Pelvioperineal reflux (PPR) is a pathological condition characterized by retrograde blood flow from the intrapelvic veins into the superficial veins of the perineum and lower extremities [1–3]. It is registered in 25-62% of patients with pelvic varicose veins (PVD) [4-6]. The clinical manifestation of PPR is varicose transformation of the veins of the external genitalia, perineum, and posteromedial surface of the thighs. Instrumental diagnosis of this pathology is based on the use of radiation research methods, the choice of which is the subject of active debate among researchers [7–9]. Some of them, in addition to ultrasound angioscanning (USAS) of the pelvic veins, consider oophorography and pelvic venography (OGTP) as a mandatory component of the examination of patients with vulvar (VV) and perineal varicose veins (PV) regardless of the presence of symptoms of pelvic venous congestion (PVP) [3 , 9-11]. Other authors [12-14], guided by the principle of reasonable sufficiency, argue that to determine treatment tactics and select a method for eliminating PPR and varicose veins of the perineum and vulva (phlebectomy or scleroobliteration) in patients with VBT without signs of TVP, it is quite sufficient to perform an ultrasound scan of the pelvic and perineal veins .
The purpose of this study is to determine the diagnostic value and feasibility of performing OGTF in patients with VBT, VV and PV.
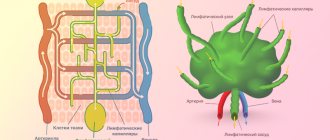
Briefly about the diagnostic method
Contrast venography (venography, ascending contrast venography or contrast venography) is an x-ray examination of deep or superficial veins using a contrast agent that provides an image of the blood vessel.
Phlebography determines the patency of the deep veins, the presence of blood clots, valve function and allows for a general assessment of the condition of the deep veins. Phlebography can be used when deep vein thrombosis is suspected, but ultrasound diagnostics cannot accurately exclude it. The study allows us to reliably assess the condition of the iliac veins in obese patients, when it is impossible to conduct a detailed ultrasound scan.
Contrast venography is most often used either during endovascular surgery on the deep veins (angioplasty or installation of a vena cava filter). We use retrograde venography to assess the integrity of venous valves when planning surgery for venous outflow reflux after venous thrombosis.
results
In both groups of patients, the main etiological factor in the development of VBT, VV and PV, according to the anamnesis, was pregnancy and childbirth. It was during pregnancy (primary or repeat) that women experienced PV and PV, and after childbirth, pelvic pain. The number of pregnancies and births in the groups did not differ statistically and ranged from 1 to 3. In table 1 presented
Table 1. Clinical characteristics of patients of groups 1 and 2 Note. Here and in the table. 2, 3: percentage in brackets. clinical characteristics of the examined patients.
Ultrasound examinations.
In group 1, 10 patients had valvular insufficiency of the left gonadal vein, 2 had bilateral lesions of the gonadal veins, and 1 had valvular insufficiency of the internal iliac veins (SIV). During ultrasound examination of the intrapelvic veins, no attempts were made to assess the condition of the parietal tributaries of the SVC (obturator, internal pudendal and inferior gluteal veins), since, firstly, their visualization is associated with significant time costs, and secondly, ultrasound examination seems to be a low-informative way to assess the valvular apparatus of the tributaries The SVC and, thirdly, all patients were scheduled to undergo radiocontrast venography, which is still considered the most accurate method for studying the intrapelvic veins, including the tributaries of the internal iliac veins. Pathology of the main superficial veins of the lower extremities was not detected in any observation (Table 2).
Table 2. Results of ultrasonography of the veins of the pelvis, perineum and lower extremities in patients of groups 1 and 2.
PPR in the 1st group was found in 16.6% of patients, in the 2nd - in 100%.
Phlebographic studies.
No complications were recorded during or after phlebography. The duration of the diagnostic procedure was 20-30 minutes.
1st group.
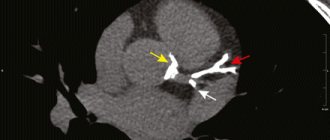
Valvular insufficiency of the gonadal veins was detected in 100% of patients: 10 (83.3%) were diagnosed with left-sided lesions, 2 (16.6%) - right-sided. In none of the cases, according to phlebography, was an anatomical connection established between the ovarian and superficial veins of the perineum or lower extremities (Fig. 1).
Rice.
1. Phlebograms. a — valvular insufficiency of the left gonadal vein (1); b — valvular insufficiency of the pampiniform venous plexus (2), parametric (3) and uterine (4) veins (indicated by arrows). Retrograde contrasting of parametric veins was found in all patients, uterine veins - in 8 (66.6%). Reflux of the contrast agent along the trunk of the left SVC was recorded in 2 (16.6%) women, along the left obturator vein - in 2 (16.6%) (Fig. 2).
Rice. 2. Pelvic venogram. Reflux of contrast agent along the left internal iliac (1) and left obturator (2) veins (indicated by arrows).
It should be noted that visualization of this parietal inflow was obtained in one woman with valvular regurgitation of the left SVC and in one without it. In the remaining patients, even selective catheterization of the SVC tributaries was not accompanied by visualization of the SPV, i.e., no reflux of the contrast agent was recorded from the internal tributaries into the superficial veins of the perineum, including 2 patients with VV.
In one case, a uretero-ovarian conflict was detected when the right ureter was compressed by the right gonadal vein, which was accompanied by impaired urine passage and pyelectasis (Fig. 3).
Rice. 3. Ovaricogram. Right ureter (1) and right gonadal vein (2), right-sided pyelectasis (3) (indicated by arrows).
Compression of the left common iliac vein by the right common iliac artery (May-Turner syndrome) was diagnosed in 2 patients (Fig. 4).
Rice. 4. Pelvic venogram. Compression of the left common iliac vein by the right common iliac artery (indicated by an arrow).
Thus, the results of the use of OGTF in the group of patients with VBT and TVP proved that this method is necessary to confirm the diagnosis, identify the features of the anatomical structure of the gonadal and intrapelvic veins, pelvic arteriovenous and ureterovenous conflicts. This is extremely important for the choice of treatment method and the order of surgical interventions.
2nd group.
Valvular insufficiency of the left gonadal vein was found in 15% of patients. An anatomical connection between the gonadal and superficial veins of the perineum and lower extremities was not identified in one of the observations. Reflux of contrast agent through the parametric veins was diagnosed in 100% of those examined, and through the uterine veins - only in 10%. Retrograde contrast of the trunk of the left SVC was recorded in 3 (15%) patients, the left obturator vein - in 2 (10%), and the left internal pudendal vein - in 1 (5%) patient (Fig. 5).
Rice.
5. Pelvic venogram. Catheterization of the trunk of the left internal iliac vein. Contrasting the internal pudendal (1) and obturator (2) veins (indicated by arrows). It should be noted that reflux of contrast agent along the SVC tributaries was detected only in the case of their selective catheterization. When contrast was introduced into the trunk of the SVC on the left and right and a Valsalva maneuver was performed with a breath hold for at least 5 s, no retrograde flow of contrast into the parietal tributaries of the SVC was detected. Visualization of the internal pudendal and obturator veins did not show further spread of the contrast agent into the superficial veins of the perineum or lower extremities, i.e., according to the results of phlebography, there was no obvious connection between the intrapelvic, vulvar and perineal veins, which means there was no continuous, permanent PPR.
Arteriovenous and ureterovenous conflicts were not identified in this group of patients.
The results of ultrasound and venographic studies showed comparable, almost identical results (Table 3).
Table 3. Results of ultrasound and radiological methods for studying the veins of the pelvis, perineum and lower extremities in patients of the 1st and 2nd groups
Preparing for diagnosis
- You cannot eat for four hours before the test, and you can only drink water.
- Patients who are allergic (particularly to iodine) or have already had a reaction to contrast should inform their doctor.
- A sedative may be prescribed to calm the patient.
- You may be asked to follow certain dietary restrictions before the test, depending on your specific procedure.
- Tell your doctor about the medications, herbs, or supplements you take. You may be advised to stop taking some of these medications before the test.
Clinical examination
The simplest and most common method for diagnosing pathology of the venous system of the body is a clinical examination of the patient. An examination and interview by a competent specialist (phlebologist, vascular surgeon, angiologist) with sufficient clinical experience will help to suspect and diagnose venous diseases in the early stages of the disease, which will significantly increase the chances of achieving the greatest success in treatment. A clinical examination involves an analysis of the patient’s complaints, a competent history taking and a thorough examination of the patient.
Complaints specific to venous diseases should be considered: pain and heaviness in the calf muscles (dull, aching in nature, more often disturbing in the evening or during prolonged static loads: long stay in the “sitting”, “standing” position); fatigue and numbness of the legs; periodic night cramps.
Objective signs of chronic venous insufficiency should be considered: the appearance of telangiectasia (spider veins, hemangiomas); varicose veins of the saphenous veins; swelling of the lower extremities; trophic skin disorders (the appearance of areas of hyperpigmentation, peeling of the skin on the legs, the appearance of trophic ulcers, varicose eczema, dermatitis)
Unfortunately, clinical examination data alone are often insufficient to determine the nosological variant of venous diseases and determine further treatment tactics. In such cases, numerous instrumental techniques for examining the patient come to the rescue.
How is the diagnosis carried out?
During the procedure, the patient lies on a special X-ray table. The area where the catheter will be inserted is cleared (usually a vein in the arm so that any necessary medications can be administered during the procedure). Sometimes local anesthesia is administered.
A contrast solution is delivered through the catheter. The dye injection causes a warm sensation that can spread throughout the body. The contrast may also cause mild nausea. About 18% of patients experience discomfort from the contrast solution. To fill the deep venous system with dye, a thick tape (or tourniquet) is sometimes placed around the ankle, or the limbs may be angled. The patient is asked to keep his leg still. The doctor watches the movement of the solution through the vein using a fluoroscope. At the same time, a series of photographs are taken.
When the study is completed, saline is injected into the same catheter to clear the veins of contrast, then the catheter is removed and a bandage is applied to the injection site.
Features of phlebography (by location of the veins being examined):
Venography of the lower extremity: the patient is positioned leaning on an inclined X-ray table. The table is tilted so that the legs are raised or lowered. The catheter is inserted into the selected leg or arm. The procedure may take 30 to 45 minutes.
Adrenal venography: the patient lies supine on the X-ray table. The catheter is inserted into the femoral vein. Under the guidance of fluoroscopic visualization, he carefully targets either the renal or suprarenal veins in the abdominal cavity. The procedure takes about 1 hour.
Abdominal venography: the patient lies supine on an x-ray table. The catheter is inserted into the femoral artery. The procedure takes about 1 hour.
Discussion
The study showed paradoxical results at first glance. Firstly, uretero-ovarian conflict and May-Turner syndrome are detected quite often in comparison with the available literature data [15, 16]. This can be explained both by chance and by a specific sample of patients, when these three rare pathologies were diagnosed within 6 months. In one patient, right-sided compression of the ureter by the gonadal vein in combination with compression of the left common iliac vein by the right common iliac artery was detected. In addition, one should take into account the peculiarity of the present study, in which a vascular surgeon and a radioangiosurgeon jointly and purposefully studied the condition of the intrapelvic veins, avoiding superficial judgments and interpretations. In everyday practice, such cooperation often does not exist.
Another unexpected finding was that none of the 32 venographic studies recorded an obvious connection between the intrapelvic and superficial veins of the perineum and lower extremities, despite the fact that selective catheterization of the SVC tributaries was performed. What does this indicate? It can be assumed, firstly, that there is a certain contingent of women in whom pregnancy is accompanied by expansion of the parietal inflows of the SVC and blood reflux through them with the formation of the PV and PV. They probably have hereditary and anatomical factors (impaired collagen III synthesis, hypoplasia or agenesis of pelvic vein valves, latent forms of May-Turner syndrome and VBT) that contribute to varicose transformation of the pelvic veins, which occur or worsen during pregnancy. Secondly, the active functioning, “opening” of the pelvic perforating veins can be considered as a compensatory phenomenon aimed at self-regulation of venous outflow from the pelvis during pregnancy. Subsequently, in the majority of women who give birth, this compensatory mechanism undergoes reverse development, and in some women it continues to function due to the above reasons, which determines the preservation of varicose veins of the perineum and external genitalia after childbirth. And finally, the third possible way of development of events is that after childbirth, blood reflux through the tributaries of the internal iliac veins disappears, and varicose veins of the perineum and vulva persist due to their significant expansion and the impossibility of reverse development of varicose veins.
The most likely assumption is that retrograde blood flow from the intrapelvic veins into the perineal and superficial veins of the lower extremities is not a constant phenomenon in a particular patient, but an episode that arose as a result of exposure to a factor (most often pregnancy) and resulted in dilation of the vulvar, perineal, and gluteal veins, which resolved after eliminating the influencing factor (childbirth). At the same time, the existence of permanent PPR cannot be completely excluded, the correction of which involves the use of endovascular occlusion of the connecting vessel. Perhaps such patients were not included in this study.
The results obtained in the study are absolutely opposite to the data of foreign authors [1, 5, 7], who identify PPR using phlebography and perform endovascular embolization of parietal tributaries of the SVC. Thus, G. Asciutto et al. [5] found valvular insufficiency of the SVC in 58% of cases, and reflux of contrast material into the groin and lower extremities in 62%. M. Greiner and G. Gilling-Smith [7] reported the detection of valvular insufficiency of the internal pudendal vein in 26%, obturator vein in 12% of patients with VVT and with relapse of varicose veins of the lower extremities (VVV). J. Lasry et al. [1] reported reflux of contrast agent during phlebography along the obturator and internal pudendal veins in 10% of the examined patients with TVP.
This dissonance can be partly explained by the inclusion in the studies of patients predominantly with symptoms of TVP and relapse of VBI, the performance of distal catheterization and contrasting of the obturator or internal pudendal veins, which in turn provides visualization of the direct connection of the intrapelvic and superficial veins of the perineum. In addition, the interpretation of data obtained from pelvic venography is important. For example, in the present study, contrasting of the obturator veins was not considered as PPR. On the contrary, in the work of M. Greiner and G. Gilling-Smith [7], such a picture was regarded precisely as PPR, although the phlebograms presented in their publication do not show images of a direct connection of the SVC tributaries with the vulvar or perineal veins. In this regard, if we interpret the visualization of the obturator and internal pudendal veins according to the pelvic venography performed in this work, the results do not differ so significantly from the data of J. Lasry et al. [1] - 15.65% of patients in the general group.
Information about the condition of the veins of the pelvis and lower extremities according to these two methods does not coincide only in the assessment of the SVC and their tributaries. USAS has advantages in studying the veins of the perineum and lower extremities. In addition, the significant pressure under which the contrast agent is injected during venography and direct catheterization of the SVC branches should be taken into account. In this regard, USAS is more physiological and closer to the natural conditions of functioning of the pelvic veins. In the work of A. Whiteley et al. [6] based only on the results of transvaginal ultrasound examination, PPR was identified in 25.6% of patients with recurrent VLNK. It is important that the authors did not study the state of the SVC tributaries using USAS and judged them by visually detected varicose veins of the perineum and lower extremities.
After diagnosis
After phlebography, observation in the clinic is necessary for at least 24 hours. Depending on the procedure, you may be advised to rest in bed for a certain period of time.
Patients should drink plenty of fluids to flush any remaining contrast solution from the body. The area where the catheter was inserted may be sore for several days. If you notice swelling, redness, pain, or fever, tell your doctor. In most cases, the patient can resume normal activities the next day.
About place, time and beneficial advantages
The clinic has a specially equipped room for conducting research. The latest CT scanner Siemens Somatom Definition AS meets the latest requirements of medical science.
The advantages of this examination method are not unique, they include:
- High information content of the method.
- CT venography data reflects the current state of your blood vessels.
- A three-dimensional image allows you to find out the cause of the pathology with maximum accuracy and select an individual treatment option.
- CT venography does not require special preparation of the patient.
Possible complications
Phlebography can cause complications such as phlebitis, tissue damage, and deep vein thrombosis in the healthy leg. Complications are quite rare, but they must be taken into account when planning treatment so that the risk of the study does not exceed the risk of the disease for which it is being performed.
A rare side effect (up to 1% of cases) is a serious allergic reaction to the contrast dye. It usually appears 30 minutes after the dye injection and requires medical attention.
Possible risks include blood clot formation in the vein, bleeding, damage to blood vessels, or infection at the site where the catheter was inserted.
Some people may experience an allergic reaction to iodine-based contrast dye. This can cause symptoms such as nausea, sneezing, vomiting, hives, and sometimes a life-threatening reaction called anaphylactic shock (especially in older patients with chronic dehydration or mild kidney failure).
MR phlebography of the brain
Magnetic resonance venography (MRF) of the brain
Introduction
MRF (MRV) stands for magnetic resonance venography. MRF is used to evaluate disturbances of venous outflow in the vessels of the brain. Two-dimensional (2D) (TOF) MR venography (MRV) and three-dimensional (3D) phase-contrast angiography (PC) are techniques commonly used to evaluate the cerebral venous sinuses because they are easy to perform and do not require contrast enhancement.
angiography ( TOF)
This is a technique for obtaining contrast between fixed tissues and blood flow by influencing the magnitude of magnetization.
The magnitude of magnetization from moving spins is very large compared to that from stationary spins. This leads to the formation of a high signal from moving blood spins and a reduced signal from stationary tissue spins. Time-of-flight (TOF) angiography uses the longitudinal vector of magnetization to manipulate images.
Phase-contrast angiography (Phase-contrast, PC)
This is a technique for obtaining contrast between fixed tissues and blood flow by influencing the magnitude of magnetization. The magnetization phase from stationary spins is zero, and accordingly the magnetization phase from moving spins is not zero. Phase is a measure of how much the magnetization process deviates in the transverse plane over time until it is detected. In order to reduce the signal from stationary tissue, a bipolar gradient pulse of equal and opposite magnitude is used.
Phase contrast angiography (PCA) uses transverse magnetization vectors. In the difference image phase, the signal is linearly proportional to the spin speed. Fast moving spins produce a larger signal and promote spins moving in one direction, indicated by bright signals and appearing white in the image, while spins moving in the opposite direction are indicated by dark signals and appear black accordingly. Phase contrast methods are sensitive to a range of speeds, so the researcher must select this value with care. To highlight different vessels, different encoding rates can be applied in different scans. High coding speed is typical for arteries (40-70 cm/sec), due to rapid arterial inflow. Low coding speed is typical for veins (10-20 cm/sec), due to slow venous outflow.
Phase contrast angiography can be used to obtain 2D or 3D images.
Indications for magnetic resonance venography (MRF) of cerebral vessels:
- Diagnosis of thrombosis;
- Tumor of the venous sinus of the dura mater;
- Drowsiness and confusion accompanying headache
Contraindications for magnetic resonance venography (MRF) of cerebral vessels:
- Any electrical, magnetic, or mechanical activated implant (eg, pacemaker, insulin pump biostimulator, neurostimulator, cochlear implant, and hearing aids);
- Intracranial (intracranial) aneurysmal clips (except titanium);
- Pregnancy (if the risk outweighs the benefit);
- The presence of ferromagnetic surgical clamps or staples;
- Presence of a metallic foreign body in the eye;
- The presence of metal shrapnel or bullets in the body.
Preparing the patient for MR venography of cerebral vessels
- Before the scanning procedure, it is necessary to obtain the patient's written consent for the study;
- Ask the patient to remove all metal objects, including keys, coins, rings, plastic cards with magnetic stripes, jewelry, hearing aids, and hairpins;
- If necessary, provide an accompanying person for patients suffering from claustrophobia (for example, a relative or employee);
- Offer the patient earplugs or headphones with music for additional comfort;
- It is necessary to explain to the patient the essence of the procedure and the procedure for its implementation;
- Warn the patient to remain calm during the procedure;
- Note the patient's weight.
Position during MRF of cerebral vessels
- Lying on your back with your head forward (towards the magnet);
- Position the head in the head coil and immobilize it with pillows;
- For additional comfort, place bolsters under the patient's feet;
- The center of the laser beam is focused over the bridge of the nose.
Recommended protocols, parameters and planning
Localizer
The first step in sequence planning is to take images in 3 planes. Exposure times are less than 25 seconds, producing low-resolution T1-weighted images.
Series T2 turbo spin echo, axial cut
Planning of axial sections on the sagittal plane; The angular position of the block should be parallel to the knee and splenium of the corpus callosum. These sections should completely cover the brain from the crown to the level of the foramen magnum. Check the location of the block on 2 other planes. An appropriate angle must be obtained in the coronal plane with the head in a tilted position (perpendicular to the line connecting the third ventricle and the brain stem).
Options
| TR | T.E. | SLICE | FLIP | PHASE | MATRIX | FOV | GAP | NXA(AVRAGE) |
| 3000-4000 | 100-120 | 5mm | 130-150 | R˃L | 320X320 | 210-230 | 10% | 2 |
Time-of-flight angiography 2D - time-of-flight (TOF) or phase - contrast angiography 3D - phase-contrast (PC)
Planning sagittal 3D or 2D blocks on the axial plane; the angular location of the block should be at an angle of 10° to the midline of the brain. Check the position of the block on the coronal plane and the position of the block at an angle of 10° to the midline of the brain. These angles must be maintained to reduce saturation effects on the plane. Place saturation bands at the bottom of the block in the sagittal and coronal planes to suppress arterial pulsatility signals. These slices should completely cover the brain from one to the opposite temporal lobe.
Options
3D phase-contrast (PC)
| TR | T.E. | FLIP | NXA | SLICE | MATRIX | FOV | PHASE | GAP | velocity |
| 68-75 | 8-9 | 15 | 2 | 1 mm | 256X256 | 280 | А˃Р | 20% | 10 |
velocity – speed
2D time-of-flight (TOF)
| TR | T.E. | FLIP | NXA | SLICE | MATRIX | FOV | PHASE | GAP | MTC |
| 28-35 | 5-8 | 60 | 1 | 2 mm | 256X256 | 250 | А˃Р | -50% | ON |
MTC (Magnetization Transfer Contrast) – magnetization transfer
Maximum Intensity Projection (MIP)
MIP is the most commonly used method for processing vascular MR imaging. MIP allows 2D image projections to be reconstructed from 3D data using a ray tracing algorithm that produces an image of white pixels as the maximum intensity signals of the region of interest.
MIP is the most commonly used post processing technique in MRI vascular studies. MIP reconstructs a 2D projection image from 3D data by a ray tracing algorithm, which produces an image of white pixels representing the highest intensity signal in that location within the examined volume.
CLICK ON THE ITEMS BELOW TO CHECK THE SCAN