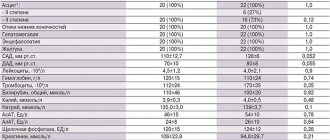
Pharmacological properties of the drug Ramipril
Antihypertensive drug, ACE inhibitor. By suppressing the synthesis of angiotensin II, it reduces its vasoconstrictor effect and stimulating effect on aldosterone secretion. Increases renin activity in blood plasma, inhibits bradykinin metabolism. Reduces peripheral vascular resistance, does not significantly change renal blood flow (and in some cases increases it) and glomerular filtration rate. It has an antihypertensive effect both in the standing and lying position of the patient, and does not cause compensatory tachycardia. It has an antihypertensive effect, both at high and low levels of renin in the blood plasma. After a single dose, the antihypertensive effect is observed within 1–2 hours, reaches a maximum after 3–6 hours and lasts for 24 hours. With daily use, the antihypertensive effect increases gradually over 3–4 weeks and persists with long-term treatment. Sudden withdrawal does not lead to a rapid increase in blood pressure. Ramipril also has a cardioprotective effect due to the inhibition of ACE in the myocardium and, possibly, due to the accumulation of bradykinin. There is evidence that ramipril promotes the reverse development of myocardial hypertrophy in patients with hypertension (arterial hypertension), reduces the frequency of arrhythmias during myocardial reperfusion. The ability of ramipril to prevent contractile changes in the vascular endothelium resulting from eating foods high in cholesterol has been described. After oral administration, it is quickly absorbed in an amount of at least 50–60% of the dose taken. Concomitant food intake does not affect the degree of absorption, but slows down the absorption of the active substance. The maximum level in blood plasma is reached 2–4 hours after administration. Primary metabolism occurs in the liver, resulting in the formation of a pharmacologically active derivative of ramipril - ramiprilat. Ramiprilat is approximately 6 times more active in inhibiting ACE than ramipril. Plasma protein binding is 73% for ramipril, and 56% for ramiprilat. In patients with liver disease, the conversion of ramipril to ramiprilat slows down, the level of ramipril in the blood plasma may increase 3 times, but the maximum concentration of ramiprilat in the blood plasma does not change. In heart failure, there is an increase in the concentration of ramiprilat in the blood by 1.5–1.8 times. In elderly people, pharmacokinetics do not change significantly.
Ramipril
The drug ramipril belongs to the group of angiotensin-converting enzyme inhibitors (ACEIs) and is used to treat a wide range of cardiovascular diseases. These drugs firmly entered into cardiological practice about three decades ago and in such a short time they became the first choice drugs in the treatment of arterial hypertension and heart failure. Despite the apparent pharmacological homogeneity of the ACEI group, there are important differences between its members that may cause the choice or refusal of one or another drug in a particular clinical case. A prominent representative among the ACE inhibitors is ramipril, synthesized in the 80s of the last century. Since then, many controlled clinical trials have been conducted, confirming its high effectiveness in the treatment of various manifestations of cardiovascular pathology. Due to the strong evidence base, ramipril is widely used in European countries where, according to statistics, it accounts for about 20% of all medical prescriptions for ACE inhibitors. In Russia, this drug is much less popular: domestic therapists and cardiologists prescribe it only in 6% of cases, preferring the good old captopril (a pioneer drug among ACE inhibitors) and enalapril.
Ramipril is a long-acting, fat-soluble ACE inhibitor. It is a prodrug and is transformed into an active form while already in the human body. After oral administration, it is quickly absorbed from the digestive tract. The bioavailability of the drug is in the range of 50–65%. Eating does not affect the complete absorption of ramipril, but reduces its speed. The active form of the drug - ramiprilat - is formed in the liver as a result of deesterification of ramipril, which, by the way, is 6 times inferior in pharmacological activity to its “alter ego”. The half-life of ramipril is 13-17 hours, which allows it to be prescribed no more than once a day.
As practice has shown, taking the drug more rarely increases adherence to treatment, making pharmacotherapy more effective.
The antihypertensive effect of the drug begins to develop 1-2 hours after administration, reaches its peak at 5-7 hours and lasts for at least one day. With regular daily intake of ramipril, its antihypertensive activity gradually increases. At 3-4 weeks, blood pressure stabilizes at the desired level, and remains there regardless of the duration of treatment (up to 1-2 years). The “effectiveness” of ramipril in terms of lowering blood pressure knows no age, gender, or constitutional (body weight) boundaries: the drug can help everyone. However, as a rule, it does not cause excessive hypotension at the beginning of treatment, and abrupt discontinuation of the drug is not fraught with the development of withdrawal syndrome. Ramipril can reduce left ventricular hypertrophy (an ominous harbinger of heart attacks and strokes). The drug improves survival in patients with myocardial infarction aggravated by acute left ventricular failure. As for the survival of patients with chronic heart failure (CHF), large-scale studies in this area have not yet been conducted. However, several small clinical trials have clearly demonstrated that ramipril effectively affects the neurohormonal link in the pathogenesis of CHF, increases the body's resistance to physical stress and, ultimately, improves the quality of life. An additional advantage of ramipril over other ACE inhibitors is its nephroprotective effect, which develops both in diabetic nephropathy (damage to the kidney vessels due to disorders of carbohydrate and lipid metabolism) and in other kidney diseases. This fact further expands the scope for prescribing ramipril to a wide variety of patient groups.
Use of the drug Ramipril
The initial dose for the treatment of hypertension (arterial hypertension) is usually 2.5 mg 1 time per day (in the morning) on an empty stomach; subsequently, if necessary, the dose is gradually increased every 2–3 weeks. In some patients, a uniform antihypertensive effect is achieved by using ramipril 2 times a day. The maximum daily dose is 10 mg, maintenance - 2.5–5 mg. In the absence of optimal blood pressure reduction, diuretics can be prescribed. For chronic heart failure, the recommended initial dose is 1.25 mg/day. Depending on the patient's response, the dose can be increased (double within 1–2 weeks). If the daily dose is 2.5 mg or more, it is taken in 1 or 2 doses. When treating patients who have suffered myocardial infarction, the recommended initial dose is 2.5 mg 2 times a day. In patients with impaired renal function (creatinine clearance from 30 to 60 ml/min), patients with diabetes mellitus, as well as in patients over the age of 65 years, the initial daily dose should not exceed 1.25 mg, and the maximum - 5 mg.
Side effects of the drug Ramipril
Arterial hypotension, collapse and associated tachycardia, arrhythmia, angina pectoris, myocardial infarction, stroke; the appearance or intensification of renal dysfunction up to the development of acute renal failure (especially with simultaneous use of diuretics), proteinuria, dry cough, bronchitis, shortness of breath, sinusitis, rhinitis, in some cases - bronchospasm; nausea, pain in the epigastric region, dyspepsia, vomiting, diarrhea, constipation, dysphagia, anorexia, increased activity of liver enzymes and bilirubin concentration in the blood serum, hepatitis, cholestatic jaundice, liver failure, pancreatitis, anxiety, depression, dizziness, increased irritability, drowsiness, sleep disturbances, amnesia, tremor, convulsions, neuralgia, neuropathy, paresthesia, hearing loss, tinnitus, blurred vision, itching, rash, urticaria, photosensitivity, angioedema, erythema multiforme, arthralgia, arthritis, myalgia, hyperkalemia, decreased hemoglobin and hematocrit levels, leukopenia, eosinophilia, edema, nosebleeds, impotence, increased sweating, general weakness, weight gain, increased concentrations of uric acid and glucose in the blood.
Special instructions for the use of Ramipril
Patients are warned to immediately consult a doctor if they develop fever, swollen lymph nodes and/or sore throat (these symptoms may be associated with the development of agranulocytosis). At the beginning of treatment and regularly during therapy, the number of leukocytes, the level of hemoglobin in the peripheral blood, the level of potassium, creatinine and liver enzymes in the blood are monitored, especially in patients with impaired renal function, diffuse connective tissue diseases, receiving immunosuppressants, cytostatic agents, allopurinol or procainamide . Patients who have a decrease in blood volume and/or sodium deficiency (for example, due to long-term use of diuretics, sodium restrictions, hemodialysis, repeated vomiting or diarrhea) are especially at risk of developing significant arterial hypotension when prescribed ramipril. Before starting the use of ramipril in such patients, blood volume and sodium levels are corrected. If ramipril is prescribed to patients already taking diuretics, in order to avoid the development of significant arterial hypotension, these drugs are discontinued within 2–3 days. Then, if blood pressure is not sufficiently controlled by taking ramipril alone, diuretics are added again. If preliminary withdrawal of diuretics is not possible, ramipril is prescribed in the minimum initial dose (1.25 mg/day). In patients with heart failure, ramipril can also cause the development of severe arterial hypotension, which in some cases is accompanied by oliguria or azotemia and (rarely) acute renal failure. Patients with an increased risk of developing arterial hypotension after taking the first dose of ramipril, as well as after increasing the dose of it or a diuretic, should be under strict medical supervision, especially in the first 2 weeks of treatment. During treatment with ramipril, hemodialysis or hemofiltration using membranes based on polyacryl metall sulfonate (for example, “AN 69”) with high ultrafiltration activity is excluded, since in this case there is a risk of developing serious anaphylactoid reactions. In the case of emergency hemodialysis, the patient is first transferred to another antihypertensive drug (but not an ACE inhibitor) or other membranes are used for hemodialysis. In case of development of angioedema, especially spreading to the tongue, pharynx or larynx, adrenaline, corticosteroids, and antihistamines are administered intravenously. Prescribe with caution to patients whose work requires increased attention and speed of mental and motor reactions, especially at the beginning of treatment and during the change of antihypertensive drugs. It is not recommended to drink alcohol at the same time.
Ramipril
Before starting treatment with Ramipril, it is necessary to eliminate hyponatremia and hypovolemia. In patients who have previously taken diuretics, it is necessary to discontinue them or at least reduce their dose 2-3 days before starting Ramipril (in this case, the condition of patients with CSI should be carefully monitored, due to the possibility of their developing decompensation during against the background of an increase in circulating blood volume (CBV)).
After taking the first dose of the drug, as well as when increasing its dose and/or the dose of diuretics (especially loop diuretics), it is necessary to ensure careful medical monitoring of the patient for at least 8 hours so that appropriate measures can be taken in a timely manner in case of an excessive decrease in blood pressure. If Ramipril is used for the first time or at a high dose in patients with increased RAAS activity, their blood pressure should be carefully monitored, especially at the beginning of treatment, as these patients have an increased risk of excessive reduction in blood pressure (see section "With caution").
In case of malignant arterial hypertension and heart failure, especially in the acute stage of myocardial infarction, treatment with Ramipril should only
in a hospital setting.
In patients with CHF, taking the drug can lead to the development of a pronounced decrease in blood pressure, which in some cases is accompanied by oliguria or azotemia and rarely by the development of acute renal failure.
Caution should be exercised when treating elderly patients, as they may be particularly sensitive to ACE inhibitors; it is recommended to monitor renal function in the initial phase of treatment (see also section "Dosage and Administration").
In patients for whom a decrease in blood pressure may pose a certain risk (for example, in patients with atherosclerotic narrowing of the coronary or cerebral arteries), treatment should begin under strict medical supervision.
Caution should be exercised during physical activity and/or hot weather due to the risk of increased sweating and dehydration with the development of arterial hypotension due to a decrease in blood volume and a decrease in sodium concentration in the blood.
During treatment with Ramipril, it is not recommended to drink alcohol (ethanol). A transient excessive decrease in blood pressure is not a contraindication for continuing treatment after stabilization of blood pressure. In case of repeated development of a pronounced decrease in blood pressure, the dose should be reduced or the drug discontinued.
The simultaneous use of Ramipril with drugs containing aliskiren or with ARA II, leading to double blockade of the RAAS, is not recommended due to the risk of an excessive decrease in blood pressure, the development of hyperkalemia and deterioration of renal function compared to monotherapy.
The simultaneous use of ACE inhibitors with drugs containing aliskiren is contraindicated in patients with diabetes mellitus and/or with moderate or severe renal impairment (GFR less than 60 ml/min/1.73 m2 body surface area) and is not recommended in other patients (see. sections “Contraindications” and “Interactions with other drugs”).
Concomitant use with ARA II is contraindicated in patients with diabetic nephropathy and is not recommended in other patients (see sections “Contraindications” and “Interactions with other drugs”).
Cases of angioedema of the face, extremities, lips, tongue, larynx and/or pharynx have been observed in patients treated with ACE inhibitors.
If swelling occurs in the face (lips, eyelids) or tongue, or difficulty swallowing or breathing, the patient should immediately stop taking the drug. Angioedema, localized in the area of the tongue, pharynx or larynx (possible symptoms: difficulty swallowing or breathing), can be life-threatening and requires urgent measures to relieve it: subcutaneous administration of 0.3-0.5 mg or intravenous drip of 0.1 mg of epinephrine (adrenaline) (under the control of blood pressure, heart rate and ECG) followed by the use of glucocorticosteroids (iv, intramuscular, or orally); Intravenous administration of antihistamines (H1- and H2-histamine receptor antagonists) is also recommended; in case of insufficiency of C1-esterase enzyme inactivators, the need to introduce C1-esterase enzyme inhibitors in addition to epinephrine (adrenaline) can be considered. The patient should be hospitalized and monitored until symptoms are completely relieved, but not less than 24 hours.
In patients receiving ACE inhibitors. There have been cases of intestinal angioedema, which was manifested by abdominal pain with or without nausea and vomiting; in some cases, angioedema of the face was simultaneously observed. If a patient develops the symptoms described above during treatment with ACE inhibitors, the possibility of developing intestinal angioedema should be considered when making a differential diagnosis.
Treatment aimed at desensitization to insect venom (such as bees, wasps) and concomitant use of ACE inhibitors can initiate anaphylactic and anaphylactoid reactions (for example, decreased blood pressure, shortness of breath, vomiting, allergic skin reactions), which can be life-threatening. During treatment with ACE inhibitors, hypersensitivity reactions to insect venom (such as bees, wasps) develop faster and are more severe. If desensitization to insect venom is necessary, the ACE inhibitor should be replaced with an appropriate drug from another group.
Life-threatening, rapidly developing anaphylactoid reactions, sometimes leading to shock, have been described with the use of ACE inhibitors during hemodialysis or plasma filtration using certain high-flow membranes (for example, polyacrylonitrile membranes) (see also membrane manufacturer's instructions). The combined use of Ramipril and the use of this type of membrane, for example, for urgent hemodialysis or hemofiltration, should be avoided. In this case, it is preferable to use other types of membranes or avoid taking ACE inhibitors. Similar reactions were observed with low-density lipoprotein apheresis using dextran sulfate. Therefore, this method should not be used in patients receiving ACE inhibitors.
In patients with impaired liver function, the response to treatment with Ramipril may be enhanced or weakened.
In addition, in patients with severe liver cirrhosis with edema and/or ascites, significant activation of the RAAS is possible, so special care should be taken when treating these patients (see also section "Dosage and Administration").
Before surgery (including dental surgery), it is necessary to warn the surgeon/anesthesiologist about taking ACE inhibitors.
It is recommended to closely monitor neonates exposed in utero to ACE inhibitors for hypotension, oliguria, and hyperkalemia. In oliguria, it is necessary to maintain blood pressure and renal perfusion by administering appropriate fluids and vasoconstrictors. These neonates are at risk of developing oliguria and neurological disorders, possibly due to decreased renal and cerebral blood flow due to the decrease in blood pressure caused by ACE inhibitors.
Monitor laboratory parameters before and during treatment with ramipril (up to 1 time per month in the first 3-6 months of treatment).
Monitoring kidney function (determining serum creatinine concentrations)
When treating with ACE inhibitors, it is recommended to monitor renal function in the first weeks of treatment and subsequently. Particularly careful monitoring is required in patients with acute and CHF, impaired renal function, after kidney transplantation, patients with renovascular diseases, including patients with hemodynamically significant unilateral renal artery stenosis in the presence of two kidneys (in such patients, even a slight increase in serum creatinine concentration may be an indicator of a decrease kidney function).
Electrolyte control
Regular monitoring of serum potassium and sodium levels is recommended. Particularly careful monitoring of potassium levels in the blood serum is required for patients with impaired renal function, significant disturbances in water and electrolyte balance, and CHF.
Monitoring of hematological parameters (hemoglobin, number of leukocytes, erythrocytes, platelets, leukocyte formula)
It is recommended to monitor the complete blood count to identify possible leukopenia.
More regular monitoring is recommended at the beginning of treatment and in patients with impaired renal function, as well as in patients with connective tissue diseases or in patients simultaneously receiving other drugs that can change the peripheral blood picture (see section "Interactions with other drugs") .
Monitoring the number of leukocytes is necessary for the early detection of leukopenia, which is especially important in patients with an increased risk of its development, as well as at the first signs of infection. If neutropenia is detected (the number of neutrophils is less than 2000/μl), discontinuation of treatment with ACE inhibitors is required.
If symptoms due to leukopenia appear (for example, fever, enlarged lymph nodes, tonsillitis), urgent monitoring of the peripheral blood picture is necessary. If signs of bleeding appear (tiny petechiae, red-brown rashes on the skin and mucous membranes), monitoring the number of platelets in the peripheral blood is also necessary.
Determination of the activity of “liver” enzymes, the concentration of bilirubin in the blood
If jaundice or a significant increase in the activity of liver enzymes occurs, treatment with ramipril should be discontinued and the patient should be monitored by a physician.
The use of ACE inhibitors in rare cases has been accompanied by the development of a syndrome starting with cholestatic jaundice or hepatitis and progressing to fulminant liver necrosis, sometimes with death. The mechanism of development of this syndrome is unknown.
Cases of cough occurring during therapy with ACE inhibitors have been observed. As a rule, the cough is non-productive, constant and stops after discontinuation of the drug. Cough associated with the use of ACE inhibitors. should be taken into account in the differential diagnosis of dry cough.
Some patients treated with ramipril experience syndrome of inappropriate antidiuretic hormone secretion (SIADH), followed by the development of hyponatremia. It is recommended to regularly monitor plasma sodium levels in elderly patients and other patients at risk of developing hyponatremia.
Ethnic characteristics
Ramipril, like other ACE inhibitors, has a less pronounced antihypertensive effect in patients of the Negroid race compared to representatives of other races. The drug should be prescribed with caution to patients of the Black race due to a higher risk of developing angioedema.
Patients after kidney transplantation
There is insufficient experience with the use of Ramipril in patients who have recently undergone kidney transplantation.
Drug interactions Ramipril
When taking antihypertensive, diuretic drugs, opioid analgesics, and anesthetics simultaneously, the antihypertensive effect of ramipril may be enhanced. With simultaneous use of NSAIDs (for example, acetylsalicylic acid, indomethacin), table salt, a decrease in the antihypertensive effect is possible. When taking potassium supplements and potassium-sparing diuretics (for example, amiloride, spironolactone, triamterene), a significant increase in potassium levels in the blood is possible. When taken simultaneously with lithium preparations, it is possible to increase the concentration of lithium in the blood (regular monitoring of lithium levels is necessary). With simultaneous treatment with antidiabetic agents (sulfonylurea derivatives, insulin), the hypoglycemic effect may be enhanced. When used simultaneously with allopurinol, cytostatic agents, immunosuppressants, procainamide, leukopenia may develop. With the simultaneous use of alcohol, a sharp increase in the effect of alcohol is possible.
Ramipril, 28 pcs., 5 mg, tablets
Contraindicated combinations - Use of certain high-flow membranes with a negatively charged surface (for example, polyacrylonitrile membranes) when performing hemodialysis or hemofiltration; use of dextran sulfate in low-density lipoprotein apheresis Risk of severe anaphylactic reactions. Not recommended combinations - With potassium salts, potassium-sparing diuretics (for example, amiloride, triamterene, spironolactone) A more pronounced increase in serum potassium levels is possible (with simultaneous use, careful monitoring of serum potassium levels is required). Combinations that should be used with caution - With antihypertensive drugs (especially diuretics) and other drugs that lower blood pressure (nitrates, tricyclic antidepressants) Potentiation of the hypotensive effect; when combined with diuretics, serum sodium levels should be monitored. — With sleeping pills, narcotic and non-narcotic analgesics. A more pronounced decrease in blood pressure is possible; - With vasopressor sympathomimetics (epinephrine) Reducing the hypotensive effect of ramipril, careful monitoring of blood pressure is required. — With allopurinol, procainamide, cytostatics, immunosuppressants, systemic glucocorticosteroids and other drugs that can affect hematological parameters. Combined use increases the risk of developing leukopenia. — With lithium salts Increased serum concentration of lithium and increased cardio- and neurotoxic effects of lithium. - With hypoglycemic agents for oral administration (sulfonylurea derivatives, biguanides), insulin. Due to the decrease in insulin resistance under the influence of ramipril, the hypoglycemic effect of these drugs may be enhanced, up to the development of hypoglycemia. Combinations that should be taken into account - With non-steroidal anti-inflammatory drugs (indomethacin, acetylsalicylic acid) The effect of ramipril may be weakened, the risk of renal dysfunction and increased potassium levels in the blood serum may be increased. — With heparin It is possible to increase the potassium content in the blood serum. — With sodium chloride Weakening the hypotensive effect of ramipril and less effective treatment of symptoms of chronic heart failure. — With ethanol Increased vasodilation. Ramipril may increase the adverse effects of ethanol on the body. — With estrogens Weakening the hypotensive effect of ramipril (fluid retention). — Desensitization therapy for hypersensitivity to insect venoms ACE inhibitors, including ramipril, increase the likelihood of developing severe anaphylactic or anaphylactoid reactions to insect venoms. With the simultaneous use of ACE inhibitors and gold preparations (sodium aurothiomalate) for intravenous administration, a symptom complex has been described, including facial flushing, nausea, vomiting and arterial hypotension
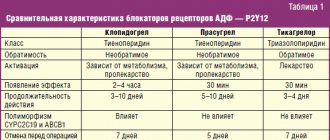

![Rice. 1. ACCOMPLISH study: the effect of the combination of ACE inhibitor* amlodipine and ACE inhibitor hydrochlorothiazide on the risk of primary endpoint events (CVE) [14]](https://expert35.ru/wp-content/uploads/ris-1-issledovanie-accomplish-vliyanie-kombinacii-iapf-amlodipin-i-iapf-330x140.jpg)