Algorithm for choosing antihypertensive therapy for metabolic syndrome and arterial hypertension
S.V.
Nedogoda, T.A. Chalyabi, A.S. Salasyuk, I.N. Barykina. D.A. Pocheptsov, A.A. Ledyaeva, V.V. Tsoma, E.V. Chumachek, Volgograd State Medical University ACE inhibitors and angiotensin II receptor blockers belong to the main classes of antihypertensive drugs used in the treatment of arterial hypertension in patients with metabolic syndrome.
Each of the representatives of this group has clinical-pharmacological characteristics and nuances of clinical use. Based on their analysis, an algorithm for differentiated selection of a specific drug is proposed. It is known that in arterial hypertension against the background of metabolic syndrome, pronounced dysfunction of the vascular endothelium (excess of vasoconstrictors and deficiency of vasodilators, primarily nitric oxide), increased activity of the renin-angiotensin-aldosterone and sympatho-adrenal systems (high levels of leptin), vasospasm against the background of increased cardiac output, increased sodium reabsorption in the nephron tubules (due to hyperinsulinemia and compression of the kidneys by adipose tissue), fluid retention and hypervolemia, increased sodium and calcium content in the vascular wall, obstructive sleep apnea. The vast majority of patients have insulin resistance. They experience early target organ damage (left ventricular hypertrophy, quickly leading to diastolic myocardial dysfunction, increased stiffness of large arteries, hypofiltration in the kidneys and microalbuminuria). Typical metabolic disorders in arterial hypertension are impaired glucose tolerance, increased levels of triglycerides, cholesterol and uric acid. All these features significantly influence the choice of antihypertensive drug for the correction of blood pressure (BP) in patients with metabolic syndrome.
For patients with arterial hypertension and metabolic syndrome, it seems fundamentally important to ensure the positive effect of antihypertensive drugs on adipokines produced by adipose tissue, which today is no longer considered as an energy depot, but as an endocrine and paracrine organ that has a humoral, prothrombogenic and pro-inflammatory effect on other organs and body systems. Among more than 50 adipokines in obesity, the greatest role in the pathogenesis of both obesity itself and associated diseases and conditions is played by resistin, ghrelin, visfatin, apelin, adiponectin, TNF-alpha and interleukin 6. However, by far the most studied and important adipokine in obesity leptin is a hormone produced by fat cells and circulating in the blood in free and bound forms. Through its influence on specific receptors of the hypothalamus, leptin changes the expression of neuropeptides that regulate energy intake and expenditure in the body. In obesity, compensatory resistance of the hypothalamus to the central action of leptin develops with the development of leptin resistance (hyperleptinemia) through a feedback mechanism. Leptin has a number of effects that can aggravate existing disorders in arterial hypertension and obesity (stimulation of the sympatho-adrenal system, increased rate of fibrosis of the heart muscle, increased platelet aggregation, sodium retention, increased insulin resistance).
It seems clinically important to find a “leader” drug, since a patient with a combination of arterial hypertension and metabolic syndrome requires not only normalization of blood pressure, but also the maximum possible correction of all other modifiable risk factors (Fig. 1 and 2). The latest 2013 European recommendations for the treatment of arterial hypertension (ESH/ESC Guidelines for the management of arterial hypertension) declare that since metabolic syndrome is often regarded as a “prediabetic” condition, preference is given to blockers of the renin-angiotensin system and calcium antagonists, so as they have the potential to improve, or at least not worsen, insulin sensitivity, whereas beta blockers (except beta blockers with vasodilating properties) and diuretics should be considered as adjunctive agents, preferably used in low doses. The recommendations in the section “Drugs of choice in certain situations” also indicate that ACE inhibitors, angiotensin II receptor blockers (ARBs) and calcium antagonists are the drugs of choice for metabolic syndrome [1].
Thus, while there is some certainty with the classes of antihypertensive drugs that have benefits in patients with arterial hypertension and metabolic syndrome, there is no certainty regarding their specific representatives. At the same time, it is quite clear that the so-called “class-specific effects” are increasingly being called into question. That is why it seems advisable to clarify the capabilities of specific drugs for this pathology and offer the doctor an algorithm for their differentiated use.
ACE inhibitors
It is no coincidence that ACE inhibitors play an important role in the treatment of arterial hypertension in patients with metabolic disorders. This is due to their positive influence on the key links in the pathogenesis of the disease. Representatives of this group of drugs reduce the activity of the RAAS both in plasma and in tissues, slow down the inactivation of bradykinin and reduce the activity of the sympathetic nervous system (primarily by reducing insulin resistance and the level of postprandial glycemia).
The undoubted advantage of drugs in this group is the absence of a negative effect on carbohydrate, lipid and purine metabolism. Large multicenter studies SOLVD, ASCOT, HOPE revealed a significant reduction in the number of new cases of diabetes among patients receiving long-term ACE inhibitors.
These drugs have an organoprotective effect: they reduce left ventricular myocardial hypertrophy, slow down myocardial remodeling and fibrosis, reduce MAU, proteinuria, and prevent nephroangiosclerosis and chronic renal failure. The high effectiveness of ACEIs in slowing the progression of kidney disease and reducing the risk of cardiovascular complications was shown in the AIRE, REIN, PROGRESS, ADVANCE studies. The vasoprotective effect of ACE inhibitors is to improve the function of the endothelium of the vascular wall, antiproliferative and antiatherogenic effects. Large clinical studies (PHYLLIS, SECURE) have found that ACE inhibitor therapy can reduce the thickness of the carotid artery intima-media complex in patients with asymptomatic or manifest atherosclerosis.
An important advantage of ACE inhibitors is the ability to prevent the development of cardiovascular complications in high- and very high-risk patients, including hypertensive patients with MS and diabetes. The studies SOLVD, EUROPA, PROGRESS, FACET, ADVANCE showed that treatment with ACE inhibitors reduces the risk of recurrent stroke, ischemic events, hospitalizations due to decompensation of CHF and other cardiovascular complications and death from them.
There are 3 groups of ACE inhibitors depending on the chemical structure: those containing an SH group (captopril, zofenopril), a carboxyl group (enalapril, quinapril, ramipril, perindopril (Prestarium A®), trandolapril, spirapril, cilazapril, lisinopril), a phosphinyl group (fosinopril) . Since in arterial hypertension with metabolic disorders there is often an increase in the volume of adipose tissue, the choice of a specific ACEI should take into account its lipophilicity, since a higher level of lipophilicity determines a greater tissue affinity of the drug (i.e., the ability to influence ACE activity not only in circulation , but also directly in tissues). Most ACEIs are eliminated by the kidneys; only 4 drugs (zofenopril, fosinopril, trandolapril, spirapril) have a dual elimination route - through the liver and kidneys.
The active metabolites of fosinopril, quinapril, trandolapril, ramipril and perindopril (Prestarium A®) are highly lipophilic; moderate - enalapril, moexipril and captopril; Lisinopril is a hydrophilic compound. It is the high lipophilicity that determines the preferred choice of drug when a patient has a combination of arterial hypertension and metabolic syndrome, since only in this case is it possible to block the release of vasoactive humoral substances (primarily angiotensin II and proinflammatory cytokines) from the adipocyte. Currently, in the absence of a large evidence base on the differentiated use of antihypertensive drugs, there are two polar points of view on the choice of the optimal drug depending on lipo- and hydrophilicity. Proponents of the predominant use of hydrophilic ACEIs in the treatment of patients with obesity and arterial hypertension believe that lipophilic ACEIs are distributed in adipose tissue, and therefore it is necessary to increase their dosage to achieve the desired therapeutic effect. They believe that lisinopril, which is the only hydrophilic ACEI and is not “absorbed” by adipose tissue, appears to be more effective in this clinical situation. In addition, the following argument is put forward in favor of lisinopril: obese patients often have concomitant liver diseases (steatosis and steatohepatitis), and therefore preference should be given to ACEIs that are not metabolized in the liver, namely lisinopril, which is the only ACEI with no hepatic metabolism. However, it should be noted that there is also a fairly strong argument in favor of the use of lipophilic ACE inhibitors. First, today adipose tissue is considered as an endocrine-active tissue, in which the secretion of adipokines is closely related to the activity of RASS (primarily angiotensin). Therefore, it is the action of ACEIs at the level of the adipocyte itself that can positively influence the production of adipokines, which hydrophilic ACEIs, by definition, cannot do.
Among ACE inhibitors with high lipophilicity, the most studied in the treatment of arterial hypertension and metabolic syndrome are perindopril (Prestarium A®) and ramipril.
Perindopril (Prestarium A®) has clearly demonstrated its advantages over enalapril and losartan in several studies [3, 4, 5]. It has been shown that therapy with perindopril (Prestarium A®) in patients with arterial hypertension and metabolic syndrome can achieve better blood pressure control than with enalapril and losartan, more pronounced cardio-, angio- and nephroprotection, improvement in lipid and carbohydrate metabolism, reduction insulin resistance and inflammation, as well as a positive effect on one of the key adipokines in obesity - leptin.
Ramipril demonstrated its benefits in patients with arterial hypertension and metabolic syndrome in the international MICRO-HOPE study and the Russian CHARISM study.
Angiotensin II receptor blockers
It is known that ARBs (sartans) suppress the activity of the renin-angiotensin-aldosterone system more completely than ACEIs and do not affect the bradykinin system. In terms of antihypertensive effectiveness, ACEIs and ARBs are equivalent, but the latter have a better tolerability profile, because do not cause cough, angioedema. ARBs provide higher adherence to therapy among patients with hypertension due to a better tolerability profile and the absence of “escape” of the hypotensive effect.
ARBs have a pronounced cardio- and nephroprotective effect: they prevent left ventricular hypertrophy, increase the duration of sinus rhythm retention in paroxysmal atrial fibrillation, reduce the degree of microalbuminuria and proteinuria, and slow the progression of heart and renal failure. With long-term use, ARBs reduce carotid intima-media thickness and the volume of large atherosclerotic plaques (MORE study with telmisartan).
A significant reduction in the risk of developing diabetes during ARB therapy was also found in the LIVE, VALUE, CHARM, and ALPIN studies. In the LIFE study, the reduction in the relative risk of developing new cases of diabetes while taking losartan was 25%, in SCOPE with eprosatartan therapy - 20%, and in CHARM with candesartan therapy - 22%. Meta-analysis data also showed that ARB therapy is associated with a reduction in the development of new cases of diabetes [2].
At the same time, a significant reduction in the incidence of cardiovascular complications was noted in the treatment of ARBs in hypertensive patients with diabetes (LIFE, IDNT, RENAAL).
An important advantage of ARB therapy in light of the metabolic syndrome epidemic is that the number of AT1 receptors and their sensitivity to angiotensin II increases sharply with obesity, hyperinsulinemia and dyslipidemia.
An important mechanism of action of ARBs is the modulation of the activity of PPARγ receptors responsible for glucose homeostasis, lipid metabolism and blood pressure regulation. This effect not only reduces blood pressure, but also normalizes levels of glucose, insulin and triglycerides. Moreover, the results of a number of studies indicate a decrease in tissue insulin resistance due to stimulation of nuclear PPARγ receptors in the cells of adipose, muscle tissue and hepatocytes, and this effect is comparable to the effect of oral hypoglycemic drugs.
Thus, there is a compelling case for widespread use of the ARB class in metabolic syndrome. At the same time, the practicing physician faces the problem of differentiated selection of a specific drug for a specific patient. In this regard, it is advisable to consider the clinical and pharmacological characteristics of ARB representatives. Moreover, significant intraclass differences between them find new confirmation [5]. Thus, in a clinical study, two drugs from the ARB group (telmisartan and losartan) were compared. The study found that telmisartan, unlike losartan, reduces the concentration of free blood glucose, unbound insulin and HbA1c, thereby identifying the main intraclass differences between drugs in the potential improvement of metabolic disorders in patients with MS [6]. Losartan
Over the past decade, numerous and convincing data have emerged on the close relationship between increased uric acid levels and an increased risk of cardiovascular complications in hypertension, metabolic syndrome, and type 2 diabetes. At the same time, a connection has been proven between impaired uric acid metabolism and endothelial dysfunction [7]. According to the PIUMA Study, the presence of hyperurecemia increases the risk of cardiovascular complications by 1.73 times, and the risk of mortality by 1.96 times. The prevalence of HU with a combination of arterial hypertension and metabolic syndrome is 37.8%, and in its absence - 22% [8]. At the same time, the duration of the disease does not affect the frequency of GU. Patients with uric acid levels above 300 µmol/l have more pronounced metabolic risk factors, which are directly dependent on the degree of increase in uric acid. Losartan blocks the two main transport systems of distal tubular epithelial cells involved in the reabsorption of urates (urate/lactate and urate/chloride) and protects the structures of the renal tubulointerstitium from the damaging effects of urates. When using losartan, the excretory pool of uric acid increases only due to inhibition of urate reabsorption without increasing filtration, which fundamentally distinguishes it from classical uricosuric drugs, the use of which increases urate filtration and increases the risk of developing nephrolithiasis. It has been shown that losartan itself, and not its active metabolite E-3174, has uricosuric activity [9]. Consequently, it is due to the characteristics of the molecule, and not to blockade of the AT1 receptor, which is fundamentally important when analyzing the class-specific effects of this group of antihypertensive drugs. The LIFE trial, which assessed the effects of losartan and atenolol on cardiovascular events and mortality in high-risk hypertensive patients with left ventricular hypertrophy, also examined the association of baseline uric acid levels with other risk factors and disease prognosis and was the first large-scale study to do so. demonstrated that a decrease in uric acid levels during losartan therapy is associated with a positive effect on the incidence of complications in the treatment of arterial hypertension.
Valsartan
The NAVIGATOR study showed that in patients with impaired glucose tolerance and risk factors, treatment with valsartan leads to a relative reduction of 14% and an absolute reduction of 3.8% in the incidence of new cases of diabetes [10].
A distinctive feature of valsartan is its positive effect on sexual function in men and women [11,12]. This is due to its ability to exhibit a direct vasodilating effect, improve endothelial function and microcirculation, reduce the volume of connective tissue in the corpora cavernosa, increase testosterone levels in men and indirectly, through metabolites of angiotensin II (angiotensin IV), influence the dopaminergic system involved in the regulation of sexual behavior.
Candesartan
The metabolic effects of long-term antihypertensive therapy with candesartan were studied in the ALPINE study [13]. When compared with hydrochlorothiazide, the candesartan group showed a decrease in insulin levels, blood glucose, triglycerides and an increase in HDL. In the hydrochlorothiazide group, compared with the candesartan group, there was also an increase in the ratios of LDL cholesterol/HDL cholesterol and apolipoprotein B/apolipoprotein A-I, indicating a deterioration in the lipid profile. Against the background of these changes, diabetes mellitus was diagnosed in 4.1% of patients in the hydrochlorothiazide group and only 0.5% of patients in the candesartan group, and metabolic syndrome was diagnosed in 18 patients in the hydrochlorothiazide group and only 5 patients in the candesartan group. A distinctive feature of the drug is the presence of an evidence base for effective use in chronic heart failure, including in the subgroup of patients with impaired carbohydrate metabolism.
Irbesartan
The nephroprotective effect and ability of the drug to reduce albuminuria has been proven in large studies (IRMA, IDNT). But an important feature of irbesartan is its ability to achieve target blood pressure in metabolic syndrome in 70% of patients. At the same time, all of them showed a decrease in waist circumference and a decrease in the initially elevated levels of insulin, glucose and blood lipids. Moreover, it should be noted that these changes are most pronounced when the patient has a combination of arterial hypertension and metabolic syndrome [14]. Similar results were obtained in another study [15]. In addition, recent studies have shown that irbesartan and telmisartan act as partial agonists of peroxisome proliferator-activated receptor γ (PPARγ) at oral concentrations and doses recommended for the treatment of hypertension, making their use possible for improving insulin sensitivity [16]. Telmisartan
Telmisartan exhibits maximum activity against PPARs compared to other ARBs and has maximum lipophilicity. PPARγ play a key role in the metabolism and energy metabolism of adipose tissue - creating lipid reserves in white adipose tissue and increasing energy expenditure in brown fat. PPARγ is also involved in adipocyte differentiation and in the regulation of glucose metabolism by improving insulin sensitivity, being a link in the metabolism of lipids and carbohydrates. The use of telmisartan may prevent the development of atherosclerosis by reducing visceral fat, suppressing vascular inflammation and increasing adiponectin levels, especially in patients with metabolic syndrome.
A recently published meta-analysis of 10 randomized trials involving 546 patients with metabolic syndrome showed that telmisartan significantly reduced fasting glucose, hyperinsulinemia, glycated hemoglobin and increased insulin sensitivity and adiponectin levels [17].
It is important to note that telmisartan also has the same positive effect on all of these indicators, as well as on lipids and resistin, during therapy with rosiglitazone [18]. In addition, only telmisartan in combination with rosuvastatin reduces insulin resistance and C-reactive protein levels, in contrast to the combination of this statin with irbesartan and olmesartan [19].
Thus, analysis of the evidence base for the use of ARBs in metabolic syndrome, the features of their clinical pharmacology and experience in practical use allows us to offer the practitioner a simple algorithm for their differentiated use in this condition (Fig. 3). Calcium antagonists
As is known, this group includes dihydropyridine (nifedipidine, amlodipine, felodipine, lacidipine) and non-dihydropyridine (verapamil and diltiazem) calcium antagonists. Representatives of the first subgroup, against the background of a decrease in blood pressure, which develops due to peripheral vasodilation, can increase the heart rate (HR) and contribute to the activation of the SAS. Representatives of the second group, while maintaining pronounced antihypertensive activity, have a significantly less pronounced peripheral vasodilating effect than dihydropyridine calcium antagonists. Moreover, they are able to reduce heart rate (by suppressing the activity of sinus node automaticity) and reduce the activity of the SAS (data from the VAMPHYR study), which is important for obese patients. It should be noted that, according to many large studies, calcium antagonists significantly reduce the number of new cases of diabetes mellitus (INVEST, INSIGHT, ALLHAT), are able to reduce left ventricular hypertrophy and have an anti-sclerotic effect (VHAS).
Diuretics
One of the main mechanisms of increased blood pressure in arterial hypertension against the background of metabolic syndrome is hypervolemia, which occurs as a result of increased reabsorption of sodium and water in the proximal renal tubules against the background of hyperinsulinemia and increased vascular resistance. Therefore, diuretics could become one of the main classes of antihypertensive drugs used for this pathology. However, the undoubted advantages of classical thiazide diuretics are clearly insufficient to compensate for their negative effects (primarily metabolic effects - hypokalemia, deterioration of carbohydrate, lipid and purine metabolism).
According to the results of clinical observations, all thiazide diuretics worsen carbohydrate metabolism, even at a daily dose of 12.5 mg. Moreover, the higher the initial level of glycemia, the more it increases with their use. In young people, impaired glucose tolerance develops on average after 5 years of continuous use of thiazide diuretics, and in older people - 1 to 2 years after the start of their use. In the case of concomitant diabetes mellitus, glycemic control worsens within the first few days of starting thiazide diuretics. In addition to the adverse effect on carbohydrate metabolism, thiazide diuretics can have a negative effect on lipid and purine metabolism.
Indapamide retard (Arifon retard) stands apart among diuretics, which, unlike classical thiazide diuretics, does not have a negative effect on the metabolism of glucose, lipids and uric acid. Thus, according to the Russian multicenter study MINOTAVR (619 patients with metabolic syndrome and arterial hypertension), it proved to be a drug that can effectively reduce blood pressure and have a positive effect on carbohydrate, lipid and purine metabolism. It must be borne in mind that indapamide retard (Arifon retard) has a proven and pronounced cardio-, angio- and nephroprotective effect, which makes it in the group of diuretics the drug of choice for the treatment of patients with obesity and disorders of carbohydrate, lipid and purine metabolism, both mono- and combination therapy.
Beta blockers
Increased activity of the SAS in obese patients dictates the need for the use of β-blockers. However, non-selective β-blockers (atenolol, propranolol) negatively affect carbohydrate and lipid metabolism. In addition, β-blockers (including selective β1-blockers in high doses), by blocking β-adrenergic receptors of the pancreas, inhibit the release of insulin. Since β-blockers cause the development of IGT and weight gain, their use in uncomplicated hypertension and obesity is not recommended as first-line therapy. However, this group of drugs can be used for obesity and arterial hypertension in cases where it is not possible to achieve the target blood pressure level due to pronounced activation of the SAS and tachycardia.
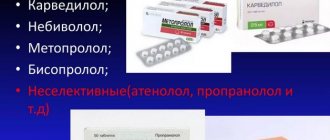
“New” highly selective β1-blockers (bisoprolol, carvedilol, nebivolol) are practically free of those adverse side effects that limited their widespread use in patients with impaired carbohydrate and lipid metabolism. In addition, nebivolol has the unique property of increasing NO production and reducing the manifestations of endothelial dysfunction, which is typical for obese patients. In addition, the drug has a beneficial effect on carbohydrate metabolism (decrease in triglyceride levels) and cerebral hemodynamics, which can significantly expand the possibilities of its use in arterial hypertension against the background of metabolic syndrome. Nebivolol does not have an “antilipase” effect and it reduces total and peripheral vascular resistance, improves regional blood flow, renal perfusion, glomerular filtration rate, and increases the sensitivity of peripheral tissues to insulin. Therefore, among beta-blockers, the drug of choice for a combination of arterial hypertension and metabolic syndrome is nebivolol.
Imidazoline receptor agonists
A prerequisite for prescribing I2-imidazoline receptor agonists to patients with obesity and arterial hypertension is their ability to increase tissue sensitivity to insulin and improve carbohydrate metabolism. At the same time, according to the Russian multicenter study ALMAZ, in patients with metabolic syndrome, moxonidine therapy significantly improved lipid and carbohydrate metabolism, tissue sensitivity to insulin, contributed to a decrease in body weight and leptin levels in the blood and improved vascular endothelial function. Moreover, these positive changes were comparable in severity to metformin therapy.
Alpha - adrenergic blockers
Alpha-blockers (despite the results of the ALLHAT study) retain their therapeutic potential in the treatment of arterial hypertension in obese patients, due to their ability to reduce insulin resistance, improve carbohydrate and lipid metabolism and have a positive effect on renal hemodynamics. However, use should be limited to use only in combination with other antihypertensive agents.
As a result of the analysis of clinical trial data, the evidence base and the clinical pharmacology of antihypertensive drugs, it is possible to offer the practitioner an algorithm for their differentiated use in arterial hypertension and metabolic syndrome (Fig. 4).
Literature
1. ESH/ESC Guidelines for the management of arterial hypertension. Journal of Hypertension 2013; 31:1281–1357. 2. Elliott W., Meyer P. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet 2010; 369: 201 – 207. 3. Krysiak1 R., Sierant1 M.., et al. The effect of angiotensin-converting enzyme inhibitors on plasma adipokine levels in normotensive patients with coronary artery disease. Pol J Endocrinol 2010; 61 (3): 280–286 4. Krysiak1 R., Sierant1 M.., et al. The effect of perindopril and enalapril on plasma resistin levels in normotensive patients with coronary heart disease. Pol J Endocrinol 2010; 61 (6): 683–690 5. Nedogoda S., Ledyaeva A., Chumachok E., Tsoma V., Mazina G., Salasyuk A., Barykina I. Randomized Trial of Perindopril, Enalapril, Losartan And Telmisartan in Overweight or Obese Patients With Hypertension // Clin Drug Investig 2013, 33:553–561 6. Vitale S, Mercuro G, et al. Metabolic effect of telmisartan and losartan in hypertensive patients with metabolic syndrome. Cardiovascular Diabetology 2005; 4:6-11. 7. Lebedeva M.V., Stakhova T.Yu., Minakova E.G., Zaitseva L.I., Severova M.M., Pulin A.A. Endothelial function in patients with arterial hypertension and uric acid metabolism disorders // Bulletin of the Russian Academy of Medical Sciences. 2010. No. 12. pp. 44-46. 8. Kobalava Zh.D., Kotovskaya Yu.V., Tolkacheva V.V., Malto A.S. Uric acid is a key component of the cardiorenometabolic continuum // Cardiovascular Therapy and Prevention 2008. No. 4.95-106. 9. Alderman M., Aiyer KJ Uric acid: role in cardiovascular disease and effects of losartan. Curr Med Res Opin 2004; 20 (3): 369–379. 10. NAVIGATOR Study Group. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med 2010; 366:1477-90. 11. Dusing R. Effect of the angiotensin II antagonist valsartan on sexual function in hypertensive men. Blood Press Suppl 2003;2:29-34. 12. Fogari R., Preti P., Derosa G. et al. Effect of antihypertensive treatment with valsartan or atenolol on sexual activity and plasma testosterone in hypertensive men. Eur J Clin Pharmacol 2002;58(3):177-80. 13. Lindholm LH, Persson M., Alaupovic P., Carlberg B. Metabolic outcome during 1 year in newly detected hypertensives: results of the Antihypertensive Treatment and Lipid Profile in a North of Sweden Efficacy Evaluation (ALPINE study). J Hypertension 2003;21(8):1563-1574. 14. Mamirbaeva K.M., Mychka V.B., Sergienko V.B., Chazova I.E. Metabolic syndrome and angiotensin II receptor antagonists. // Cardiovascular therapy and prevention 2007. No. 2. P.42-51. 15. Kintscher U., Bramlage P., et al. Irbesartan for the treatment of hypertension in patients with the metabolic syndrome: A sub analysis of the Treat to Target post authorization survey. Prospective observational, two armed study in 14,200 patients. Cardiovascular Diabetology 2007; 6: 12-16. 16. Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW: Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension 2004, 43(5):993-1002 17. Takagi H, Niwa M, Mizuno Y, Goto SN, Umemoto T; ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. Telmisartan as a metabolic sartan: the first meta-analysis of randomized controlled trials in metabolic syndrome. J Am Soc Hypertens. 2013,7(3):229-35 18. Derosa G, Fogari D, et al. Metabolic effects of telmisartan and irbesartan in type 2diabetic patients with metabolic syndrome treated with rosiglitazone. Journal of Clinical Pharmacy and Therapeutics 2007; 32: 261–268. 19. Rizos CV, Milionis HJ, Kostapanos MS, et al. Effects of rosuvastatin combi-ned with olmesartan, irbesartan, or telmisartan on indices of glucose metabolism in Greek adults with impaired fasting glucose, hypertension, and mixed hyperlipidemia: a 24-week, randomized, open-label, prospective study. Clin Ther 2010; 32:492-505.
Spurs. Pharmacology. / agonists to imidazoline receptors
Imidazoline receptor agonists
Mechanism of action
Imidazoline receptors are localized both in the central nervous system (in the nuclei of the reticular formation, rostral ventrolateral region of the medulla oblongata) - subtype 1, and on the periphery (for example, in the kidneys, pancreas) - subtype 2. The latter are also found on mitochondria. Another type of receptor is described that does not belong to any of the mentioned types and is localized in sympathetic nerve endings. Their activation leads to a decrease in the production of norepinephrine.
Activation of imidazoline receptors leads to an increase in the synthesis of arachidonic acid and inhibition of Na+/H+ ion exchange channels. Activation of central I1 receptors leads to a decrease in blood pressure and a decrease in heart rate, due to a central suppressive effect on the peripheral sympathetic nervous system. Differences in the therapeutic and hemodynamic effect of centrally acting drugs are due to unequal affinities for different types of receptors. The first-generation centrally acting drug clonidine has an affinity for two types of receptors: central α-aderonoreceptors and imidazoline receptors. Its hypotensive effect is largely due to stimulation of imidazoline receptors, while the main side effects are mediated by cortical α1-adrenergic receptors.
Rilmenidine and moxonidine are highly selective for I1 receptors. Their affinity for I1 receptors is more than 100 times greater than their affinity for α2 adrenergic receptors. Both drugs are characterized by a pronounced hypotensive effect, sometimes accompanied by a slight sedative effect. The hypotensive effect of imidazoline receptor agonists and the resulting decrease in peripheral vascular resistance are associated with their pronounced peripheral sympatholytic activity. In this case, stimulation of I1 receptors causes only a slight decrease in heart rate (HR). It has been shown that bradycardia when using clonidine is largely associated with stimulation of α-adrenergic receptors.
Pharmacokinetics
Rilmenidine (1-2 mg/day once, Bioavailability 100% Plasma protein binding 10% T_ - about 8 hours, The main route of elimination is through the kidneys unchanged, Onset of action - after 1 -1.5 hours, Maximum - 2- 5 hours Duration - 24 hours, No withdrawal syndrome and orthostatic hypotension)
Moxonidine (0.2-0.4 mg/day once, Bioavailability 90% Plasma protein binding - 8%, half-life - 2-3 hours, Main route of elimination is renal excretion, Onset of action - 0.5 hours Maximum action - 2- 5 hours Duration - 24 hours, No tolerance with long-term use No withdrawal syndrome
Both I1 receptor agonists have similar pharmacokinetic characteristics. It should be noted that despite the relatively short half-life, the hypotensive effect of the drugs after a single dose persists throughout the day. The maximum decrease in diastolic blood pressure at the peak concentration was 30.9 mmM. Hg Art. A single dose of moxonidine, according to 24-hour blood pressure monitoring, while providing a long-term hypotensive effect, does not change the circadian rhythm of blood pressure [14]. The duration of the therapeutic effect of imidazoline agonists is associated with their accumulation in the nuclei of the brain.
Side effects, tolerability
The most common side effects of rilmenidine are dry mouth (4.9%), asthenia (4.1%), insomnia (4.5%). Their severity depends on the dose of the drug and decreases with long-term therapy. When using moxonidine, dry mouth was most often observed (in 12.9% of patients). However, in general, numerous registration and post-marketing studies indicate that moxonidine is very well tolerated - it caused dry mouth and sedation in less than 10% of patients, which is much less common than with other centrally acting antihypertensive drugs. In addition to dry mouth and sedation, insomnia (5-8%) and headache (6%) were observed with moxonidine use. The highest incidence of side effects was in patients of older age groups.