What is cardiogenic shock
Cardiogenic shock develops mainly against the background of small-focal or extensive myocardial infarction. As a result, blood circulation throughout the body is sharply disrupted. When this condition develops, it is possible to save the patient’s life only in 10% of cases, despite timely assistance and resuscitation measures.
A dangerous condition occurs due to a sharp disruption of the contractile function of the myocardium. This can be provoked by myocardial infarction, dilated cardiomyopathy, aortic stenosis, damage to the interventricular septum and other diseases. Cardiogenic shock entails a critical decrease in blood pressure. At the same time, activation of the sympathetic nervous system occurs, which provokes excitation of cardiac activity.
A sharp decrease in cardiac output is accompanied by a decrease in the amount of blood in the arteries, this entails fluid retention in the body, the load on the heart muscle increases, and pulmonary edema develops. In turn, the accumulation of under-oxidized metabolic products causes metabolic acidosis.
Publications in the media
Cardiogenic shock is shock that occurs as a result of a sudden decrease in cardiac output.
Etiology • As a rule, it occurs with extensive MI against the background of multivessel lesions of the coronary arteries. Shock develops when more than 40% of the myocardial mass is involved, and is observed in 5–20% of patients with infarction • Other causes •• Acute myocarditis •• Severe, acute aortic or mitral stenosis •• Severe, acute aortic or mitral regurgitation •• Rupture interventricular septum •• Arrhythmias.
Risk factors • Old age • Decrease in left ventricular ejection fraction below normal (according to EchoCG) • Extensive MI (according to ECG, infarct changes in 8-9 leads; large zone of akinesia according to EchoCG), • Previous MI • Diabetes.
Pathogenesis . Severe impairment of myocardial contractile function with the additional addition of factors that aggravate myocardial ischemia.
• Activation of the sympathetic nervous system due to a drop in cardiac output and a decrease in blood pressure leads to an increase in heart rate and increased myocardial contractility, which increases the heart's need for oxygen.
• Fluid retention due to decreased renal blood flow and an increase in blood volume, which increases preload on the heart, contributes to pulmonary edema and hypoxemia.
• Increased peripheral vascular resistance due to vasoconstriction, leading to increased afterload on the heart and increased myocardial oxygen demand.
• Violation of diastolic relaxation of the left ventricle of the myocardium due to impaired filling and decreased compliance, which causes an increase in pressure in the left atrium and contributes to increased blood stagnation in the lungs.
• Metabolic acidosis due to prolonged hypoperfusion of organs and tissues.
Clinical manifestations
• Arterial hypotension - systolic blood pressure less than 90 mm Hg. or by 30 mm Hg. below normal levels for 30 minutes or more. Cardiac index less than 1.8–2 l/min/m2.
• Impaired peripheral perfusion •• kidneys - oliguria •• skin - pallor, increased humidity •• CNS - workload, stupor.
• Pulmonary edema as a manifestation of left ventricular failure.
When examining the patient, cold extremities, impaired consciousness, arterial hypotension (average blood pressure below 50–60 mm Hg), tachycardia, muffled heart sounds, oliguria (less than 20 ml/min) are detected. Auscultation of the lungs may reveal moist rales.
Special research methods
• Installation of a Swan-Ganz catheter to monitor central hemodynamics, measurement of cardiac output - by echocardiography or invasively.
• Arteriovenous difference in oxygen is more than 5.5 ml%.
Diagnostics . It is necessary to exclude other causes of arterial hypotension: hypovolemia, vasovagal reactions, electrolyte disturbances (for example, hyponatremia), side effects of drugs, arrhythmias (for example, paroxysmal supraventricular and ventricular tachycardias).
TREATMENT
An emergency condition requires urgent treatment. The main goal of therapy is to increase blood pressure.
Drug therapy . The following drugs are used (preferably administered through special dispensers). With their help, blood pressure should be increased to 90 mm Hg. and higher • Dobutamine (selective b1-adrenergic agonist with a positive inotropic effect and minimal positive chronotropic effect, i.e. the effect of increasing heart rate is insignificant) at a dose of 2.5–10 mcg/kg/min • Dopamine (has a more pronounced positive chronotropic effect , i.e., it can increase heart rate and, accordingly, myocardial oxygen demand, thereby slightly aggravating myocardial ischemia) at a dose of 2–10 mcg/kg/min with a gradual increase in dose every 2–5 min to 20–50 mcg/kg/min • Norepinephrine at a dose of 2–4 mcg/min (up to 15 mcg/min), although it, along with increased myocardial contractility, significantly increases peripheral vascular resistance, which can also aggravate myocardial ischemia.
Intra-aortic balloon counterpulsation ( mechanical injection of blood into the aorta using a pump during diastole, which increases blood flow in the coronary arteries ) . Carry out in the presence of appropriate equipment and the ineffectiveness of drug treatment for cardiogenic shock.
Percutaneous transluminal coronary angioplasty - restoration of the patency of the coronary arteries with its help in the first 4-8 hours from the onset of a heart attack not only preserves the myocardium, but also interrupts the vicious circle of pathogenetic mechanisms of cardiogenic shock.
Observation . In case of cardiogenic shock, constant monitoring of blood pressure, heart rate, diuresis (indwelling urinary catheter), pulmonary capillary wedge pressure (balloon catheter in the pulmonary artery), as well as monitoring of cardiac output using echocardiography or radionuclide angiography is recommended.
Forecast . The mortality rate for cardiogenic shock is 50–90%.
ICD-10 • R57 . 0 Cardiogenic shock
How to recognize a dangerous condition
The sooner help is provided for cardiogenic shock, the greater the chance of saving the patient’s life. The clinic always depends on the condition that caused the shock. With myocardial infarction, a person experiences severe pain in the chest, a feeling of fear and panic appears. If the heart rhythm is abnormal, the patient notices a pain syndrome in the chest, the heart sinks or, conversely, the heart rate increases. If the cause of cardiogenic shock is pulmonary embolism, the person suffocates, becomes weak, and sometimes coughs up blood.
Cardiogenic shock causes severe chest pain and other symptoms
Further development of shock is accompanied by the following symptoms:
- the appearance of cold sticky sweat;
- blue lips, nose, fingertips;
- pale skin;
- the patient’s anxiety or lethargy;
- swelling of the neck veins;
- decreased temperature of the extremities;
- feeling of panic and fear.
With pulmonary thromboembolism, the skin on the head, chest and neck becomes earthy or marbled in color.
Important! In the absence of the necessary help, the patient loses consciousness, cardiac and brain activity stops, and death occurs.
Help with cardiogenic shock, prevention
Medical care is to increase blood pressure to normal levels. For this purpose, dopamine or dobutamine is used. In case of ventricular fibrillation, defibrillation is performed, and in case of cardiac arrest, indirect cardiac massage is performed.
Help with cardiogenic shock in a hospital setting: oxygen therapy, prescription of vasopressors, cardiac glycosides, analgesics, prednisolone, heparin, diuretics. The dosage and treatment regimen are prescribed by the doctor in each specific case individually.
Prevention:
- healthy lifestyle, giving up bad habits;
- balanced diet;
- adequate sleep, stress management;
- moderate physical activity;
- timely treatment of any cardiac pathology.
Once a year, undergo a medical examination with a cardiologist. If you have heart disease, preventive examination is recommended every six months. In our center you can undergo a full examination of the body and receive a transcript of the results. During the consultation, the doctor will answer your questions and tell you about the symptoms of cardiogenic shock during myocardial infarction. Registration online and by phone.
Pre-hospital emergency care
If signs of cardiogenic shock are detected, it is necessary to call an ambulance as soon as possible and provide emergency assistance to the person. To do this, follow these steps:
- Place the patient on any surface, the body should be in a horizontal position, legs slightly elevated. This position ensures better blood flow to the brain.
- During emergency care, it is important to provide fresh air into the room. To do this, you need to open the window or front door. Crowds should not be allowed near the victim.
- The person's neck and chest must be freed from clothing. If there is a tight collar, tie, scarf or other items, they should be removed.
- At the initial stage, you need to measure the patient's blood pressure. In cardiogenic shock it is always reduced. To normalize the indicators, you need to give the patient a drug that contains dopamine, metazone or hydrocartisone.
- If the person is conscious, taking analgesic medications is allowed.
After this, you should wait for the ambulance, and after the doctors arrive, tell them under what circumstances the shock developed.
First aid for the development of shock should be immediate.
Resuscitation measures
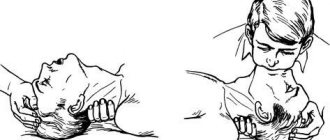
In case of loss of consciousness and respiratory arrest, urgent resuscitation measures must be performed. Artificial respiration is performed mouth to mouth. To do this, the person’s head needs to be tilted back, placing a cushion made of a towel or any other fabric under the neck. The person performing resuscitation must inhale air, cover the victim's nose with his fingers, and exhale air through the victim's mouth. You need to take up to 12 breaths in one minute.
During the provision of first aid, it is necessary to monitor the patient’s pulse. If a person loses consciousness and heartbeats cannot be heard, chest compressions must be performed. To perform it, the patient is placed on his back, the surface must be hard. The person performing the massage should position himself to the side of the patient. The heels of your palms should press down on the chest area in the middle. Pushes are performed with straight arms; there is no need to bend them. The frequency of pressing is at least 60 shocks per minute. If an elderly person is being resuscitated, the number of pushes per minute is up to 50, for children - 120 pushes. Important! When performing artificial respiration and chest compressions simultaneously, you should alternate 2 breaths with 30 shocks.
Helping a patient in a hospital setting
The algorithm of actions of doctors depends on the characteristics of the patient’s condition. The first medical measures are carried out in the ambulance. The following methods are used here:
- the use of oxygen therapy - the procedure helps to support the patient’s breathing and maintain vital functions until arriving at the hospital;
- use of narcotic analgesics. This event helps reduce severe pain. Medicines such as Droperidol, Promedol, Fentanyl and others are used here;
- to eliminate the risk of blood clots in the arteries, a person is given heparin;
- solutions of Dobutamine, Dopamine, Norepinephrine help normalize the heart rate;
- Injecting insulin with glucose helps improve the nutrition of the heart muscle;
- Panangin, Gilurythmal, Lidocaine help eliminate tachyarrhythmia;
- sodium bicarbonate solution is administered to improve the body's metabolic processes.
Further treatment of cardiogenic shock in a clinical setting involves continuation of therapy started at home and in the ambulance. Upon admission of the patient to the hospital, an immediate comprehensive examination of the body is carried out. This helps to identify contraindications and the risk of side effects that may complicate the situation.
In a hospital setting, resuscitation measures are carried out aimed at restoring the patient’s vital functions
The further standard of care depends on the disease that caused the development of shock:
- a condition in which pulmonary edema occurs requires the prescription of Nitroglycerin, the use of alcohol solutions, and diuretics;
- severe pain is relieved with the help of strong narcotic analgesics, which include Morphine, Promedol, Fentanyl;
- Treatment of severely low blood pressure is carried out using Dopamine solution;
- to preserve breathing in an unconscious patient, tracheal intubation is performed;
- Oxygen therapy helps prevent oxygen starvation of the brain and other organs.
If a person’s condition is serious, it is necessary to use a heart-lung machine and artificial ventilation. During this period, the patient should be provided with the necessary nursing care. It consists of performing hygiene procedures, regularly measuring blood pressure, body temperature and feeding the patient.
How to treat cardiogenic shock during myocardial infarction?
N
Despite significant advances in the treatment of patients with acute myocardial infarction (MI), which has significantly reduced mortality in this disease,
cardiogenic shock
(CS)
still remains the main cause of death in patients with MI even in the so-called “thrombolytic era”
. CABG occurs on average in 5-10% of patients with MI. According to Golbert (1991), the mortality rate in patients with MI complicated by CABG in the period from 1975 to 1988 was 78%. And the results of the National Registry of Myocardial Infarction (NRMI 2), which tracked the outcomes of myocardial infarction in 23 thousand patients with CABG in 1400 US hospitals from 1994 to 2001, showed that mortality in recent years has decreased slightly and amounted to about 70%.
CABG is a complication of MI associated with a decrease in cardiac output with adequate intravascular volume, leading to hypoxia of organs and tissues. As a rule, shock develops in patients as a result of serious dysfunction of the left ventricle due to significant damage to the myocardium. At autopsy in patients who died from CABG, the size of the MI ranges from 40 to 70% of the mass of the left ventricular myocardium. This article will discuss the principles of treatment of patients with true CABG. Other clinical variants of CABG, for example, those associated with the development of arshock or hypovolemia, as well as with internal or external myocardial ruptures and acute mitral regurgitation, require other pathogenetic approaches to treatment.
Criteria for diagnosing cardiogenic shock:
- systolic blood pressure is less than 90 mmHg. for 1 hour or more;
- signs of hypoperfusion - cyanosis, cold, moist skin, severe oliguria (urination less than 20 ml per hour), congestive heart failure, mental disorders;
- heart rate above 60 beats. per minute;
- hemodynamic signs - wedge pressure in the pulmonary artery more than 18 mm Hg, cardiac index less than 2.2 l/min/sq.m.
Standard treatment for cardiogenic shock
Patients with MI complicated by CABG must be in an intensive care unit and require careful constant monitoring of a number of parameters: general clinical condition; blood pressure level - preferably by a direct method, using, for example, catheterization of the radial artery; water balance - with mandatory measurement of hourly diuresis; ECG monitoring. If possible, it is advisable to monitor central hemodynamic parameters using a Swan-Ganz catheter or at least central venous pressure.
Conventional therapy for CABG includes oxygen therapy
.
Patients should receive oxygen through intranasal catheters or a mask, and in cases of severe respiratory dysfunction, transfer to artificial ventilation. As a rule, patients require therapy with inotropic drugs: intravenous infusion of dopamine
at a rate necessary to control blood pressure levels (on average, it is 10-20 mcg/kg per minute);
in case of insufficient effectiveness and high peripheral resistance, a dobutamine infusion
of 5-20 mcg/kg per minute is started.
Subsequently, it is possible to replace one of these drugs with norepinephrine in increasing doses from 0.5 to 30 mcg/kg per minute or adrenaline. In some cases, these drugs help maintain blood pressure at a level of at least 100 mm Hg. Art. diuretics
are usually prescribed . The use of nitro drugs and other peripheral dilators should be avoided due to their hypotensive effect. CABG is an absolute contraindication for the use of b-blockers.
It must be said that standard drug therapy, as a rule, turns out to be either ineffective or gives a short-term effect, so we will not dwell in detail on the detailed characteristics of well-known drugs - we will discuss those treatment methods that, according to modern ideas, can change the prognosis in patients with CABG.
Thrombolytic therapy
Most often, CABG develops with thrombotic occlusion of a large subepicardial coronary artery, leading to myocardial damage and ischemic necrosis. Thrombolytic therapy (TLT) is one of the modern methods of treatment
, allowing to restore perfusion in the ischemic focus and save viable (hibernated) myocardium.
The first encouraging results were obtained in the large-scale study GUSTO-I
(1997), where more than 40 thousand patients with MI were examined.
It turned out that in patients receiving tissue plasminogen activator (t-PA) therapy, shock developed in the hospital in 5.5% of cases, and in the group treated with streptokinase - in 6.9% of cases (p<0.01). The 30-day mortality rates were 57% and 58%, respectively. That is, t-PA therapy can prevent the development of CABG in patients with MI in the hospital. The next generations of tissue plasminogen activator - alteplase and retiplase, which have a number of advantages over t-PA (rapid thrombus destruction and ease of administration), were studied in the GUSTO-III
(1999). When alteplase and retiplase were administered, shock developed in a hospital setting in 5.3 and 5.5% of cases, and 30-day mortality was 65% and 63%, respectively. Thus, the next generation of thrombolytics were not as effective as expected in preventing the development of CS in hospital patients. Among patients included in GUSTO-I and GUSTO-III, upon admission to hospital, signs of CABG were recorded in 0.8% and 11%, respectively. Their mortality rate was: in the t-PA group - 59%, streptokinase - 54%, retiplase - 58%. Thrombolytic therapy slightly reduces the mortality rate of patients with MI complicated by CABG, and t-PA appears to reduce the incidence of its development. Research in this direction continues. It is possible that not only the use of new thrombolytics (mutant molecules, etc.), but also other methods of optimizing the treatment of patients can improve the outcome of the disease. It is known that combined therapy with thrombolytics and low molecular weight heparins, for example, enoxaparin (ASSENT-3, AMI-SK, HART II), significantly improved the prognosis of MI, reducing mortality, the number of recurrent MIs and the need for revascularization. It is possible that treatment of CABG using thrombolytic drugs and low molecular weight heparins will be more effective, although at present this is only a hypothetical consideration.
The use of thrombolytics for cardiogenic shock can improve survival in patients with MI, and in some cases prevent the development of this complication. However, the use of this treatment method alone is unlikely to significantly change the existing situation. This is due to the fact that low systemic pressure leads to low perfusion pressure in the coronary arteries and a significant decrease in the effectiveness of thrombolysis.
Intra-aortic balloon counterpulsation
Intra-aortic balloon pumping (IABP) is used to stabilize the condition of patients with CABG and increase the effectiveness of thrombolytic therapy. This is due to the fact that IABP improves myocardial perfusion in diastole, reduces systolic afterload, and does not change the myocardial oxygen demand. The GUSTO-I study showed a decrease in mortality by the 30th day of MI and after 1 year of the disease in cases where IABP was used in patients with CABG. Analyzing the results of NRMI-2
, compared the effectiveness of treatment of patients with CABG with and without IABP. It should be noted that these data were obtained as a result of the treatment of CABG in more than 20 thousand patients, not in a specially designed study, but in US healthcare practice over the past 6 years. Data on the percentage of patients who received thrombolytics, primary angioplasty (TBCA) or who did not receive “reperfusion” therapy are presented in Figure 1. It turned out that in the group receiving thrombolytic therapy, the use of IABP significantly reduced hospital mortality from 70% to 49%. The use of IABP during primary angioplasty did not significantly change in-hospital mortality. Mortality with CABG (Fig. 2) in patients after primary coronary angioplasty was 42% and was lower than with any other treatment methods.
Rice. 1. Percentage of patients treated with TLT or primary TBKA, or without these interventions, depending on the implementation of IABP NRMI 2
Rice. 2. In-hospital mortality in the group of patients with TLT or primary TBKA depending on the IABP NRMI 2
Thus, the use of IABP during CABG allows not only to stabilize the condition of patients during its implementation, but also to significantly improve the effectiveness of thrombolytic therapy and patient survival.
Non-drug restoration of coronary blood flow
The possibilities of non-drug restoration of blood flow in MI complicated by CABG, primarily using primary coronary angioplasty and emergency CABG, have been actively studied in the last decade. It is known that with successful primary angioplasty it is possible to achieve a more complete restoration of coronary blood flow, a smaller diameter of residual stenosis and improved survival of patients with CABG (S-MASH, GUSTO-1).
The most convincing results were obtained in the multicenter SHOCK
, conducted in 30 centers in the USA and Canada from 1993 to 1998, 302 patients with true CABG were randomized into groups of intensive drug treatment (n=150) and early revascularization (n=152). Patients of groups 1 and 2 received therapy with inotropic drugs in 99%, IABP in 86%, and thrombolytics in 63% and 49% of cases, respectively. In the second group, 97% underwent emergency coronary angiography and early revascularization was achieved in 87% of cases (intracoronary intervention - 64%, surgical - 36%).
The mortality rate in patients in the drug treatment group by the 30th day of MI was 56%, and in the early revascularization group it was 46.7%. By the 6th month of the disease, mortality in group 2 was significantly lower (63% and 50%, respectively), and these differences persisted until the 12th month of the disease (Fig. 3). Analysis of the effectiveness of aggressive management of patients with MI with CABG showed that the survival results of patients were better in cases of revascularization
in almost all subgroups (Fig. 4). The exceptions were elderly patients (over 75 years of age) and women in whom drug treatment was preferable. The SHOCK study was brilliantly designed. For example, the average time from decision to angioplasty was 0.9 hours, and to coronary surgery was 2.7 hours.
Rice. 3. Mortality of patients in the drug treatment (group 1) and early revascularization (group 2) groups SHOCK Study
Rice. 4. Comparative risk of 30-day mortality in subgroups of the SHOCK Study
Thus, early revascularization in patients with MI complicated by CABG apparently leads to restoration of the function of the hibernated myocardium and allows for a significant reduction in mortality in these patients. It should be noted that 55% of patients with CABG in the SHOCK study were transferred to specialized centers, where they underwent appropriate invasive interventions. This tactic for managing patients with CABG may be the most promising in our country.
Metabolic therapy
When a coronary artery is occluded, serious disturbances in the structure and function of the myocardium occur. Disorders of myocardial metabolism that develop during prolonged ischemia and systemic hypotension, even with restoration of coronary blood flow, can interfere with the normalization of recovery of cardiac function. In this regard, numerous attempts have been made to restore myocardial metabolism in patients with CABG using a number of drugs - glucose-insulin-potassium mixture, adenosine, Na+H+ channel blockers, L-carnitine. It was assumed that they would help increase the viability of ischemic myocardium. Despite the theoretical premises, today, from the standpoint of evidence-based medicine, no data has been obtained that would allow recommending the use of metabolic drugs in clinical practice for the treatment of patients with CABG.
New approaches to treatment
Encouraging results have been obtained using the latest generation of antiplatelet drugs - blockers of IIb-IIIa glycoprotein platelet receptors. Use of eptifibatide
in the subgroup of patients with CABG in the
PURSUIT
(2001) in patients with acute coronary syndromes without ST elevation led to a significant increase in survival compared to the control group. Perhaps this effect is partly due to the ability of this group of drugs to improve blood circulation in the microvasculature by eliminating platelet aggregates.
Treatment after discharge from hospital
Significant impairments in myocardial contractility persist in the majority of surviving patients with MI complicated by CABG. Therefore, they need careful drug monitoring and active therapy after discharge from the hospital. The minimum possible therapy, the purpose of which is to reduce the processes of myocardial remodeling and manifestations of heart failure (ACE inhibitors, diuretics, b-blockers and cardiac glycosides), reduce the risk of thrombosis and thromboembolism (acetylsalicylic acid, coumarin derivatives - warfarin, etc.), should be used by the treating physician doctor in relation to each patient, taking into account the developing clinical situation.
Emergency surgical treatment
If a patient's condition in cardiogenic shock does not improve after using drug therapy and resuscitation measures, doctors use surgery to help save the person's life. The operation is performed exclusively in a hospital setting using the necessary medical equipment.
To combat the symptoms of cardiogenic shock, the following techniques are used:
- coronary artery bypass grafting – consists of creating an additional bloodstream, which is used as a bridge before the upcoming myocardial transplantation;
- intra-aortic balloon counterpulsation - the technique is carried out by introducing a special balloon, which inflates when the heart muscle contracts. A procedure is performed to normalize blood pressure;
- percutaneous transluminal coronary angioplasty - involves restoring the integrity of blood vessels, which ensures normal contractile function of the heart and maintaining vital body processes at the proper level.
In the absence of timely resuscitation measures, severe consequences of cardiogenic shock develop. These include heart failure, cerebral vein thrombosis, trophic ulcers of the stomach, intestines and other conditions. Even with timely and competent medical care, death occurs in 90% of cases. This is explained by the severe course of cardiogenic shock and its frequent complications. To avoid this condition, it is necessary to direct efforts towards its prevention. In this case, preventive measures should be aimed at the root cause, that is, at preventing pathologies that cause the risk of developing shock. Proper treatment of cardiovascular diseases and timely seeking medical help will help significantly reduce the risk of cardiogenic shock.
Despite numerous studies and the use of the latest modern drugs, technologies and devices, cardiogenic shock (CS) remains the main and leading cause of death in patients with acute myocardial infarction (AMI) and a number of other acute heart diseases [12, 14]. This severe cardiac complication is stage IV of acute heart failure according to the classification of T. Killip (1967), which is characterized by systolic blood pressure less than 90 mm Hg, signs of systemic hypoperfusion and peripheral vasoconstriction, oliguria, cyanosis, sweating. Pulmonary rales may be heard, but may be absent. In ⅓ of patients with CABG, pulmonary edema is not diagnosed either clinically or according to instrumental methods [24, 30].
The incidence of CABG in patients with ST-segment elevation AMI (STEMI) according to various authors, studies and international registries is different and varies from 3.4 to 15% [60]. The largest studies (NRMI-2, 3, 4), which analyzed data from 1,970,000 patients in the United States with STEMI obtained in 1994-2004, demonstrated an overall rate of CABG of 8.6%, with no trend to decrease or increase over time. specified period [3].
A slightly lower incidence of CABG is observed in patients with non-ST segment elevation AMI (NSTEMI). In the GUSTO IIb study, which included 7986 patients with NSTEMI, CABG developed in 2.6% [26], the same incidence of CABG (2.5%) in NSTEMI was reported in the PURSUIT study [19]. In the more recent GRACE registry, the incidence of CABG over a 6-year period (1999–2006) decreased from 2.1 to 1.8% [13].
Mortality in CABG ranges from 50 to 90% in all age groups [12], while the majority of patients with CABG are women, elderly and senile people, patients with concomitant diabetes mellitus and arterial hypertension [14].
Unfortunately, a generally accepted optimal treatment strategy for patients with CABG has not yet been developed [29].
Myocardial revascularization—percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)—is one of the main and defining moments in the treatment of patients with AMI complicated by CABG. Revascularization can significantly reduce mortality and improve treatment outcomes in this group of patients [12, 29, 57] both in the early postoperative period and in the long term [24, 33]. According to the 2013 ACC/AHA guidelines for the treatment of STEMI, urgent revascularization through PCI or CABG for CABG is recommended for all eligible patients with CABG events, regardless of the timing of disease onset (Evidence Level IB). If it is impossible to perform myocardial revascularization by any method and in the absence of contraindications, thrombolytic therapy is recommended (level of evidence IB) [36].
The problem regarding the volume of myocardial revascularization during CABG remains unresolved. The 2012 ACC/AHA/SCAI guidelines indicate that in multivessel disease, revascularization of infarct-unrelated arteries may be necessary to maximize myocardial perfusion. As an alternative strategy, in this case, CABG is preferable [32]. Complete myocardial revascularization during CABG according to D. Mylotte et al. [33] improved 6-month survival: 43.9% (revascularization of the infarct-related artery (ISA) only) versus 20.4% ( p
<0,002).
In the GUSTO-I trial, PCI resulted in a 22% reduction in 30-day mortality. Moreover, mortality was lower both among patients in whom CABG developed before admission and in the group of patients in whom CABG occurred after admission [25]. An analysis of 2000 patients with CABG demonstrated the advantage of the myocardial revascularization strategy: 30-day mortality in the PCI and CABG group was 38%, while drug therapy was associated with 62% of deaths [4]. A recent registry [60] that analyzed in-hospital mortality among 1333 CABG patients undergoing primary PCI reported a mortality rate of 46%. However, all these studies and works were non-randomized. In addition, patients included in the revascularization group were obviously younger and, accordingly, had a lower risk of death, which influenced the treatment results [21]. An analysis of the SHOCK registry showed that mortality in older patients (76%) was higher than in younger patients (55%; p
<0.001).
However, the mortality rate of operated patients of senile and elderly age was significantly less than in patients of the same age category, but without myocardial revascularization: 48% versus 81%; p
=0.0002 [11].
According to J. Hochman et al. [24], exclusion criteria for performing PCI in CABG are stenosis of the ISA of less than 70% with TIMI III blood flow through it, as well as an angiographic picture of the lesion with a possible high risk of developing microcirculatory obstruction syndrome (no-reflow). Left main coronary artery disease, unsuitable anatomy for PCI, multiple coronary artery disease, mechanical complications of AMI, or failure of PCI are indications for CABG [24].
However, there are studies that do not demonstrate the benefits of invasive treatment for CABG. One of the small randomized trials, SMASH (The Swiss Multicenter trial of Angioplasty Shock), which included 55 patients, was stopped early: 30-day mortality in the PCI group was 69% versus 78% in patients who received medical therapy. After 12 months, mortality was 74 and 83%, respectively. The authors were unable to demonstrate the benefit of emergency PCI in improving survival outcomes after CABG [53].
The SHOCK trial randomized 302 patients to emergency revascularization (PCI or CABG) with medical stabilization (thrombolytic therapy, inotropic and vasopressor support) followed by long-term revascularization. There were no statistically significant differences in 30-day mortality between the revascularization and drug stabilization groups: 46.7% versus 56% ( p
=0.11). However, within 6 months and over the course of a year, the differences in mortality between these two groups became statistically significant in favor of patients with emergency revascularization: 53% versus 66% (
p
<0.03) [23].
Although PCI and CABG are common approaches in the treatment of coronary artery disease, each of these myocardial revascularization strategies has different objectives in patients with CABG. Stenting of the coronary arteries does not always achieve a high percentage of technical success, accompanied in some cases by the development of no-reflow syndrome, which only worsens the results of PCI in patients with CABG [22]. Thus, TIMI III blood flow in 1333 patients with CABG, according to German authors, was achieved only in 75.2% of cases [60]. According to I. Porto et al. [38], additional perioperative myocardial damage as a result of distal embolism (studied using magnetic resonance imaging) during PCI was diagnosed in 23% of patients; in another 11% of cases, the cause of perioperative myocardial infarction was the “snow-plowing” mechanism, when as a result of implantation stent, the atherosclerotic plaque is displaced with compromise of the mouths of the side branches of the ISA or collaterals [38]. In addition, most patients with CABG have three-vessel or left main coronary artery disease, making them candidates for CABG [24, 56]. Additional advantages of CABG for CABG, according to some authors, are the protection of damaged and ischemic myocardium with the help of cardioplegia, cardiopulmonary bypass to unload both ventricles of the heart and revascularization of non-infarcted areas of the heart. Chronic occlusions of the coronary arteries and mechanical complications of AMI are also successfully corrected thanks to open cardiac surgery [24]. In the SHOCK study, 47 patients with CABG underwent CABG, representing 36% of the total number of patients undergoing revascularization. Survival after CABG at 30 days (57.4%) did not differ from that in the PCI group (55.4%; p
=0.86).
At one year, survival after CABG was 46.8% versus 51.9% in patients after PCI ( p
= 0.71) [58]. According to J. Hochman et al. [24], in the future, with the development of anesthesia, the advantages of cardioplegia, the development of new and improvement of existing devices for circulatory support will significantly improve the results of cardiac surgical treatment for CABG [24].
An important point in the treatment of patients with ACS is the identification of factors whose presence increases the risk of developing CABG [37]. Clinical prognostic factors for the occurrence of CS include female gender, elderly and senile age, acute disturbance of cerebral blood supply, angina pectoris and AMI in history, low left ventricular ejection fraction (<35%), extensive nature of the infarction (according to dynamic assessment of cardiac enzymes), diseases peripheral arteries and diabetes mellitus [18, 31]. The PURSUIT study assessed risk factors for the development of CABG in patients with non-ST segment elevation ACS. ST segment depression on the initial electrocardiogram is also a risk factor for the development of CABG [19]. According to the GUSTO-I study [45], His bundle branch block in patients with AMI resulted in higher mortality and incidence of CABG. A sharp increase in the incidence of CABG (12% vs. 6%; p
<0.035) was observed in patients with elevated blood glucose concentrations on admission. According to the analysis, hyperglycemia was the strongest prognostic factor for the development of CABG [59].
A number of studies have attempted to identify various prognostic factors for death in patients with CABG. Among the independent factors influencing mortality in CABG, different authors [1, 14] indicate age, history of angina pectoris, creatinine level and hyperlipidemia. TIMI 0-I ISA blood flow after thrombolysis and TIMI blood flow less than 3 after PCI are also unfavorable prognostic factors in patients with CABG [14]. Older age, left main coronary artery disease, three-vessel coronary artery disease, and a significant period from the onset of shock to PCI according to another registry are also independent prognostic factors for death [60]. In the work of R. Andrié et al. [1] analyzed various factors, including humoral ones, that can influence the results (primarily 30-day mortality) of treatment for CABG. Among the plasma factors, the authors identified interleukin-6, procalcitonin and the N-terminal precursor of brain natriuretic peptide. The concentration of these markers in shock patients who underwent revascularization and intra-aortic balloon counterpulsation (IABP) was determined upon admission, after 24 and 72 hours. Interleukin-6 turned out to be the most significant prognostic factor of early postoperative mortality in CABG [1]. Among other factors influencing the results of treatment in CABG, a number of authors highlight interferon-γ, tumor necrosis factor α, macrophage inflammatory protein-1β, granulocyte colony-stimulating factor, etc. [41].
In the long-term period after PCI, prognostic factors for unfavorable outcome are age over 65 years, high initial levels of creatinine and glucose [5].
A number of works [14, 57] are devoted to the use of various drugs in the treatment of patients with CABG. Inotropic support (dobutamine, norepinephrine) is a necessary component in the complex treatment of CABG; in the case of catecholamine-resistant CABG, the administration of levosimendan is recommended [57].
The use of glycoprotein IIb/IIIa platelet receptor blockers, according to a number of studies, improves the results of treatment of STEMI, but its role in the complex treatment of AMI complicated by CABG remains unknown [2, 9, 16, 27]. The PURSUIT study [19] showed that shock patients treated with eptifibatide had a 50% reduction in 30-day mortality. In another study [2], in the group of patients with abciximab, 30-day mortality was 18% versus 42%, where this drug was not used. A. Chan et al. [9] compared the effectiveness of abciximab in 4 groups of patients with CABG: the stenting + abciximab group, the isolated stenting group, the balloon angioplasty + abciximab group, and the isolated balloon angioplasty group. Efficiency was assessed over 2.5 years. The mortality rate for the specified period of time was 33, 43, 61 and 68%, respectively. TIMI III blood flow according to ISA was achieved more often in patients using abciximab: 85% versus 65%; p
=0.048 [9].
Positive results from the use of abciximab (in terms of the frequency of achieving TIMI III blood flow, mortality, recurrent infarctions) were also obtained in the work of S. Giri et al. [16]. However, in a study by P. Tousek et al. [51], which compared the effectiveness of abciximab in 2 groups (usual use and selective use), the benefit of its usual use was not demonstrated for any of the 30-day outcome measures (death, reinfarction, stroke, renal failure). However, significant hemorrhagic complications occurred more than 2 times more often in patients with routine use of abciximab: 17.5% versus 7.5% ( p
= 0.31) [51]. Despite the majority of beneficial effects of IIb/IIIa receptor blockers in the treatment of CABG, no randomized trials have been conducted on this issue.
The use of IABP in CABG has also been the subject of many studies, the results of which are contradictory [10, 12, 20, 39]. Class I recommendations and level of evidence The benefits of using IABP in the complex treatment of CABG are available according to European guidelines, while the ACC/AHA recommendations indicate class IIa and level B if CABG is refractory to medical treatment [36]. The intra-aortic balloon is placed in the descending aorta, just below the origin of the left subclavian artery. The principle of operation of the device is to mechanically pump blood in both the proximal and distal directions, due to the inflation of the balloon during diastole. This leads to an increase in blood flow through the coronary arteries, myocardial perfusion and support for the pumping function of the left ventricle. Sharp deflation of the balloon during presystole reduces afterload on the left ventricle of the heart, reducing myocardial oxygen consumption. The incidence of IABP use in patients with CABG ranges from 20 to 98% [47, 50, 58]. In Germany, IABP is used in approximately ¼ of patients with CABG [61]. In the work of G. Fornaro et al. [12] presented the experience of treating 20 patients with CABG, in whom myocardial revascularization (endovascular interventions or CABG), elimination of atrial septal defects and mitral insufficiency were carried out against the background of IABP. The mortality rate was 35%: 7 patients died (4 from CABG, 1 from a hemorrhagic complication, 1 from septic shock, 1 from heart failure after CABG). According to the authors, IABP is a useful and necessary component in the complex treatment of patients with CABG, allowing to stabilize their condition and improve the results of myocardial revascularization [12]. In the IABP SHOCK Trial, patients with AMI complicated by CABG and undergoing PCI were randomized into 2 groups: with or without IABP. It was shown that the use of IABP had a moderate positive effect on the condition of patients in accordance with the APACHE II scale, improved cardiac index, reduced the systemic inflammatory response, and reduced the content of brain natriuretic peptide compared to patients in whom IABP was not used [39]. In a more recent study, the authors assessed the effect of using an IABP in 40 patients with CABG who also underwent PCI. While causing a temporary improvement in the main hemodynamic parameters in patients with CABG, IABP did not lead to statistically significant differences in these parameters between patients with IABP and those receiving only drug treatment. According to the authors [40], the effectiveness of using IABP in patients with CABG remains unknown [40].
In a study conducted by T. Sanborn et al. [43], four groups of patients with CABG were compared: 1st - without thrombolytic therapy and IABP, 2nd - only with IABP, 3rd - only with thrombolysis and 4th - with IABP and thrombolytic therapy. Thrombolysis compared with no thrombolysis reduced in-hospital mortality (54% vs. 64%; p
=0.005).
The same situation was observed in patients with CABG and IABP, in whom mortality (50%) was lower, in contrast to patients with CABG and without IABP (72%) [43]. K. Sjauw et al. [47] demonstrated experience in treating 292 patients with CABG. In the group of patients ( n
=93) in whom IABP was not used, mortality was 47%, while among patients (
n
=199) with IABP it was 28%.
Among 199 patients with IABP, two groups were also identified: 1st - with IABP before PCI and 2nd - IABP after PCI, while mortality was higher in group 1: 62% versus 40% [47]. One of the latest and largest studies (prospective, open, multicenter and randomized) assessing the effectiveness of IABP in shock patients was conducted by H. Thiele et al. [50]. The work analyzed the results of treatment of two groups: 1st ( n
= 298) - with the use of IABP and 2nd (
n
= 300) - optimal drug therapy.
Myocardial revascularization was carried out in all studied patients. At the same time, no significant differences were found between the two groups either in 30-day mortality or in the incidence of hemorrhagic and ischemic complications, sepsis or stroke. The use of IABP did not reduce 30-day mortality in CABG (39.7% vs. 43.1%; p
= 0.69) [34]. The lack of reduction in mortality and the incidence of various cardiac and vascular complications in the case of the use of IABP has been demonstrated in other studies. In turn, the feasibility of using IABP for CABG against the background of mechanical complications of AMI (rupture of the interventricular septum, chordal avulsion, etc.) is beyond doubt [7].
Recent studies have questioned the effectiveness of IABP in the treatment of CABG, and the scope of its application may be reduced to a minimum [20, 61].
A. Jacobs et al. [29] compared the results of treatment with CABG in 881 patients with STEMI ( n
=729) and STEMI (
n
=152).
The authors found that patients with CABG and NSTEMI had a significantly more severe clinical condition and aggravated medical history: these were patients of older age groups who more often had AMI, heart failure, reconstructive vascular surgery, and a history of peripheral arterial disease. Myocardial revascularization was performed in both groups in approximately the same proportion: 36.8% in NSTEMI and 41.9% in NSTEMI ( p
= 0.277). Mortality in the STEMI group was 62.5%, which was not statistically significantly different from the rate for STEMI (60.4%). Despite almost the same mortality, the authors [29] point to patients with NSTEMI and CABG as the most difficult cohort of patients requiring earlier intervention and aggressive reperfusion therapy.
In recent years, studies have appeared demonstrating the equal effectiveness and safety of radial access compared to femoral for PCI in the treatment of patients with CABG [5, 15, 42]. In addition to the comparable effectiveness of using radial access, according to T. Fujii et al. [15], it also demonstrated fewer complications. Mortality during CABG in the radial approach group did not differ from that in patients with femoral access: 28.9% versus 25.5% ( p
=0.7) [15].
Comparing both approaches in the treatment of patients with CABG, I. Bernat et al. [5] showed that radial access is effective and safe in more than 50% of patients presenting with clinical signs of CABG. In addition, the use of radial access led, according to the authors [5], to a decrease in one-year mortality from 64% for the femoral approach to 44% for the radial approach ( p
= 0.0044).
According to O. Rodriguez-Leor et al. [42], radial access was successfully used in ⅔ of patients with CABG, demonstrating an advantage over femoral access in terms of the incidence of complications at the puncture site, the need for transfusion of blood components, and mortality (32.5% versus 64.3%; p
= 0.001) , as well as in the incidence of major cardiovascular complications (death, heart attack, stroke, severe bleeding and posthypoxic encephalopathy) - 43.8% versus 73.8% (
p
= 0.001).
A number of works have also been devoted to the use of extracorporeal membrane oxygenation (ECMO) in CABG, but most of them study the use of this method in severe respiratory disorders or post-cardiotomy CABG [6, 44, 49, 52]. The device takes venous blood, saturates it with oxygen using a membrane oxygenator and returns it to the systemic arterial circulation. The technique is most suitable in cases of severe right-left ventricular and cardiopulmonary failure, cardiac arrest. Peripheral cannulation involves access through the femoral or jugular vein with drainage of blood into the femoral artery; in the case of central cannulation (mainly in cardiac surgery), venous blood is taken from the right atrium with subsequent drainage into the ascending aorta [24]. The combined use of ECMO and IABP in patients with CABG treated with PCI increased 1-year survival to 63.4%, while isolated use of IABP was associated with only 24% 1-year survival [52]. The need to use ECMO in order to improve the results of treatment of CABG for thrombosis of the left main coronary artery is indicated by F. Hussain et al. [28]. Among 105 patients, the technical success of using ECMO was achieved in 95%, the overall mortality rate was 7.6%, while among patients younger than 75 years and without intervention on the left main coronary artery, the mortality rate was 2.6% [55]. According to F. Shawl et al. [46], ECMO is often the only alternative in the treatment of refractory CABG, allowing ⅓ of patients to be discharged from the hospital. The disadvantages of this technique are the high risk of hemorrhagic and ischemic complications due to the large diameter of the devices used, which are installed in the vessels (16-18 F) [24].
Moderate hypothermia (32-34 °C) in the complex treatment of patients with CABG is beginning to take a confident position, demonstrating effectiveness both in experimental and clinical conditions. There are invasive methods (using special heat-exchange catheters installed in the femoral vein) and non-invasive methods of hypothermia, when cold water circulates through a special heat-exchange blanket covering at least 70% of the patient's body [8, 17, 35, 48]. Moderate hypothermia improves treatment outcomes in CABG by reducing oxygen consumption and using vasopressor support, which makes it possible to recommend the use of the method in hemodynamically unstable patients [62]. The technique also improves metabolic and hemodynamic parameters in patients with CABG [54], positively affecting survival in CABG patients [48]. However, the effectiveness of hypothermia in the treatment of patients with CABG has not been sufficiently proven.
Thus, cardiogenic shock still remains a severe and in most cases fatal complication of AMI and a number of other heart diseases. Despite the development of interventional cardiology, cardiac surgery and the emergence of various circulatory support devices, cardiogenic shock and methods of its treatment require further study with additional randomized studies.