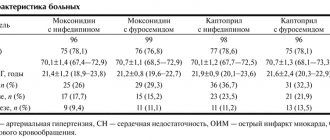
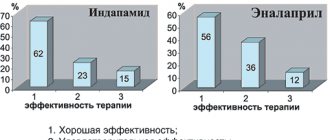
Why does discomfort occur?
The fact is that blood circulates in the human body all the time, even when the person is absolutely not susceptible. While blood enters the ventricles of the heart, the heart muscle spasms, and the blood in the ventricles under pressure comes out into the vessels. The process of filling the vessels occurs so quickly that pressure is created.
As soon as a failure occurs in this mechanism, a person begins to feel a deterioration in his general condition.
We can conclude that blood pressure depends on how intensely the heart contracts. As soon as you see that your blood pressure has deviated from the norm, you should immediately consult a doctor. It is possible that this is due to heart disease. If no heart problems are found, it is worth looking for a problem in other areas of the cardiovascular system.
Blood pressure, which is recorded at the moment the heart muscle pushes blood out of the heart, is called symbolic or upper pressure.
what is the difference between high and low blood pressure
Using systolic pressure, you can accurately determine the heart rate. Normally it is about 110-130.
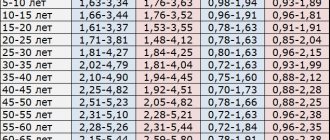
As a person ages, a person's blood pressure increases. After 50 years, a blood pressure of 140 is considered normal.
But the pressure on the walls of the arteries at the moment of complete relaxation of the heart muscle is called diastolic or lower pressure.
From this indicator one can judge the force with which blood moves through the vessels of the body. If a person is absolutely healthy, then the indicator can be 65-80.
As you noticed, the difference between blood pressure and heart pressure
65 and 80 is significant. It is determined by the individual characteristics of each individual person. If your vessels are in good tone and elastic enough, then the pressure will be about 75-80 units.
How does it all work? A few words about the structure of the heart and its parts
The structure of the heart in mammals with 2 circles of blood circulation is approximately the same. The heart consists of two atria (the first chambers along the path of incoming blood), two ventricles, valves between these chambers, and vessels entering and leaving the heart with valves at their beginnings.
Between the right atrium and the right ventricle is the right atrioventricular
, or a
trioventricular
, which consists of 3 valves.
Therefore, it is called tricuspid
, or
tricuspid
.
Between the left atrium and the left ventricle is the left atrioventricular valve
, which consists of two valves and is called
mitral
.
Valves
, located at the mouths of the vessels extending from the heart, or the main vessels, namely
the aorta
and
pulmonary artery
, are called
“aortic”
and
“pulmonary”
.
Atrioventricular valves
– casement, i.e. their structure resembles doors on the wings: opened and closed, down - up.
Aortic valves
and
the pulmonary artery are different in structure
. Each of them consists of 3 semilunar valves that close in the center. When they open, they press against the wall of their vessel (aorta or pulmonary artery), and close, completely closing the lumen of the vessel. Moreover, their appearance resembles a brand name.
The tissue of the valves themselves, both atrioventricular and semilunar, is thin, even transparent in children, but amazingly elastic and durable, designed by nature for continuous rhythmic work, amounting to billions of monotonous actions.
Between the cavities of the heart, or its chambers, there are partitions that separate the flow of venous and arterial blood. This is the interatrial septum, i.e. between the right and left atria, and the interventricular septum - between the right and left ventricles. In a normal, formed heart, they are completely closed, there are no holes or defects in them
and thus blood never flows from one half of the heart to the other.
Let us dwell in more detail on the anatomical structure of the heart and its chambers. After all, even those that have the same name (atria or ventricles) are structured completely differently and perform different functions.
The shape of the heart resembles a pear lying somewhat on its side, with the top located to the left and below, and the base to the right and above. The apex of the heart is the part of the heart whose movements can be felt if you place your palm on the chest in the fifth intercostal space to the left of the sternum. You can easily feel its push both in yourself and in your child. These are the movements of the apex of the heart with each contraction. The contractions are almost synchronous with the pulse, which can also be easily felt on the arm (where the forearm meets the hand) or on the cervical vessels. The pulse is the filling of the vessels with a wave of blood coming from the heart with each contraction. Pulse frequency and its rhythm are an indirect and easily accessible reflection of the activity of the heart itself.
The apex is the most mobile part of the heart, although the whole thing, all its departments, are in constant motion.
The work of the heart, its movement, consists of two alternating phases - contraction (systole) and relaxation (diastole).
The rhythmic, constant alternation of these phases, necessary for normal functioning, is ensured by the occurrence and conduction of an electrical impulse through a system of special cells - through the nodes and fibers of the conduction system of the heart
.
Impulses arise first in the uppermost, so-called sinus node
, then pass to the second,
atrioventricular node
, and from it - along thinner fibers - to the muscles of the right and left ventricles, causing contraction of all their muscles.
Right atrium
accepts venous blood from the vena cava, i.e. from the whole body and in addition the venous blood of the heart itself. This is the largest chamber in volume and, perhaps, the most stretchable chamber of the heart. If necessary, it can accommodate several times more blood than under normal conditions, i.e. has a gigantic “reserve” of volume. The wall of the right atrium consists of a layer of thin muscle fibers. In addition to the function of “receiving” venous blood, the right atrium performs the function of a cardiac pacemaker. Both main nodes of the conduction system of the heart lie in its walls.
The right atrium connects or, more precisely, opens into the right ventricle through the atrioventricular foramen
regulated
by the tricuspid valve
. This hole is wide enough to allow the entire volume of blood to pass from the atrium into the right ventricle during the period of relaxation of its muscles, i.e. in the diastole phase, and fill its cavity.
The right ventricle is a much thicker-walled muscular structure than the atrium. This is the most anterior part of the heart, lying immediately under the sternum. It is relatively stretchable when needed. The shape of its cavity resembles a new moon appearing in the sky. If you look closely, you can see how the luminous stripe of the month in a semicircle covers a large dark ball of the unlit part of the Moon. Likewise, the right ventricle flows around the powerful cylindrical left ventricle with its cavity.
Inside, this ventricle consists of two cones that extend into each other: the inlet cone and the outlet cone. They converge at their apices at the apex of the heart and are separated at the top by a muscular ridge, the so-called supraventricular crest.
The right ventricle opens into the pulmonary artery, which, together with the aorta, is the so-called main, or “great” vessel. At the transition from the ventricle to the pulmonary artery there is a tricuspid, semilunar valve of the pulmonary trunk, which allows blood to pass in one direction - to the lungs.
The left atrium is the most posterior of the cardiac chambers. It receives oxygenated, arterial blood from the pulmonary veins. There are only four veins and they flow into the posterior wall of the left atrium. The chamber of this atrium is much smaller than the right one, and its ability to stretch is significantly less.
The left atrium opens through the atrioventricular orifice into the left ventricle. This hole contains a bicuspid - mitral - valve, the opening and closing of which regulates the process of filling and emptying of the ventricle in the systole and diastole phases.
The left ventricle is the main one in the heart, and in the entire circulatory system. This is a powerful muscular chamber, the walls of which are 3-4 times thicker than those of its right neighbor. It is a compact cone with the inlet (with the mitral valve) and outlet (with the tricuspid aortic semilunar valve) lying next to each other and closely interconnected.
In order for this entire complex system to work harmoniously and efficiently, it must receive constant necessary nutrition in the form of oxygen and nutrients, and waste products must be removed. For this purpose, there are arterial and venous systems of the heart itself.
The arterial system of the heart itself consists of two - the left
and
the right
-
coronary (coronary) arteries
, which depart at the very beginning, at the mouth of the ascending aorta. These are its first branches. They immediately divide into smaller ones and distribute blood to all parts of the continuously moving heart. The “spent” blood, which has given up oxygen, flows through numerous small veins, which gather into one large one - the coronary sinus - and flow into the cavity of the right atrium. Thus, the heart feeds itself, and its function directly depends on the correct position and condition of the coronary arteries.
So, let's summarize. Anatomically, the heart is a powerful muscular organ with four chambers and four valves. The structure of the chambers and valves is different from each other, because subordinated to performing various tasks. The right parts of the heart are separated from the left by partitions and do not communicate with each other.
Quoted from the book by G. E. Falkovsky, S. M. Krupyanko. The heart of a child. A book for parents about congenital heart defects
How to get treatment at the Scientific Center named after. A.N. Bakuleva?
Online consultations
What is the normal difference between systolic and diastolic pressure?
Let's find out what difference in pressure is considered normal
. If we take the norm, which for an adult is 120/80 mm. rt. Art. , then it is easy to calculate the difference, which is 40 units.
But, if this difference becomes more than 65, this is a signal about the possibility of the rapid development of cardiovascular diseases. In addition, incorrect functioning of the heart and blood vessels leads to their early staining and, accordingly, acceleration of the aging process.
A difference of more than 45 or less than 35 units may indicate the appearance of pathological processes in the body. Symptoms may not appear with such a difference; sometimes general weakness and drowsiness occur.
If we talk about older people, then a difference of up to 50 units between systolic and diastolic pressure is considered the age norm. After all, their tissues are worn out enough and have lost the elasticity of blood vessels.
Diastolic heart failure and vascular stiffness
Academician Ivashkin V.T.: – I ask Oksana Mikhailovna to make another message. "Diastolic heart failure and vascular stiffness." Please. Professor Drapkina O.M.: – Thank you very much. Dear colleagues, today I talked about myocardial fibrosis, and, in principle, when talking about myocardial fibrosis, one way or another you are talking about diastolic heart failure. We at the clinic and at the department are dealing with this problem, accordingly, now there are also certain opportunities to share the experience that we have accumulated.
We are talking about diastolic heart failure, we are talking about arterial hypertension and we say that, after all, impaired diastole is the fate of patients with a high risk of arterial hypertension. But when the risk of arterial hypertension according to SCORE is high, we know that this is severe target organ damage and more than three risk factors or, for example, metabolic syndrome, when there are other risk factors (Sergei Rudzherovich spoke today, and we together recalled the Jupiter study ", where the marker in healthy people was an increase in high-sensitivity C-reactive protein).
So the question arises. We say all the time: “Patients come to us with myocardial infarction because we treated arterial hypertension incorrectly.” What if we treated arterial hypertension correctly? Then you get myocardial hypertrophy and, accordingly, an increase in rigidity. So what happens that chronic heart failure with preserved ejection fraction is the correct treatment for arterial hypertension and that there is no other way?
Of course this is not true. But nevertheless, with this slide I would like to draw attention to this postulate: delaying myocardial infarction in a patient with arterial hypertension is not our only task. The range of our tasks is much larger, including preventing this stage, when the patient moves from simply being hypertensive to being hypertensive with chronic heart failure, usually with preserved ejection fraction.
Therefore, once again a few words about terminology. We will talk about heart failure with preserved ejection fraction. In principle, this is the correct term, but we, the medical community, allow each other to call this heart failure diastolic heart failure - probably just for shorthand.
The epidemiology is known. There are slightly different data – European and ours. According to the EPOCHA study, more than 70% of patients with heart failure have preserved ejection fraction. Of course, this is the lot of the elderly, since we are all fighting for the fact that our population is getting older, we are all fighting for life expectancy. This will lead to the fact that there will also be more patients with diastolic heart failure, and we will need to manage them somehow for many years.
Here, in my opinion, is interesting data. The annual mortality rate of patients with diastolic heart failure is indeed somewhat lower. But when we looked at mortality after three years, it almost leveled off. But, most importantly, statistics say that among patients with chronic heart failure with an ejection fraction of more than 50%, less than 60% remain alive after three years, which means 40% die.
But here, I would say, is data that shocks me. If the prognosis improves with treatment for heart failure with preserved ejection fraction (and we see here the range since 1987, when we mainly talked about the cardiorenal theory of chronic heart failure), then please note, dear colleagues, nothing happens with the improvement in prognosis patients with preserved ejection fraction - just as 60% of them died three years later, strictly speaking, after diagnosis, everything remains so. This raises the question that we may not yet know exactly how to treat this form of chronic heart failure.
Well, very briefly I would like to remind you of the basic knowledge that we receive from the physiology course in the third year, the so-called equator (students say “equator”; having completed the third year means that you have completed half of your studies at the institute). This is the pressure-volume curve in the left ventricle, and it is the change in this curve that characterizes the main changes that occur in patients with rigid myocardium and first with diastolic dysfunction, and then with diastolic heart failure.
So, the end of diastole marks the phase of isovolumic contraction. We see that here the pressure in the left ventricle is rapidly increasing without changing its volume. The aortic valve opens and the same systole, or ejection phase, occurs. It was precisely in the shadow of this systole that there was always diastole, since we assessed the work of the heart precisely by how it contracts, by its contractile function.
Then the period of systole closes, the aortic valve naturally closes, and exactly the most interesting stage at the moment, at the time of my story today, occurs - this is diastole. The first period, it is precisely characterized by the stage of isovolumic relaxation (we see that it is very similar to the stage of isovolumic contraction), that is, a sharp decrease in pressure in the left ventricle, the mitral valve opens, and finally the filling stage occurs. You must understand that this stage is a fairly energy-intensive process, therefore, in order for diastole to be perfect, a sufficient number of macroergs is needed.
Well, the mitral valve closes, and we see (where the point that shows the mitral valve closes) that the end of diastole occurs at such a positive end-diastolic pressure. So, if the left ventricle is rigid, then this slight rise will be steeper, and the pressure that will be in the left ventricle will be higher.
The graph is two-dimensional, so it is impossible here - at least I could not - show the time, the so-called “tau”, of isovolumic relaxation. This is a very important indicator, and we will return to it below.
Thus, we can summarize those factors that influence diastole. We can summarize them by dividing them into two, in my opinion, absolutely unequal, but quite important groups. The first is, of course, the work of the ventricle, this is the early diastolic filling of the ventricle. And it is precisely factors such as: elastic return of blood to the left ventricle, contractile function of the left ventricle, stiffness of the left ventricle in diastole, properties of the pulmonary veins, area of the mitral valve opening that make an 80 percent contribution to full diastole.
But there is still a small contribution, and nevertheless, we should not forget about it either, especially when, for example, a patient comes to us with rhythm disturbances, and chronic heart failure becomes decompensated. This is the contribution of the atrium. And here the following are very important: the PQ interval, the contractile function of the atrium itself (now there are methods that evaluate this contractile function, they are not invasive - echocardiographic), heart rate (of course, the less often the heart beats, the better the diastole) and the activity of the sympathetic nervous system .
Thus, the portrait of a typical patient with arterial hypertension and diastolic heart failure is an elderly woman (usually a woman, although, of course, it can also be a man) with a long history of arterial hypertension, which is still poorly treated; with a bouquet of other pathologies, which also contributes to the possible processes of fibrosis in the heart of such patients: this is diabetes mellitus, this is coronary heart disease, this is supraventricular rhythm disturbance, kidney dysfunction, which we determine by the glomerular filtration rate, and with vascular fibrosis.
There are key differences, and we can detect them already at the stage of getting to know the patient and at the stage of his examination. Muffled heart sounds are characteristic of systolic heart failure with reduced ejection fraction. With the ejection fraction preserved, the tones can be quite decent, and even the first tone can be preserved.
Most often, we hear a gallop rhythm in both cases, and just if we are talking about systolic heart failure, then it manifests itself with the appearance of a proto-diastolic gallop rhythm - due to S3, an additional tone, a third tone. And finally, diastolic heart failure, where the greater contribution of the atria, exhausted by high end-diastolic pressure in the left ventricle, is S4.
Diastolic heart failure sometimes progresses rapidly to pulmonary edema. And here is also amazing data. Dmitry Alexandrovich, I want to discuss them with you. It turns out (I came across this article) when... Patients with pulmonary edema, completely clinically justified pulmonary edema, are admitted to the intensive care unit, and they all had their left ventricular ejection fraction measured. Then the pulmonary edema was stopped and the left ventricular ejection fraction was measured again. So, there was no pattern. It would seem that if pulmonary edema was stopped, the ejection fraction should increase, or if you were admitted with pulmonary edema, the ejection fraction should be low. There were patients with a high ejection fraction during the culmination of pulmonary edema, and vice versa.
How do you think we, as clinicians, should evaluate this, since we so often rely on this parameter - the ejection fraction?
Professor Zateyshchikov D.A.: – Well, as they say, so much the worse for the ejection fraction. In fact, many have been saying for a long time that the ejection fraction is, so to speak, not a very good parameter. And, obviously, with the advent of new non-invasive techniques - tomography, magnetic resonance imaging - we will have a more accurate method of assessment. And, perhaps, what we today accept as this very diastolic dysfunction will partially move into another category, into the systolic category. Hard to say.
But in any case, in my opinion, this once again shows that this is not the only parameter: systole is not only systole, but also something else.
Professor Drapkina O.M.: – And yet the clinic, the clinical picture is what should mainly determine our attitude towards this patient and his prognosis.
Let's get back to the slides. Thus, there are disputes, they are not over. Is this one syndrome, in which provoked diastole is, as it were, a precursor to reduced ejection fraction, or are there two syndromes? There are both supporters and opponents of various theories. To be honest, I take the position that these are still two different syndromes. Because even the portrait of these hearts, which we see during a pathological autopsy, is so different that the question immediately arises: how can such different hearts be treated with the same approaches? Well, this question is probably still eternal, there is no answer to it.
Nevertheless, we must treat such patients; we must diagnose heart failure with preserved ejection fraction. As always, algorithms come to the rescue.
The algorithm of the European Society of Cardiology also places the clinical picture of chronic heart failure at the forefront. But translating into our daily practice, this is an obese patient who came in with shortness of breath. Then normal or only slightly reduced systolic function. Let us again translate to our realities, this is a woman who came with shortness of breath, we listened to her, examined her, we see that she is obese, we see that there are fine rales in the lungs.
We send her for an echocardiographic study to clarify the diagnosis and are surprised to see that the ejection fraction is normal. This is not a reason to send this patient to a pulmonologist, this is a reason to give such a patient at least a six-minute walk test. And we will see that she may not walk the same 450 meters that would not classify her as a patient with heart failure.
Then there's really a lot of focus on biomarkers of chronic heart failure, particularly natriuretic peptide and its terminal fragment. If it is greater than 220 and we have Doppler signs, that is, we do a regular Doppler study and see when assessing flows that the E to A ratio is less than 0.5, or just the left atrium is large, or the patient has atrial fibrillation, then we can say that we have a patient with diastolic heart failure.
But, in fact, there are more difficulties than any substantiated facts, since none of the echocardiographic criteria is sufficiently accurate today. We say that tissue Doppler is very good, and this ratio E/E' is the best, but also not ideal, characterizes diastole. It is not readily available to conduct (exactly what we were talking about) MRI and various invasive hemodynamic studies. Well, in my opinion, the exact threshold values of various biological markers of heart failure have not been determined.
This raises the question: maybe we should look for possible mechanisms of diastole development in the periphery? Why? Firstly, with diastolic heart failure, certain moments of aortic stiffness also arise, but the aorta is a second heart if it is elastic. If there is an increase in the stiffness of blood vessels in general, then these reflected waves (we’ll talk about them a little later), which gather at the periphery, go to the center, to the heart. They arrive, it turns out, just at the moment of systole, they arrive a little later than they should have arrived, and collide with the newly formed pulse wave.
Again the question: does diastolic heart failure begin in the periphery? We know that blood vessels in arterial hypertension are both the culprits and the victim. Because the high pressure itself acts on the blood vessels, and at the same time the stiffness of the blood vessels, for other reasons, spasm can lead to high blood pressure.
If we talk about the main mechanisms, then, probably, the main mechanism that is responsible for the development of diastolic heart failure is precisely the increase in preload due to the stiffness of peripheral vessels. As I have already said, the speed of propagation of pulse waves increases, the preload on the left ventricle increases and the central pulse pressure increases. Right now, central pulse pressure, or pressure in the aorta, and pressure variability are given the greatest importance in the prognosis of hypertensive patients.
How does this happen? Let's imagine our vessels. Basically, these are tubes. They are elastic. And if they are elastic, as shown in the first figure, to release a blood flow - a large blood flow, since blood spreads at high speed... An elastic vessel, as it were, dampens this wave, that is, it expands a little, and then returns to normal, and we see such a schematic pulse wave, which is shown under the elastic aortic capillary tube.
If the vessel is rigid, then it cannot dampen the wave, it cannot relax a little and then contract again. And this leads to the so-called pulse wave augmentation phenomenon.
Here, dear colleagues, we can consider this phenomenon in this diagram. The gray line shows the pulse wave, which reflects the ejection of blood from the left ventricle into the aorta. The dotted line, which is shifted slightly to the right, depicts a reflected wave that comes from the periphery, from our vessels, which have damping abilities, to the center. And when these two waves are layered on top of each other, the presence of a type “C” wave occurs, or a pulse wave of type “C”, which characterizes all healthy people.
Now the arrows will show what happens in hypertensive patients, what happens with a stiff aorta. So, this graph shifts to the left, this graph shifts upward, and the formation of the so-called “A” or “B” wave occurs, which characterizes an increase in preload on the left ventricular myocardium and a decrease in diastolic blood pressure.
Thus, the analysis of this problem, in my opinion, makes it possible to provide a diagram to help the practicing physician. An increase in vascular stiffness leads to an increase in central systolic pressure in the aorta and a decrease in diastolic pressure, hence the pulse pressure increases. Increased systolic pressure in the aorta leads to increased preload on the left ventricle. Hypertrophy and the preload itself on the left ventricle and impaired relaxation lead to subendocardial ischemia. Low aortic diastolic pressure results in decreased coronary perfusion because the coronary artery fills during diastole. This diastolic pressure, which is not very low, is also important. A study of the aorta confirmed that reducing diastolic pressure below 70 for cardiovascular events is bad. Accompanying myocardial fibrosis leads to diastolic dysfunction.
We decided to conduct a pilot study in order to: evaluate the degree of damage to the endothelium and vessels of different sizes in patients with diastolic heart failure; compare different methods for assessing endothelial function - photoplethysmography and AngioScan; and see what happens during therapy based on different classes of drugs - ACE inhibitors and calcium channel blockers.
We did not forget to pay attention to biological markers. Along with the well-known N-terminal prohormone brain natriuretic peptide, we also looked at the level of galectin-3 in patients with diastolic heart failure. This is a marker, or a biological substrate that is largely responsible for fibrosis - everything: the liver, blood vessels, heart, and so on.
The study design is presented. Patients are high risk with preserved ejection fraction. We analyze endothelial function using all possible methods, randomize patients to a treatment group based on calcium channel blockers and on the basis of ACE inhibitors (amprilan was chosen as the inhibitor). We observe patients for 3-5 weeks in the hospital and then repeat the same studies.
Dear colleagues, I will not dwell on the general characteristics of the patient groups, I will only say that they were comparable in age, body mass index, and various clinical characteristics.
The first day of the study, or the start of the study, was extremely important for us, since we determined all those indicators that can be viewed during contour analysis of the pulse wave: the duration of systole, the age of the vascular wall, the augmentation index, and the stiffness index. In addition, on the same day we did an occlusion test, as if we confirmed it with each other - both by the photoplethysmographic method and according to ultrasound data.
Of course, doing this showed us that it is extremely difficult to look at endothelial function and occlusion test using ultrasound: sit in a certain position for five minutes, clamp the artery for five minutes, pump 300 mm Hg... Therefore, of course, the photoplethysmographic method won us over namely simplicity.
This is what the “C” wave approximately looked like in normal vessels.
Results. The first conclusion we made was that all patients with diastolic heart failure have pulse wave changes. Waves of type “B” and “A” are recorded, which are characterized by a high amplitude and a large range between systole and diastole.
Results of pulse wave contour analysis before the start of therapy. We see that our patients do not differ, either in the stiffness index, or in the reflection index, or in the pressure in the central vessel.
What happened during therapy? It's good to have both. That is, the lower we reduce the pressure, the better for the vessel of our hypertensive patient. Nevertheless, amprilan had a slightly greater impact on the hardness index. And amprilan also had a slightly greater effect on pressure in the aorta.
The results of the test with reactive hyperemia are an occlusion test. We also see that both groups demonstrated good results. However, a slightly greater effect of ACE inhibitors on the vascular wall was also proven in this study.
The average level of NT-proBNP hormones was 153, the average level of galectin-3 was 0.98.
The assessment of endothelial vascular function occurred in this way. We see that after an occlusion test that lasted five minutes... we would like to see an increase in diameter in the brachial artery, and below exactly what happens to the pulse waves is shown.
We found that there was an association between NT-proBNP levels and a lot of parameters, such as the 6-minute walk test, the patient's Clinical Status Rating Scale, which we use in patients with heart failure.
There was no correlation between NT-proBNP levels and left ventricular ejection fraction and parameters of diastolic dysfunction.
As for galectin-3, it turned out that once galectin-3 increased, it did not change. Which, in general, is consistent with literature data. And therefore, we propose the combined use of galectin-3 and natriuretic peptide; this can be assessed to a greater extent as a prognosis for the course of the disease.
This is a combined application. In the first column - the meaning, in the second column - the interpretation. And the risk of an unfavorable outcome, we see, is highest when there are high levels of galectin-3 and natriuretic peptides.
And finally, a clinical example. An elderly patient, over 60 years old. We see that her vessels are stiff. Both the rigidity index, the reactivity index, and the augmentation index are high. Against the background of the use of amprilan, we see a change in the pulse wave, this is visible, so to speak, visually. In addition, this, of course, can be seen from the calculated indices that exist. Please note that the reactivity index decreased from 24 to almost 8.9, and how much the central pressure decreased!
This is naturally reflected in improved endothelial function. That is, an increase... the top picture compared to the bottom picture... we see an increase in the amplitude of pulse waves by 1.3 times.
Thus, dear colleagues, the analysis of this problem makes it possible to draw such preliminary conclusions for now. It is probably true to say that diastolic heart failure begins from the periphery; in any case, vascular stiffness makes a certain contribution to changes in diastole.
The second conclusion is that biological markers of chronic heart failure are again worthy of further study, and the new marker galectin-3 for the prognosis of patients with diastolic heart failure is likely to be very promising.
And finally, our study demonstrated that lower the pressure with something, lower it to normal values, to the values that are given to us by recommendations. But still, the use of ACE inhibitors, and in particular amprilan, in our study suggested a better effect on endothelial function and a better effect on the function of vascular resistance in patients with arterial hypertension and diastolic heart failure. Thank you for your attention.
Conclusion
The optimal difference between systolic and diastolic pressure is 70
is 40 mmHg. But a slight deviation of 10 units up or down is also within acceptable limits.
blood pressure prevention
If the pressure changes by 20 more or less, and you feel normal, most likely there was an incorrect pressure measurement. If your health really gets worse, you should immediately consult a doctor.
In case of difference between systolic and diastolic pressure
is sixty or more, this is the risk of developing heart and vascular diseases. In this case, you must constantly consult your doctor. To make an appointment with our specialist, leave a request on the website or call.
Myocardial ischemia and impaired left ventricular diastolic function
In recent years, the attention of many researchers has been attracted by the possibility of studying myocardial function in the diastole phase, i.e. diastolic function of the left ventricular myocardium.
Interest in this problem is based on the fact that a number of studies have demonstrated the leading role of impaired diastolic function of the left ventricle in the development of heart failure in many diseases. It is also known that some rhythm disturbances are accompanied by symptoms of diastolic dysfunction. All of the above makes the problem of studying the process of relaxation of the left ventricle very relevant.
The data accumulated to date indicate that the diastolic filling of the left ventricle is determined by many factors, among which the greatest importance is given to the active relaxation of the left ventricular myocardium in the early phase of diastole, the elastic properties of the myocardium itself, in particular, the degree of its rigidity, the pressure that is created in the left the atrium at the time of its systole, the state of the mitral valve and associated subvalvular structures [1-4]. In various heart diseases, pathological changes in the left ventricular myocardium itself can lead to disruption of the diastolic function of the left ventricle.
It is customary to distinguish the following periods of diastole:
the period of early diastolic filling of the left ventricle, which consists of a phase of fast and slow filling, and the period of late diastolic filling of the left ventricle, coinciding with the systole of the left atrium [8,13]. The volume of blood flow through the mitral valve and its velocity during early diastolic filling is determined by the active energy-dependent relaxation of the left ventricular myocardium, chamber stiffness [2,3] and the level of pressure in the left atrium at the beginning of left ventricular diastole [4,5]. A number of studies have shown that relaxation of the left ventricle in early diastole is an active energy-dependent process controlled by such basic mechanisms as the load of contraction, relaxation, and heterogeneity of load distribution [2]. The period of early diastolic filling of the left ventricle is influenced by the diastolic deformation of the ventricular cavity, as well as intraventricular pressure at the moment of opening of the mitral valve [1,2]. The combination of the effects of these factors creates the so-called suction function of the left ventricle, which determines the movement of part of the blood volume from the cavity of the left atrium to the cavity of the left ventricle. At the end of rapid filling, the pressure difference between the left chambers decreases, and a slow filling phase begins, during which the gradient between the atrium and the ventricle is small and the blood flow from the atrium to the ventricle is small. By the time of left atrium systole, this gradient begins to increase again, which is manifested in the re-acceleration of blood flow through the mitral valve [5].
During atrial systole, the volume of transmitral blood flow entering the left ventricular cavity depends on the pressure in the left atrium during systole, on the rigidity of the walls of the left ventricle, and the end-diastolic pressure in the ventricular cavity. An additional factor influencing the filling process should also be considered blood viscosity [1,2]. Normally, the volume and velocity of blood flow through the mitral valve during early diastole significantly exceed these values during atrial systole.
Methodological issues for determining diastolic function
In recent years, with the introduction of Doppler cardiography into widespread practice, it has become possible to measure transmitral blood flow velocities in various periods of diastole non-invasively.
It should be noted that a Doppler study of transmitral blood flow can reliably verify only the phase of early fast diastolic filling and the phase of atrial systole, since the L wave, reflecting slow diastolic filling, can be detected on a Dopplerogram only in 25% of cases and, moreover, is very variable in magnitude and duration [1].
In the absence of disturbances in the diastolic function of the left ventricle in healthy young and middle-aged individuals, the peak velocity E (Emax) and the area under the curve E (velocity integral E, denoted Ei) exceed the value of the peak and integral velocities A
(Amax and Ai, respectively) [1.6-8]. According to various authors, the ratio of the velocities of the periods of early and late diastolic filling of the left ventricle ranges from 1.0 to 2.2 for velocity integrals and from 0.9 to 1.7 for peak velocities. The time of isometric relaxation of the left ventricular myocardium, measured by simultaneous recording of mitral and aortic flows, also largely depends on age, most often it is 74 ± 26 ms [1,2].
A number of studies have also shown the relationship between the increase in the contribution of the atrial component of diastolic filling of the left ventricle and the age of the subjects, which is expressed by a decrease in the ratio of the rates of the early and late diastolic filling periods due to an increase in the rates of the atrial systole period and a decrease in the rates of the early diastolic filling period. It should also be noted that the data on phase analysis of diastole in the literature are incomplete and heterogeneous in terminological definition, which requires further study of this issue.
Based on the above, we can conclude that normally the diastolic function of the left ventricle is determined by the following most significant points:
diastolic deformation of the left ventricle, pressure in its cavity at the time of opening of the mitral valve, rigidity of the walls of the left ventricle, preservation of the structures of the mitral complex and the rheological properties of the blood itself.
Impaired diastolic function in myocardial ischemia
In the presence of chronic myocardial ischemia, the rigidity or rigidity of its walls increases [4,6,9]. In particular, a number of researchers have convincingly shown the existence of a close correlation between the diastolic properties of the heart and the maximum oxygen consumption of the myocardium at rest and during exercise.
At the current level of development of this issue, the pathogenetic mechanism of impaired diastolic relaxation of the left ventricle is as follows:
insufficient oxygen supply to the myocardium leads to a deficiency of high-energy compounds, which in turn leads to a slowdown in the process of early diastolic relaxation of the left ventricle.
These changes affect the process of filling the ventricular chamber in early diastole: due to a slower than usual decrease in pressure in the left ventricular chamber, the moment when the pressure levels between the ventricle and atrium are comparable is reached later. This leads to an increase in the duration of the period of isometric relaxation of the left ventricular myocardium. Once the mitral valve opens, the pressure gradient between the ventricle and atrium is less than normal and, therefore, early diastolic filling flow is reduced. A kind of compensation is provided during atrial systole, when the volume of blood required for adequate filling of the left ventricle enters during active contraction of the atrium chamber. Thus, the atrial contribution to the formation of the stroke volume of the chamber increases [1–4, 6, 8, 10]. The above hemodynamic changes are attributed to the early type of ventricular diastole disorder, in which there is no significant increase in pressure in the chamber of the left atrium, and, accordingly, changes in the hemodynamics of the pulmonary circulation and signs of congestive heart failure are not observed [2,11].
The explanation of the pathogenetic aspects of the influence of ischemia in patients with impaired diastolic function of the restrictive type looks much more complicated. For the formation of this type of diastole disorder, the following main points are necessary: high end-diastolic pressure in the cavity of the left ventricle, formed by the significant rigidity of its myocardium [2,11,12], high pressure in the cavity of the left atrium [4,11,13], ensuring adequate filling of the ventricle in early diastole, decreased systolic function of the left atrium. Most authors in this regard point to the rather rare occurrence of a restrictive type of diastole impairment in patients with coronary artery disease [4, 6], since high myocardial stiffness is more often associated with its organic damage, for example, with restrictive cardiomyopathy, infiltrative cardiopathy [7,8]. Patients with coronary heart disease are characterized by the presence of focal myocardial pathology and the formation of high myocardial stiffness
due to prolonged, chronic ischemia and the development of fibrosis.
Thus, today it is quite obvious that myocardial ischemia has a negative effect on the process of diastolic filling of the left ventricle. Therefore, it is advisable to also touch upon the issues of diagnosing impaired diastolic function in the category of patients under consideration.
Diagnostics
Doppler cardiography has become increasingly important in recent years.
[8,11,12]. It is generally accepted today to distinguish 2 types of impaired diastolic function of the left ventricle according to Doppler cardiography data [8].
1st type
, in which, as a result of a violation of the early phase of ventricular diastole, the speed and volume of blood flow through the mitral orifice in the early phase of diastole (Epic) decrease and the volume and speed of blood flow increase during atrial systole (Apic), while an increase in the time of isometric relaxation of the left ventricular myocardium is noted ( VIRM) and prolongation of the deceleration time (SD) of flow E.
Type 2, designated pseudonormal
, or restrictive, which assumes the presence of significant rigidity of the ventricular myocardium, which leads to an increase in diastolic pressure in the ventricular chamber, and then in the atrium, and the pressure in the atrium chamber can significantly exceed the pressure in the ventricular cavity by the time the latter begins diastole, which ensures the presence of significant pressure gradient between chambers at the beginning of diastole; at the same time, the nature of the transmitral blood flow changes: Epik increases and Apik decreases, and the previously indicated time intervals (VIRM and VZ) are shortened.
A number of authors suggest dividing disorders of left ventricular diastolic function into 3 types: early, pseudonormal and restrictive
. Thus, E. Braunwald [2] proposes to differentiate the pseudonormal type of disorder from the normal and restrictive type based on the duration of the slowdown of the E peak of early filling, which, as is known, is shortened in pseudonormal and restrictive types of diastole disorder. The validity of this approach is questionable in light of the presence in the literature of data on a significant influence on the duration of diastole time intervals of heart rate at the time of the study.
Other authors point out the possibility of differentiating between the pseudonormal type of disorder and the norm by assessing flows in the pulmonary veins. With the pseudonormal type, there is an increase in pressure in the left atrium, which affects the filling nature of the left atrium [11].
The role and place of color Doppler M-modal echocardiography in the differential diagnosis between the above types of left ventricular filling is not entirely clear today. A number of authors believe that this technique helps to distinguish the pseudonormal type of filling from the restrictive and normal ones [1,2], while at the same time the question remains open about the degree and nature of the influence of factors such as heart rate, blood viscosity on the accuracy of measurements in this mode , the state of the myocardium of the left atrium, etc. It seems that color Doppler mapping in this situation does not have any fundamental advantages over a conventional Dopplerogram, because with M-modal scanning of a color Doppler image, the time intervals described above are also measured, which means that the influence remains all previously mentioned limiting factors.
It is important to study segmental diastolic function
using the Doppler tissue imaging method with M-modal scanning [8,11]. The use of this method makes it possible to assess not only the general state of diastolic function, but also the nature of relaxation of individual segments, which is especially important when assessing the effect of myocardial ischemia on these parameters at rest and during stress tests.
Clinical significance of left ventricular diastolic dysfunction and the possibility of drug intervention
IHD is one of the most common causes of left ventricular diastolic dysfunction [1,4,6] due to impaired early diastolic relaxation against the background of acute or chronic ischemia, increased myocardial stiffness at the site of the post-infarction scar and the formation of connective tissue against the background of chronic ischemia. In addition, an increase in the stiffness of hypertrophied intact myocardium in patients with coronary artery disease may be associated with ischemia due to coronary insufficiency
due to stenosis of the artery supplying blood to this area of the myocardium, and as a result of relative coronary insufficiency, which often occurs with hypertrophy. It is also known that diastolic dysfunction can occur without impairment of left ventricular systolic function [2,14]. But impaired diastolic function, even in isolated form, leads to a significant deterioration in central hemodynamics and may contribute to the onset or progression of pre-existing systolic heart failure [11,14].
The prognosis for patients with coronary heart disease who have diastolic dysfunction is more unfavorable [1,2,14], which makes the problem of its drug correction urgent.
Few studies have been devoted to the issues of drug therapy for impaired diastolic function in patients with coronary artery disease. In addition, to date there is no large study on this issue. In recent years, the scientific literature has published mainly experimental work on animals devoted to studying the effect of antianginal drugs of various groups
, as well as
ACE inhibitors (enalapril - SOLVD - investigators)
on the process of diastolic relaxation of the myocardium [15,16,17,18].
According to the results of these studies, the greatest effectiveness was noted with the use of calcium antagonists, b-blockers, and ACE inhibitors
.
For example, E.Omerovic et al. (1999) demonstrated the positive effect of the selective b1-blocker metoprolol
on the state of systolic and diastolic function of the left ventricle during myocardial infarction.
There are also separate clinical works devoted to this issue. A. Tsoukas et al. (1999), studying the effect of combination therapy with diuretics and ACE inhibitors
on the state of central hemodynamics in patients with a restrictive type of transmitral blood flow and reduced left ventricular ejection fraction (<40%), noted a positive effect of this combination of drugs in 25% of patients.
Elimination of diastolic dysfunction in the presence of myocardial ischemia is largely determined by the adequacy of individually selected antianginal therapy or surgical revascularization of the myocardium [7,14,16].
For this purpose,
calcium antagonists (in particular amlodipine), b-blockers, and nitrates are most often used.
The data of C. Stanescu et al. are also interesting. (published in the proceedings of the 21st Congress of the European Association of Cardiology in 1999) on the frequency of prescription of various groups of drugs in patients with heart failure of various etiologies (coronary artery disease - 35%, hypertension - 24%, valvular heart disease - 8%, cardiomyopathies - 3 %, other reasons - 17%). According to these authors, of 1360 patients hospitalized for heart failure, diastolic heart failure was diagnosed in 38% of cases. After an echocardiographic study, the frequency of prescription of various drugs in these patients was as follows: diuretics - 57%, calcium antagonists - 44%, b-blockers - 31%, ACE inhibitors - 25%, cardiac glycosides - 16%. While before echocardiographic examination and determination of the presence of diastolic form of heart failure, the frequency of prescription of the above drugs in these patients was as follows: diuretics - 53%, calcium antagonists - 16%, b-blockers - 10%, ACE inhibitors - 28%, cardiac glycosides - 44%. Thus, after the echocardiographic study, calcium antagonists were prescribed 3 times more often, and cardiac glycosides - less often than before the study.
In conclusion, it is advisable to note that the problem of correcting diastolic dysfunction in coronary patients is far from being resolved.
Some issues regarding the diagnosis of diastolic dysfunction remain controversial, and there is no consensus regarding drug therapy. It seems that many aspects of this problem will be resolved when the results of large studies appear on the effect of therapy on the state of diastolic function in coronary patients. Literature
1. Barats S.S., Zakroeva A.G. Diastolic cardiac dysfunction in terms of transmitral blood flow and flow in the pulmonary veins: controversial issues of pathogenesis, terminology and classification. Cardiology 1998; 5: 69-76.
2. E. Braunwald ed., Heart disease, 5th Ed., WB Saunders company 1997.
3. Caash WH, Apstein CS, Levine HJ et al. Diastolic properties of the left ventricle. In.- The LV-basic and clinical aspects. Ed. HJLevine. Boston. 1985; 143.
4. Choong CY Left ventricle: diastolic function — its principles and evaluation.-Principles and practice of echocardiography. Ed. A. Weiman. Philadelphia. Lea and Febiger. 1994; 1721-9.
5. Bonow PO, Frederick 1.M., Bacliarach SJ et al. Atrial systole and left ventricular filling in Hypertrophic cardiomyopathy: effect of verapamil. Amer J Cardiology 1983; 51:1386.
6. Baschinsky S.E., Osipov M.A. Diagnostic value of studying left ventricular diastolic function during stress Doppler echocardiography in patients with coronary heart disease. Cardiology 1991; 9: 28-31.
7. Bessen M., Gardin JN. Evaluation of left ventricular diastolic function. Cardiol.Clinics 1990; 18: 315-32.
8. Feigenbaum H. Echocardiography.- 5th Edition.- Lea & Ebiger.-Philadelphia. 1994; 166-72,189-91.
9. Zhelnov V.V., Pavlova I.F., Simonov V.I., Batishchev A.A. Diastolic function of the left ventricle in patients with coronary heart disease. Cardiology 1993; 5:12-4.
10. Dobrotvorskaya T.E., Suprun E.K., Shukov A.A. The effect of enalapril on systolic and diastolic function of the left ventricle in congestive heart failure. Cardiology 1994; 12: 106-12.
11. Christopher P., Appleton MD Doppler assessment of left venricular diastolic function: the refinements continue. JACC 1993; 21(7): 1697–700.
12. Cecconi M., Manfrin M., Zanoli R. et al. Doppler echocardiographic evaluation of left ventricular end-diastolic pressure in patients with coronary artery disease. J Am Soc Echocardiol 1996; 110: 241–50.
13. Castello D, Vaughn M, Dressler FA et al. Relation between pulmonary venous flow and pulmonary weige pressure: influence of cardiac output. Amer Heart J 1995; 130: P.127-31.
14. Vasan RS, Benjamin EJ, Levy D. Congestive heart failure with normal left ventricular systolic function. Arch Intern Med. 1996: 156: 146-57.
15. Barbier R., Tamborini G., Alioto G., Pepi M. Acute filling pattern changes of the failing left ventricle after captopril as related to ventricular structure. Cardiology 1996; 87: 153–60.
16. Goldstein S. Beta-blockers: insights into the mechanism of action in patients with left ventricular dysfunction. J Heart Failure. 1996: 13: 115.
17. Poultur H., Rousseau M.F., van Eyll C., et. al. Effects with long-term enalapril therapy on left ventricular diastolic properties in patients with depressed ejection fraction. SOLVD Investigators. Circulation 1993 Aug 88: 2 481-91
18. Sasaki M., Oki T., Inchi A., Tabata T., et. al. Relationship between the angiotensin converting enzyme gene polymorphism and the effects of enalapril on left ventricular hypertrophy and impaired diastolic filling in essential hypertension: M-mode and pulsed Doppler echocardiographic studies. J Hypertens 1996 Dec 14:12 1403-8
Enalapril –
Ednit (trade name)
(Gedeon Richter)
Amlodipine –
Amlovas (trade name)
(Unique Pharmaceutical Laboratories)
| Applications to the article |
| Diastolic filling of the left ventricle is determined by many factors, among which the greatest importance is given to the active relaxation of the myocardium of the left ventricle in the early phase of diastole, the elastic properties of the myocardium itself, in particular, the degree of its rigidity, the pressure that is created in the left atrium at the time of its systole, the state of the mitral valve and associated subvalvular structures |
| IHD is one of the most common causes of left ventricular diastolic dysfunction due to disruption of early diastolic relaxation against the background of acute or chronic ischemia, increased myocardial stiffness at the site of the post-infarction scar and the formation of connective tissue against the background of chronic ischemia |
| In patients with coronary heart disease who have diastolic dysfunction, the prognosis is more unfavorable, which makes the problem of its drug correction relevant. |
Pericarditis - symptoms and treatment
The first non-pharmacologic recommendation for patients is to limit physical activity to normal sedentary activities until symptoms resolve and CRP levels return to normal. This often happens within a few days [9][10].
Drug therapy
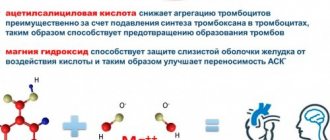
Anti-inflammatory therapy is the leading method of treating acute pericarditis. Acetylsalicylic acid (ASA), Ibuprofen and Colchicine are used. They are recognized as the main drugs for the treatment of acute pericarditis not associated with specific infections, such as tuberculosis. ASA is considered the preferred drug. If you are intolerant, take Ibuprofen.
NSAIDs are also used to treat recurrent pericarditis. In addition to Ibuprofen, the anti-inflammatory drug Indomethacin is used. The dosage of medications and duration of use are determined by the attending physician.
NSAIDs increase the risk of developing gastrointestinal bleeding, therefore, starting from the first hours of treatment for acute pericarditis, be sure to take medications that protect the stomach from erosions and ulcers.
Colchicine is recommended in patients with acute pericarditis in addition to ASA or other NSAIDs. The drug increases the effectiveness of drug therapy and reduces the risk of relapse. The course lasts until symptoms completely disappear and CRP drops below 3.0 g/l (usually at least six months). Long-term use of Colchicine and its gradual withdrawal can reduce the risk of relapse [9][10].
Glucocorticosteroids (GCS) are used to treat acute pericarditis if ASA and NSAIDs are contraindicated, are not effective enough, or for specific indications (for example, pericarditis caused by autoimmune diseases). GCS, in particular Prednisolone in low doses, reduce the risk of relapse, complications after treatment and other side effects.
GCS therapy quickly eliminates the symptoms of pericarditis. To reduce the risk of relapses, and especially in case of recurrent pericarditis, the dose of GCS is reduced slowly. In case of relapse, every effort should be made not to increase the dose of the drug or restart corticosteroid therapy.
Taking corticosteroids often causes steroid dependence, which is manifested by inflammation even with a slight reduction in doses. Side effects of GCS include fluid retention, edema and electrolyte imbalance.
The use of Azathioprine in combination with GCS is most effective for autoimmune causes of pericarditis. Common side effects include mild increases in aminotransferases, decreased white blood cell counts, and digestive disturbances [7][9][10].
Anakinra (IL-1 receptor antagonist) is effective for recurrent pericarditis: the drug reduces the risk of relapse by six times, hospitalization by seven times, and admission to the intensive care unit by 11 times, and also significantly reduces the need for GCS [10].
Intravenous immunoglobulin is widely used in the treatment of autoimmune diseases. The drug is quite safe, but the effect of therapy is short-lived, and it is rarely used for pericarditis.
Specific antimicrobial treatment is indicated for purulent pericarditis. This rare disease is life-threatening, but with adequate treatment, most patients recover. Intravenous antimicrobial therapy until culture results are obtained is prescribed empirically, i.e., depending on the body's response to therapy. To prevent the appearance of closed “pockets” in the pericardial cavity (often found in purulent pericarditis), it is necessary to promptly drain the pericardial cavity .
Pericardial drainage is indicated for the treatment of cardiac tamponade [11].
In the early stages of pericarditis, intrapericardial thrombolysis is performed - this is a drug therapy that helps prevent the development of constrictive pericarditis.
Specific antiviral treatment is indicated for confirmed viremia, a condition in which viruses enter the bloodstream and spread throughout the body. This therapy is especially important for immunodeficiency [10].
Surgery
In many cases, surgery becomes the only suitable method of treatment, but it is performed according to strict indications: if pericarditis affects intracardiac circulation, with constrictive pericarditis and calcification of the pericardium (“armored” heart).
Pericardectomy (removal of the pericardium) may be recommended for patients with chronic recurrent exudative pericarditis with shortness of breath, chest pain and inability to perform household activities. It is also performed for severe chest pain that is not relieved by drug therapy.
After pericardiectomy, intracardiac circulation is restored in 60% of patients. Pain is usually significantly reduced, but residual chest pain may persist[3][10].
The risk of death during surgery is 10–20%. It is especially high in congestive heart failure, renal failure, diabetes mellitus, chronic obstructive pulmonary disease, chest irradiation, and previous cardiac surgery [10].
Diastole
A Wiggers diagram showing various events during diastole. During early ventricular diastole -
see the vertical bar labeled “Isovolumetric Relaxation”—the pressure in each ventricle (blue curve) begins to fall rapidly from the wave height reached during systole.
When ventricular pressure drops below the atrial chambers, the atrioventricular (mitral and tricuspid) valves open, causing the volume of blood (red trace) in the atria to flow into the ventricles. + In late ventricular diastole,
the two atrial chambers begin to contract (atrial systole), causing an increase in blood pressure in both atria and forcing additional blood volume into the ventricles. This onset of atrial systole is known as the atrial kick—see the Ventricular Volume graph (red) just above the P wave on the electrocardiogram graph (dark blue).
For a healthy human heart, the entire cardiac cycle usually lasts less than one second. That is, for a typical heart rate of 75 beats per minute (beats per minute), the cycle requires 0.3 seconds of ventricular systole (contraction) - pumping blood to all body systems from the two ventricles; and 0.5 seconds in diastole (dilatation), refilling the four chambers of the heart, for a total of 0.8 seconds to complete the cycle. [2]
Early ventricular diastole
During early ventricular diastole, the pressure in the two ventricles begins to fall from the peak reached during systole. When the pressure in the left ventricle falls below that in the left atrium, the mitral valve opens due to a negative pressure difference (suction) between the two chambers, causing blood in the atrium (accumulated during atrial diastole) to flow into the ventricle (see figure above ). Likewise, the same phenomenon occurs simultaneously in the right ventricle and right atrium through the tricuspid valve.
Ventricular filling flow (or flow from the atria to the ventricles) has an early (E) diastolic component caused by ventricular suction, followed by a late component produced by atrial systole (A). The E/A ratio is used as a diagnostic measure because its decrease indicates possible diastolic dysfunction. [3]
Late ventricular diastole
Early diastole is the mechanism of suction between the atrial and ventricular chambers. [4] Then, during late ventricular diastole, the two atrial chambers contract (atrial systole), causing an increase in blood pressure in both atria and forcing additional blood flow into the ventricles. This onset of atrial systole is known as the atrial impulse -
see Wiggers diagram.
The atrial impulse does not
provide a greater volume of blood flow (during the cardiac cycle), since about 80 percent of the collected blood volume enters the ventricles during the period of active absorption. [5]
Atrial diastole
At the beginning of the cardiac cycle, all four chambers of the heart, two atria and two ventricles synchronously approach relaxation and expansion, or diastole. The atria fill with separate volumes of blood returning to the right atrium (from the vena cava) and to the left atrium (from the lungs). Once chamber pressure and back pressure are equalized, the mitral and tricuspid valves open and returning blood flows through the atria into the ventricles. When the ventricles are completely filled, the atria begin to contract (atrial systole), forcing blood under pressure into the ventricles. The ventricles now begin to contract, and as the pressure inside the ventricles increases, the mitral and tricuspid valves close.
As the pressure inside the ventricles continues to rise, they exceed the "back pressure" in the aortic trunk
and pulmonary arteries
of the trunk
. The aortic and pulmonary valves open and blood is pumped out of the heart. The expulsion causes the pressure in the ventricles to drop, and at the same time the atria begin to fill (atrial diastole). Finally, the pressure inside the ventricles drops below the back pressure in the aortic trunks and pulmonary arteries, and the aortic and pulmonary valves close. The ventricles begin to relax, the mitral and tricuspid valves begin to open, and the cycle begins again. [6]
Thus, when the ventricles are in systole and contracting, the atria are relaxed and collect returning blood. When the ventricles become fully dilated in late diastole (referred to as LVEDV and RVEDV in the images), the atria begin to contract, pumping blood into the ventricles. The atria provide a constant flow of blood to the ventricles, thereby serving as a reservoir for the ventricles and ensuring that these pumps never run dry. [7] This coordination ensures that blood is pumped and circulated efficiently throughout the body. [8]