin, it can be several feet long, which seems unusual when you consider that the axon is just part of a single nerve cell.
Depolarization that occurs in any part of the nerve cell spreads along the fiber at high speed. A wave of depolarization propagating along the processes of a nerve cell is called a nerve impulse. The pulse can travel along the fiber in any direction; Thus, if a stimulus is applied to the middle of the fiber, the impulse will spread in both directions. However, in living systems it almost always turns out that impulses propagate along the dendrites in only one direction - towards the cell body. Along the axon, the impulse always propagates from the cell body.
The speed of impulse propagation along a nerve fiber was first measured in 1852 by the German scientist Hermann Helmholtz. To do this, he applied stimuli to the nerve fiber at different distances from the muscle and recorded the time after which the muscle contracted. If the distance increased, then the delay lengthened, after which contraction occurred. The delay corresponded to the time it took for the impulse to travel the additional distance.
It is quite interesting that six years before Helmholtz’s experiment, the famous German physiologist Johannes Müller, in a fit of conservatism so characteristic of scientists in the twilight of their careers, categorically stated that no one would ever be able to measure the speed of impulse transmission along a nerve.
In different fibers, the speed of impulse conduction is not the same. First, the speed at which an impulse travels along an axon depends roughly on its thickness.
The thicker the axon, the greater the speed of impulse propagation. In very thin fibers, the impulse moves through them quite slowly, at a speed of two meters per second or even less. No faster than, say, a wave of depolarization propagates through muscle fibers. Obviously, the faster the body must react to a particular stimulus, the more desirable is the high speed of impulse conduction. One way to achieve this state is to increase the thickness of the nerve fibers. In the human body, the thinnest fibers have a diameter of 0.5 microns (a micron is one thousandth of a millimeter), and the thickest are 20 microns, that is, 40 times larger. The cross-sectional area of thick fibers is 1600 times greater than the cross-sectional area of thin fibers.
One might think that since mammals have a better developed nervous system than other groups of animals, their nerve impulses travel at the highest speed and their nerve fibers are thicker than those of all other species. But in reality this is not the case. Lower animals, cockroaches, have thicker nerve fibers than humans.
The most developed of mollusks, squid, have the thickest nerve fibers. Large squids in general are probably the most developed and highly organized animals of all invertebrates. Given their physical size, we are not surprised that they require high conduction speeds and very thick axons. The nerve fibers going to the squid muscles are called giant axons and reach a diameter of 1 millimeter. This is 50 times the diameter of the thickest axon in mammals, and the cross-sectional area of squid axons is 2,500 times larger than mammalian axons. Giant squid axons are a godsend for neuroscientists, who can easily perform experiments on them (for example, measuring potentials on the axon membranes), which is very difficult to do on the extremely thin axons of vertebrates.
Nevertheless, why did invertebrates surpass vertebrates in the thickness of nerve fibers, although vertebrates have a more developed nervous system?
The answer is that the speed of impulses along nerves in vertebrates depends not only on the thickness of the axons. Vertebrates have at their disposal a more sophisticated way of increasing the speed of impulses along axons.
In vertebrates, nerve fibers in the early stages of organism development are surrounded by so-called satellite cells. Some of these cells are called Schwann cells (named after the German zoologist Theodor Schwain, one of the founders of the cellular theory of life). Schwann cells wrap around the axon, forming a tighter and tighter spiral, covering the fiber with a fat-like sheath called the myelin sheath. Ultimately, the Schwann cells form a thin sheath around the axon called the neurilemma, which nevertheless contains the nuclei of the original Schwann cells. (By the way, Schwann himself described these neurilemmomas, which are sometimes called Schwann’s membrane in his honor. It seems to me that the term used to designate a tumor arising from a neurilemma is very unmusical and insulting to the memory of the great zoologist. It is called schwannoma.)
One individual Schwann cell envelops only a limited portion of the axon. As a result, Schwann sheaths enclose the axon in separate sections, between which there are narrow sections in which the myelin sheath is absent. As a result, under a microscope, the axon looks like a bunch of sausages. The unmyelinated areas of this ligament are called nodes of Ranvier, after the French histologist Louis Antoine Ranvier, who described them in 1878. Thus, the axon is like a thin rod threaded through a sequence of cylinders along their axes. Axis
in Latin means “axis”, hence the name of this process of the nerve cell.
The suffix -on
is added, apparently, by analogy with the word “neuron”.
The function of the myelin sheath is not entirely clear. The simplest assumption regarding its function is that it serves as a kind of insulator for the nerve fiber, reducing the leakage of current into the environment. Such leakage increases as the fiber becomes thinner, and the presence of an insulator allows the fiber to remain thin without increasing potential loss. Evidence for this is based on the fact that myelin is predominantly composed of lipid (fat-like) materials, which are indeed excellent electrical insulators. (It is this material that gives the nerve its white color. Those about the nerve cell are colored gray.)
However, if myelin performed only the functions of an electrical insulator, then simpler fat molecules could do the job. But as it turned out, the chemical composition of myelin is very complex. Of every five myelin molecules, two are cholesterol molecules, another two are phospholipid molecules (fat molecules containing phosphorus), and the fifth molecule is cerebroside (a complex fat-like molecule containing sugar). Myelin also contains other unusual substances. It seems very likely that myelin performs more than just the functions of an electrical insulator in the nervous system.
It has been suggested that myelin sheath cells maintain the integrity of the axon because it extends such a large distance from the nerve cell body that it is likely to lose normal communication with its nerve cell nucleus. It is known that the nucleus is vital for maintaining the normal functioning of any cell and all its parts. Perhaps the nuclei of Schwann cells take on the function of nannies that nourish the axon in the areas they envelop. After all, the axons of nerves, even those without myelin, are covered with a thin layer of Schwann cells, which, naturally, have nuclei.
Finally, the myelin sheath somehow speeds up the conduction of impulses along the nerve fiber. A fiber covered with a myelin sheath conducts impulses much faster than a fiber of the same diameter that lacks the myelin sheath. This is why vertebrates won the evolutionary battle with invertebrates. They retained thin nerve fibers, but significantly increased the speed of impulses through them.
Mammalian myelinated nerve fibers conduct nerve impulses at a speed of about 100 m/s, or, if you prefer, 225 miles per hour. This is a pretty decent speed. The biggest challenge that mammalian nerve impulses have to overcome is the 25 meters that separate the blue whale's head from its tail. A nerve impulse travels this long path in 0.3 s. The distance from the head to the big toe in a person, an impulse travels along a myelinated fiber in one fiftieth of a second. With regard to the speed of information transmission in the nervous and endocrine systems, there is a huge and quite obvious difference.
When a baby is born, the process of myelination of the nerves in his body is not yet complete, and various functions do not develop properly until the necessary nerves are myelinated. So, the child does not see anything at first. The function of vision is established only after myelination of the optic nerve, which, fortunately, will not keep you waiting. Similarly, the nerves to the muscles of the arms and legs remain unmyelinated during the first year of life, so the coordination required for independent ambulation is only established by this time.
Sometimes adults suffer from the so-called "demylenizing disease", in which areas of myelin degenerate with subsequent loss of function of the corresponding nerve fiber. One of these diseases that has been best studied is known as multiple sclerosis. This name is given to this disease because with it, foci of myelin degeneration appear in various parts of the nervous system with its replacement by denser scar tissue. Such demyelination can develop as a result of the action of some protein present in the patient’s blood on the myelin. This protein appears to be an antibody, a member of a class of substances that normally interact only with foreign proteins but often cause symptoms of the condition we know as allergies. In fact, a person with multiple sclerosis develops an allergy to himself, and this disease may be an example of an autoallergic disease. Because the sensory nerves are most often affected, the most common symptoms of multiple sclerosis are double vision, loss of touch sensation, and other sensory disturbances. Multiple sclerosis most often affects people between the ages of 20 and 40. The disease can progress, that is, more and more nerve fibers can be affected, and eventually death occurs. However, progression of the disease can be slow, and many patients live more than ten years from diagnosis.
Spreading
Spread into unmyelinated fibers
In unmyelinated (without pulp) nerve fibers, the AP spreads from point to point, since excitation can be registered as one that gradually “runs” along the entire fiber from its point of origin. Sodium ions entering the excited area serve as a source of electric current for the occurrence of AP in adjacent areas. In this case, the impulse occurs between the depolarized section of the membrane and its non-excited section. The potential difference here is many times higher than necessary for membrane depolarization to reach the maximum level. The speed of pulse propagation in such fibers is 0.5-2 m/s
Spread in myelinated fibers
The nerve processes of most somatic nerves are myelinated.
Only very small areas of them, the so-called node interception (interception of Ranvier), are covered with a normal cell membrane. Such nerve fibers are characterized by the fact that voltage-dependent ion channels are located on the membrane only at the interceptions. In addition, this shell increases the electrical resistance of the membrane. Therefore, when the membrane potential shifts, the current passes through the membrane of the intercepting area, that is, by jumping (saltatory) from one interception to another, which allows you to increase the speed of the nerve impulse, which ranges from 5 to 120 m/s. Moreover, the action potential that arose in one of the nodes of Ranvier causes action potentials in neighboring nodes due to the emergence of an electric field, which causes an initial depolarization in these nodes. The parameters of the EMF field and the distance of its effective action depend on the cable properties of the axon. Types of nerve fibers, impulse conduction speed, depending on myelination
| Type | Diameter (µm) | Myelination | Conduction speed (m/s) | Functional purpose |
| A alpha | 12-20 | strong | 70-120 | Mobile fibers of the somatic NS; proprioceptor sensory fibers |
| A beta | 5-12 | strong | 30-70 | Sensory fibers of skin receptors |
| A gamma | 3-16 | strong | 15-30 | Sensory fibers of proprioceptors |
| A delta | 2-5 | strong | 12-30 | Sensitive fibers of thermoreceptors, nociceptors |
| IN | 1-3 | weak | 3-15 | Preganglionic fibers of the sympathetic nervous system |
| WITH | 0,3-1,3 | absent | 0,5-2,3 | Postganglionic fibers of the sympathetic nervous system; sensory fibers of thermoreceptors, nociceptors of some mechanoreceptors |
Delayed afterdepolarization and triggered sustained rhythmic activity
frequency leads to a decrease in amplitude. In addition, if a premature action potential during stimulation occurs at a constant frequency, then the subsequent afterdepolarization has a greater amplitude than that observed after a regular action potential. Moreover, the earlier during the main cycle the premature action potential occurs, the greater the amplitude of the premature afterdepolarization.
At a high enough frequency of sustained stimulation or after a sufficiently early premature stimulus, afterdepolarization can reach threshold and produce unstimulated action potentials. The first spontaneous impulse is observed after a shorter interval compared to the duration of the main cycle, since the post-depolarization due to which it arose begins soon after the repolarization of the previous action potential.
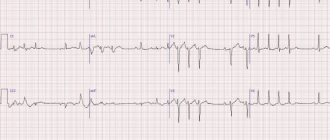
Consequently, the spontaneous impulse causes another post-depolarization, which also reaches the threshold level, causing the appearance of a second spontaneous impulse (see Fig. 3.8). This last impulse causes the next afterdepolarization, which initiates the third spontaneous impulse, and so on throughout the duration of the trigger activity.
Triggered activity may cease spontaneously and, if it does, the last unstimulated pulse is usually followed by one or more subthreshold afterdepolarizations. Fig. 3.8. Induction of trigger activity in the atrial fiber of the mitral valve in a monkey. Each fragment shows only the lower part of the action potentials.
The horizontal lines in fragments I and II are drawn at a level of -30 mV, and in fragment III - at a level of -20 mV. fragment IA and 1B: trigger activity resulting from a reduction in the duration of the main stimulation cycle. IA: stimulation cycle duration is 3400 ms; and each action potential is followed by a subthreshold delayed afterdepolarization.
in the occurrence of trigger activity due to premature stimulation. IIIA: A premature impulse (arrow) is produced during the repolarization phase of the afterdepolarization, and the amplitude of the subsequent afterdepolarization increases. IIIB: the premature impulse (large arrow) is followed by a post-depolarization that reaches the threshold (small arrow) and leads to the appearance of a series of trigger impulses [40].
Action potential propagation between cells
At a chemical synapse, after the action potential wave reaches the nerve terminal, it causes the release of neurotransmitters from the presynaptic vesicles into the synaptic cleft. Transmitter molecules released from the presynapse bind to receptors on the postsynaptic membrane, resulting in the opening of ion channels in the receptor macromolecules. Ions begin to enter the postsynaptic cell through open channels, change the charge of its membrane, which leads to partial depolarization of the membrane and, as a consequence, provoking the generation of an action potential in the postsynaptic cell.
In the electrical synapse there is no “mediator” of transmission in the form of a neurotransmitter. But the cells are connected to each other using specific protein tunnels - conexons, so ionic currents from the presynaptic cell can stimulate the postsynaptic cell, causing the generation of an action potential in it. Thanks to this structure, the action potential can propagate in both directions and much faster than through a chemical synapse.
- Scheme of the process of nerve signal transmission at a chemical synapse
- Diagram of the structure of an electrical synapse
Action potential in different cell types
Action potential in muscle tissue
The action potential in skeletal muscle cells is similar to the action potential in neurons. Their resting potential is typically -90 mV, which is less than the resting potential of typical neurons. The action potential of muscle cells lasts approximately 2–4 ms, the absolute refractory period is approximately 1–3 ms, and the conduction velocity along the muscle is approximately 5 m/s.
Action potential in cardiac tissue
The action potential of the cells of the working myocardium consists of a phase of rapid depolarization, an initial rapid repolarization, which turns into a phase of slow repolarization (plateau phase), and a phase of rapid final repolarization. The phase of rapid depolarization is caused by a sharp increase in the permeability of the membrane for sodium ions, causes a rapid incoming sodium current, when the membrane potential reaches 30-40 mV it is inactivated and subsequently the calcium ion current plays a major role. Depolarization of the membrane causes activation of calcium channels, resulting in an additional depolarizing incoming calcium current.
The action potential in cardiac tissue plays an important role in coordinating cardiac contractions.
B) trace repolarization phase (potential)
The change in membrane permeability to Na does not last long. It starts to increase for K and decreases for Na. This corresponds to the repolarization phase. The descending part of the curve corresponds to the trace potential and reflects the recovery processes that occur after irritation. The following phases are distinguished on the action potential curve: 1.
Local response (local depolarization) preceding the development of AP.2. Depolarization phase. During this phase, MP rapidly decreases and reaches zero level. The level of depolarization increases above 0. Therefore, the membrane acquires the opposite charge - it becomes positive inside and negative outside.
The phenomenon of changing the membrane charge is called membrane potential reversal. The duration of this phase in nerve and muscle cells is 1-2 ms. 3. Repolarization phase. It begins when a certain MP level is reached (approximately 20 mV). The membrane potential begins to quickly return to resting potential. Phase duration is 3-5 ms.4.
Phase of trace depolarization or trace negative potential. The period when the return of the MP to resting potential is temporarily delayed. It lasts 15-30 ms. 5. The phase of trace hyperpolarization or trace positive potential. During this phase, the MP for some time becomes higher than the initial level of PP. Its duration is 250-300 ms.
The amplitude of the action potential of skeletal muscles is on average 120-130 mV, neurons 80-90 mV, smooth muscle cells 40-50 mV. When neurons are excited, AP occurs in the initial segment of the axon - the axon hillock. The occurrence of AP is caused by a change in the ionic permeability of the membrane during excitation. During the period of local response, slow sodium channels open, while fast ones remain closed, and temporary spontaneous depolarization occurs.
the higher it is, the lower the CUD and vice versa. When the depolarization value approaches the equilibrium potential for sodium ions (20 mV), the strength of the sodium concentration gradient decreases significantly. At the same time, the process of inactivation of fast sodium channels and a decrease in sodium conductivity of the membrane begins.
Depolarization stops. The output of potassium ions sharply increases, i.e. potassium outgoing current. In some cells this occurs due to the activation of special potassium outward current channels. This current, directed out of the cell, serves to quickly shift the MP to the level of the resting potential. Those. the repolarization phase begins.
An increase in MP leads to the closure of the activation gates of sodium channels, which further reduces the sodium permeability of the membrane and accelerates repolarization. The occurrence of the trace depolarization phase is explained by the fact that a small part of the slow sodium channels remains open. The trace hyperpolarization is associated with increased, after AP, potassium conductivity of the membrane and the fact that the sodium-potassium pump works more actively, removing sodium ions that entered the cell during PD.
By changing the conductivity of fast sodium and potassium channels, one can influence the generation of APs, and therefore the excitation of cells. When sodium channels are completely blocked, for example by tetrodont fish poison - tetrodotoxin, the cell becomes inexcitable. This is used clinically. Local anesthetics such as novocaine, dicaine, lidocaine inhibit the transition of sodium channels of nerve fibers to an open state.
Therefore, the conduction of nerve impulses along the sensory nerves stops, and anesthesia of the organ occurs. When potassium channels are blocked, the release of potassium ions from the cytoplasm to the outer surface of the membrane is hindered, i.e. restoration of MP. Therefore, the repolarization phase is prolonged. This effect of potassium channel blockers is also used in clinical practice.
Molecular mechanisms of action potential generation
The active properties of the membrane that ensure the occurrence of an action potential, based mainly on the behavior of voltage-gated sodium (Na +) and potassium (K +) channels. The initial phase of AP is formed by the input sodium current, later potassium channels open and the output K + -current returns the membrane potential to the initial level. The initial ion concentration is then restored by the sodium-potassium pump.
During the PD, channels pass from state to state: in Na + channels there are three main states - closed, open and inactivated (in reality everything is more complicated, but these three states are enough for description), in K + channels there are two - closed and open.
The behavior of the channels involved in the formation of PD is described in terms of conductivity and calculated through transfer coefficients.
Carryover coefficients were derived by Alan Lloyd Hodgkin and Andrew Huxley.
Conductivity for potassium GK per unit area
| , |
| Where: |
| an — Transfer coefficient from closed to open state for K+ channels [1/s]; |
| bn — Transfer coefficient from open to closed state for K+ channels [1/s]; |
| n — Fraction of K+ channels in the open state; |
| (1 - n) - Fraction of K + channels in the closed state |
The conductivity for sodium G Na per unit area
is more difficult to calculate, since, as already mentioned, in voltage-dependent Na + channels, in addition to the closed / open states, the transition between which is a parameter, there are also inactivated / not inactivated states, the transition between which is described through a parameter
| , | , |
| Where: | Where: |
| am — Transfer coefficient from closed to open state for Na + channels [1/s]; | ah — Transfer coefficient from inactivated to non-inactivated state for Na + channels [1/s]; |
| bm — Transfer coefficient from open to closed state for Na + channels [1 / s]; | bh — Transfer coefficient from non-inactivated to inactivated state for Na + channels [1 / s]; |
| m — Fraction of Na + channels in the open state; | h — Fraction of Na + channels in a non-inactivated state; |
| (1 - m) - Fraction of Na + channels in the closed state | (1 - h) - Fraction of Na + channels in the inactivated state. |
Story
The main provisions of the membrane theory of excitation were formulated by the German neurophysiologist Yu. Bernstein
In 1902, Julius Bernstein put forward a hypothesis according to which the cell membrane allows K+ ions into the cell, and they accumulate in the cytoplasm. The calculation of the resting potential value using the Nernst equation for the potassium electrode satisfactorily coincided with the measured potential between the muscle sarcoplasm and the environment, which was about -70 mV. According to the theory of Yu. Bernstein, when a cell is excited, its membrane is damaged, and K + ions leave the cell along a concentration gradient until the membrane potential becomes zero. The membrane then restores its integrity and the potential returns to the resting potential level.
This model was developed in their 1952 work by Alan Lloyd Hodgkin and Andrew Huxley, in which they described the electrical mechanisms responsible for the generation and transmission of a nerve signal in the squid giant axon. For this, the authors of the model received the Nobel Prize in Physiology or Medicine for 1963. The model is called the Hodgkin-Huxley model
In 2005, Thomas Heimburg and Andrew D. Jackson proposed the soliton model, based on the assumption that the signal propagates through neurons in the form of solitons - stable waves propagating along the cell membrane.
The effect of certain substances on the action potential
Some substances of organic or synthetic origin can block the formation or passage of PD:
- Batrachotoxin has been found in some representatives of the leaf climber genus. Sustainably and irreversibly increases the permeability of membranes to sodium ions.
- Poneratoxin was found in ants of the genus Paraponera. Blocks sodium channels.
- Tetrodotoxin was found in the tissues of fish of the Skelezubovi family, from which the Japanese delicacy Fugu is prepared. Blocks sodium channels.
- The mechanism of action of most anesthetics (Procaine, Lidocaine) is based on blocking sodium channels and, accordingly, blocking the conduction of impulses along sensitive nerve fibers.
- 4-Aminopyridine - reversely blocks potassium channels, prolongs the duration of the action potential. Can be used in the treatment of multiple sclerosis.
- ADWX 1 - reversely blocks potassium channels. Under experimental conditions, it alleviated the course of acute disseminated encephalomyelitis in rats.