© Author: Z. Nelly Vladimirovna, laboratory diagnostics doctor at the Research Institute of Transfusiology and Medical Biotechnology, especially for SosudInfo.ru (about the authors)
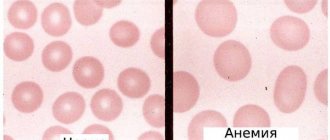
The term “macrocytosis” refers to a phenomenon in which large quantities of erythrocytes (red blood cells) are present in the blood, slightly or significantly changing their size towards an increase. Anemic syndrome, caused by the presence of a large number of enlarged formed elements of the erythrocyte series, is classified as macrocytic anemia.
Macrocytosis is detected by an automatic analyzer (erythrocyte indices MCV and RDW), and then by a doctor in a stained blood smear when studying the morphological characteristics of red blood cells.
Cells with a diameter of 8 microns and above fall into the category of macrocytes (the diameter of healthy red blood cells is ≈ 7-8 microns - normocytes, everything below is microcytes).
Meanwhile, in certain pathological conditions (lack of vitamin B12 and folic acid, for example), cells appear in the blood that are larger in size than macrocytes, their diameter is more than 10 microns - these are megalocytes . The anemia that results from this will also be macrocytic, but more often it is called megaloblastic in order to emphasize its origin. It should be borne in mind that not all macrocytic anemias are megaloblastic; in other pathological conditions caused by other reasons, red blood cells do not increase to such enormous sizes and remain macrocytes.
Enlarged cells usually carry a larger amount of red blood pigment, chromoprotein - hemoglobin, so their color may be more intense than those red blood cells that are saturated with hemoglobin correctly or even less than necessary. This intense staining of macrocytes is called hyperchromia. Macrocytosis with hyperchromia are the main laboratory signs of hyperchomic macrocytic anemia.
blood is normal and with hyperchromic macrocytic anemia
Macrocytosis of erythrocytes in a general blood test
The key point in the diagnostic search (in relation to macrocytic anemia) is a general blood test (hemogram), or rather, its individual parameters:
- Morphological characteristics of red blood cells (detection of macrocytes, poikilocytosis);
- MCV indicator (mean blood cell volume) - it shows values above 120 femtoliters with macrocytosis of erythrocytes;
- Hematocrit level (in severe vitamin deficiency anemia for a long time, the hematocrit drops to 30-25%, although in other forms of macrocytic anemia it may remain within normal values).
It should be noted that the nature of the process is significant, since it is the chronic course of the pathology that creates the preconditions for the disappearance of normal red cells and their replacement by macrocytes.
In patients suffering from anemic syndrome for a shorter period of time, the indicator may only slightly exceed the upper limit of normal values or even be within the normal range. Then you will have to take into account other circumstances that could create conditions for the development of macrocytic anemia. Macrocytosis, caused by the use of chemotherapy drugs or damage to the hepatic parenchyma, is usually mild or moderate. Reticulocytosis with a more or less uniform increase in red blood cells usually also does not raise doubts with the doctor. But with regard to vitamin deficiency conditions, questions always arise. However, the table below will help you compare and evaluate laboratory parameters for different conditions.
Table: options for increasing the size of red blood cells
| Pathological condition | MCV, fl | Morphological characteristics of cells |
| Megaloblastic anemia HCT (hematocrit) below 30% | 100 — 130 | Hyperchromia, macrocytes, poikilocytosis (ovalocytes, schizocytes) |
| Reticulocytosis (RET more than 10%) | 100 — 110 | Polychromatophilic elements - macrocytes (immature forms) |
| Lesions of the liver parenchyma | 90 — 110 | Macrocytes, uniform increase in cell diameter and volume (all red blood cells are approximately the same size) |
Macrocytosis caused by a lack of cyanocobalamin - vitamin B12, or folic acid - vitamin B9 (or both) has noticeable differences. When studying a smear morphologically:
blood for B12 deficiency anemia
- Poikilocytosis of erythrocytes;
- The presence of fragments of destroyed cells in the smear (schizocytosis);
- Hyperchromia;
- Changes in the “white” blood - a significant increase in the content of neutrophilic leukocytes, the nucleus of which has 5 or 6 segments, while normally the number of cells containing more than five segments in their nucleus is unlikely to reach 5% of the total number of segmented neutrophils .
The presence of a huge number of polysegmented neutrophil leukocytes is a kind of equivalent of poikilocytosis of red blood cells and is an important laboratory symptom of megaloblastic anemia.
Complete blood count with leukocyte formula
Complete Blood Count
A routine screening blood test, which includes determining the concentration of total hemoglobin, the number of red blood cells, leukocytes and platelets per unit volume, the hematocrit value and erythrocyte indices (MCV, MCH, MCHC).
Hemoglobin (Hb, Hemoglobin)
A respiratory pigment in the blood, which is contained in red blood cells and is involved in the transport of oxygen and carbon dioxide.
It consists of a protein part - globin - and an iron-containing part - heme. Hemoglobin is a protein of quaternary structure, formed by four polypeptide chains. Iron in heme is in divalent form. The hemoglobin content in the blood of men is slightly higher than that of women. In children of the first year of life, a physiological decrease in hemoglobin concentration is observed. An increase in hemoglobin concentration is observed when the blood thickens or is the result of an increase in the formation of red blood cells. A pathological decrease in hemoglobin content in the blood (anemia) may be a consequence of increased losses of hemoglobin during various bleedings, the result of accelerated destruction (hemolysis) of red blood cells, impaired formation of red blood cells, or other reasons. Anemia can be either an independent disease or a symptom of a general chronic disease (anemia of chronic diseases). As an independent disease, anemia develops with a lack of iron necessary for the synthesis of hemoglobin, with a deficiency of vitamins involved in the formation of red blood cells (mainly vitamin B12, folic acid), due to increased destruction of red blood cells in the peripheral blood (hemolytic anemia) or impaired formation of blood cells in the bone marrow for specific hematological diseases.
Hematocrit (Ht, Hematocrit)
The share (%) of all formed elements of the total blood volume.
This indicator, along with hemoglobin and red blood cells, is used to monitor the condition of the erythrocyte system. Hematocrit reflects the volume of all the formed elements in the blood - mainly red blood cells - and not their number. Changes in hematocrit do not always correlate with changes in total red blood cell count. For example, in patients in shock due to blood thickening, the hematocrit may be normal or even high, although due to blood loss, the total number of red blood cells may be significantly reduced. Therefore, the hematocrit value is not indicative when assessing the degree of anemia immediately after blood loss or blood transfusion.
Red Blood Cells (RBC)
Highly specialized anucleate blood cells containing hemoglobin, the main function of which is to transport oxygen from the lungs to the tissues and carbon dioxide from the tissues to the lungs.
Red blood cells are formed in the red bone marrow from stem cells. For normal development of red blood cells, vitamin B12, folic acid and an adequate supply of iron are necessary. The formation of red blood cells is stimulated by erythropoietin, which is produced in the kidneys. The level of erythropoietin increases with tissue hypoxia. The average lifespan of red blood cells in the vascular bed is 120 days. Old cells are destroyed in the reticuloendothelial system and spleen, and the iron in hemoglobin is used to form new red blood cells. In one day, about 1% of red blood cells are renewed. An increase in the number of red blood cells above normal levels is called erythrocytosis, a decrease in the number of red blood cells (and hemoglobin) is called anemia,
For the differential diagnosis of anemia, in addition to determining the number of red blood cells, an assessment of their morphological characteristics is used. Normally, the diameter of erythrocytes is 7.2-7.5 microns, the volume is 80-100 fl. Red blood cells with a diameter of less than 6.7 microns and a volume of less than 80 fL are called microcytes; red blood cells with a diameter of more than 7.7 microns and a volume of more than 100 fl - macrocytes; red blood cells larger than 9.5 microns in diameter are called megalocytes. Anisocytosis is the presence of red blood cells of different sizes in the blood. Depending on the predominance of certain forms of erythrocytes, they are distinguished: macrocytosis - a condition when 50% or more of the total number of erythrocytes are macrocytes (noted in B12 and folate deficiency anemia, liver diseases); microcytosis is a condition in which 30-50% are microcytes (observed in iron deficiency anemia, microspherocytosis, heterozygous thalassemia, lead intoxication).
The hemoglobin content in erythrocytes is assessed in terms of: normochromia, hypochromia, hyperchromia. To assess the morphology of erythrocytes, erythrocyte indices are used, which are obtained from a blood test using an automatic analyzer (MCV, MCH, MCHC - see below).
A more detailed description of the morphology of erythrocytes: changes in cell shape - poikilocytosis (presence of ovalocytes, schizocytes, spherocytes, target-like erythrocytes, etc.); presence of inclusions in erythrocytes; the content of nuclear forms of the erythroid series in peripheral blood; changes in cell coloring, etc. - if necessary, done by a hematologist when viewing a blood smear under a microscope. This information is reflected in the comments to the analysis.
Erythrocyte indices (MCV, MCH, MCHC)
Indices that allow quantitative assessment of the main morphological characteristics of red blood cells.
MCV - Mean Cell Volume
A quantitative indicator of the volume of red blood cells, a more accurate parameter than a visual assessment of the size of red blood cells when viewing a smear under a microscope. However, it should be taken into account that this parameter is an average value, and with pronounced anisocytosis, as well as in the presence of a large number of red blood cells with an altered shape, it does not sufficiently reflect the true size of the cells. Based on the MCV value, anemia is distinguished between microcytic, normocytic and macrocytic anemia. Microcytosis is characteristic of iron deficiency anemia, heterozygous thalassemia; macrocytosis - for B12 and folate deficiency anemia. Aplastic anemia can be normo- and macrocytic.
MCH - mean hemoglobin content in a red blood cell (Mean Cell Hemoglobin)
Calculated in absolute units, calculated by dividing the hemoglobin concentration by the number of red blood cells per unit volume. This parameter determines the average hemoglobin content in an individual red blood cell and is clinically similar to the color index. Based on this index, anemia can be divided into normo-, hypo- and hyperchromic.
MCHC - mean hemoglobin concentration in erythrocytes (Mean Cell Hemoglobin Concentration)
It is calculated by the ratio of blood hemoglobin to hematocrit and reflects the saturation of red blood cells with hemoglobin. This is a concentration index that does not depend on cell volume, unlike MCH. MSHC is a sensitive indicator of changes in hemoglobin formation, in particular, in iron deficiency anemia, thalassemia, and some hemoglobinopathies (decrease in MSHC).
White Blood Cells (WBC)
Blood cells that ensure the recognition and neutralization of foreign components, the elimination of altered and decaying cells of the body's own, effectors of immune and inflammatory reactions, the basis of the body's antimicrobial defense.
The formation of leukocytes (leukopoiesis) takes place in the bone marrow and organs of the lymphatic system. This is a group of cells heterogeneous in origin, structure and properties. There are 5 main types of leukocytes: neutrophils, eosinophils, basophils, lymphocytes, monocytes, which perform different functions. Differential calculation of the content of these forms is carried out when prescribing a leukocyte formula test. The total number of leukocytes can change under the influence of various factors. A physiological increase in the level of leukocytes occurs after eating, after physical activity, and due to various types of stress. Reactive physiological leukocytosis is ensured by the redistribution of stationary and circulating neutrophils, the mobilization of mature leukocytes from the bone marrow. In women, a physiological increase in the number of leukocytes can be observed in the premenstrual period. The number of leukocytes normally increases in the second half of pregnancy and during childbirth.
A pathological increase in the number of leukocytes in the blood is observed under the influence of various infectious agents, poisons, under the influence of inflammatory factors and tissue necrosis, endogenous toxins. These factors stimulate the formation of leukocytes, which is a protective reaction of the body.
With some viral infections, under the influence of cytotoxic drugs, leukopenia can develop - a decrease in the level of white blood cells. Significant changes in the number of leukocytes are observed in specific hematological diseases, which can manifest themselves as a significant increase in the content of leukocytes, or a sharp decrease in their number. Important diagnostic information in these cases is provided by determining the differential leukocyte formula by viewing a blood smear under a microscope.
Platelet count
Formed elements of blood involved in ensuring hemostasis. Platelets are small anucleate cells, oval or round in shape; their diameter is 2-4 microns. Platelets are formed in the bone marrow from megakaryocytes. In a calm state (in the bloodstream), platelets have a disc-shaped shape. When activated, platelets acquire a spherical shape and form special projections (pseudopodia). With the help of such outgrowths, blood platelets can connect with each other (aggregate) and adhere to the damaged vascular wall (adhesion ability).
When stimulated, platelets have the property of releasing the contents of their granules, which contain coagulation factors, the enzyme peroxidase, serotonin, calcium ions - Ca2*, adenosine diphosphate (ADP), von Willebrand factor, platelet fibrinogen, platelet growth factor. Platelets can carry some coagulation factors, anticoagulants and other substances on their surface. The properties of platelets interacting with the components of the vessel walls make it possible to form a temporary clot and stop bleeding in small vessels (platelet-vascular hemostasis). A temporary increase in platelet count can be observed after intense physical activity. A slight physiological decrease in platelet levels is observed in women during menstruation. A moderate decrease in platelet count can sometimes be observed in apparently healthy pregnant women.
Clinical signs of a decrease in platelet count - thrombocytopenia (increased tendency to intradermal hemorrhage, bleeding gums, menorrhagia, etc.) - usually occur only when the platelet count decreases below 50x103 cells/μl.
A pathological decrease in the number of platelets occurs due to their insufficient formation in a number of diseases of the blood system, as well as with increased consumption or destruction of platelets (autoimmune processes). After massive bleeding followed by intravenous infusions of plasma substitutes, the platelet count may decrease to 20-25% of the original value due to dilution.
An increase in the number of platelets (thrombocytosis) can be reactive, accompanying certain pathological conditions (as a result of the production of immunomodulators that stimulate platelet formation) or primary (due to defects in the hematopoietic system).
Neutrophils
They make up 50-75% of all leukocytes. In peripheral blood, two morphological types of these cells are normally found: band (younger) and segmented (mature) neutrophils. Less mature cells of the granulocytic series - young (metamyelocytes), myelocytes, promyelocytes - are normally found in the bone marrow and appear in the peripheral blood only in case of pathology. The appearance of the latter in the peripheral bloodstream indicates either stimulation of granulocyte formation in the bone marrow (reactive changes) or the presence of hemoblastosis. Mature neutrophils circulate in the blood for 8-10 hours, then enter the tissues. The lifespan of a neutrophil granulocyte in tissues is 2-3 days. The number of neutrophils, if necessary, can quickly increase due to the mobilization of mature cells from the parietal pool of the vascular bed or bone marrow reserve, or due to increased hematopoiesis. The main function of neutrophils is to participate in the fight against microorganisms through their phagocytosis. The contents of the granules are capable of destroying almost any microbes. Neutrophils contain numerous enzymes that cause bacteriolysis and digestion of microorganisms.
Options for changing (shifting) the leukocyte formula.
Neutrophilia (an increase in the number of neutrophils) can be reactive (associated with infection, inflammation, tumor or endocrine disorders) or associated with primary disorders of hematopoiesis (hemoblastoma).
Neutropenia (a decrease in the absolute neutrophil count to less than 1800/μl) can be caused by depletion of the neutrophil reserve (for example, due to septicemia), autoimmune diseases (agranulocytosis, sometimes caused by drugs), diseases of the blood system and other pathological conditions.
“Shift to the left”: (“rejuvenation” of neutrophils): an increased number of band neutrophils is present in the blood, the appearance of metamyelocytes (young) and myelocytes is possible.
Eosinophils
Eosinophils make up 0.5-5% of all blood leukocytes; they are in circulation for about 30 minutes, after which they enter the tissues, where they remain for about 12 days. Changes in the content of eosinophils in peripheral blood are the result of a balance in the production of cells in the bone marrow, their migration into tissues and destruction.
Eosinophils contain a significant amount of granules that contain a special group of bactericidal proteins, including eosinophil cationic protein, eosinophil peroxidase, etc. Having weak phagocytic activity, these cells cause extracellular cytolysis and participate in anthelmintic immunity. Chemotaxis of eosinophils and recognition of parasites is carried out due to factors produced by inflammatory cells and waste products of parasites. Eosinophils play an important role in allergic reactions. Eosinophilia (an increase in the number of eosinophils in the blood by more than 5% - 0.4x10°/l) often accompanies allergic diseases of various localizations (bronchial asthma, atopic eczema, hay fever, food allergies). In all inflammatory diseases, autoimmune processes, malignant neoplasms, chronic infections, skin diseases, the pathogenesis of which includes an allergic component determined by the hyperformation of IgE, eosinophilia is observed. Activated Eosinophils produce large amounts of proinflammatory mediators that are toxic to tissues, thereby maintaining chronic inflammation. Eosinophilia is detected in infectious diseases during the period of a full-blown clinical picture (scarlet fever, infectious mononucleosis, gonorrhea).
Assessing the dynamics of changes in the number of eosinophils during the inflammatory process has a certain prognostic value.
Eosinopenia (a decrease in the number of eosinophils in the blood to less than 0.2x10'/l) is often observed at the onset of inflammation. An increase in the number of eosinophils (> 5%) accompanies the onset of recovery. However, a number of infectious and other diseases with high levels of IgE are characterized by eosinophilia even after the end of the inflammatory process, which indicates an incomplete immune response. At the same time, a decrease in the number of eosinophils in the active phase of the disease often indicates the severity of the process and is an unfavorable sign.
Basophils
The smallest population of leukocytes. Basophils account for an average of only 0.5% of the total number of blood leukocytes. Ripe baeophils enter the bloodstream, where they circulate for about 6 hours. Then they migrate into tissues, where they die 1-2 days after performing their function. These are cells related to tissue mast cells. Basophils are capable of phagocytosis. Their granules contain sulfated or carboxylated acidic proteins, such as heparin, which acquire a blue color when stained with Giemsa, and other biologically active substances.
Basophils participate in allergic reactions involving IgE-dependent mechanisms and initiate the development of an immediate-type anaphylactic hypersensitivity reaction.
Basophilia (basophil content >0.15x10'/l) can be associated with allergic reactions, viral diseases, chronic infections, inflammatory processes, and cancer.
Lymphocytes
Lymphocytes make up 20-40% of the total number of leukocytes and represent a heterogeneous population of leukocytes. They belong to agranulocytes (do not contain granules in the cytoplasm). Different subpopulations of lymphocytes perform different functions. These include: recognition of various antigens due to the expression of unique antigen receptors on the surface of cells, formation of a humoral immune response through the synthesis of antibodies to foreign proteins (immunoglobulins of various classes), provision of cellular immunity - destruction of various cells directly by effector cytotoxic lymphocytes (transplant rejection, antitumor immunity, immunity against intracellular parasites, including antiviral). Some lymphocytes are memory cells that retain information about a previously encountered antigen. They proliferate rapidly and produce large quantities of antibodies when they encounter a known antigen again.
Lymphocytes have the ability to synthesize and secrete into the blood various protein regulators - cytokines, through which they coordinate and regulate the immune response. An increase in the content of lymphocytes is observed as a reaction to acute viral infections, chronic infections (tuberculosis and syphilis), this may also be a consequence of specific hematological diseases.
It should be borne in mind that the leukocyte formula reflects the relative (percentage) content of different types of leukocytes, and an increase or decrease in the percentage of lymphocytes can be both absolute and relative. Thus, a high percentage of lymphocytes in the formula may be a consequence of true (absolute) lymphocytosis, when the content of blood lymphocytes exceeds 3000/μl, or a decrease in the absolute number of leukocytes of other types (usually neutrophils) - in this case, lymphocytosis is relative. Lymphopenia (decrease in the number of lymphocytes) can also be absolute, when the number of cells drops below 1000/μl, or relative - be a consequence of an increase in the number of granulocytes.
Monocytes
Monocytes are the largest cells among leukocytes, make up 2-10% of all leukocytes, and belong to agranulocytes. In peripheral blood, monocytes are 80-600x10'/l. Monocytes circulate in the blood from 36 to 104 hours, then leave the vascular bed. In tissues, monocytes differentiate into organ- and tissue-specific macrophages. The lifespan of tissue macrophages (histiocytes) is calculated in months and years. Macrophages participate in the formation and regulation of the immune response, performing the function of presenting antigen to lymphocytes and being a source of biologically active substances (including regulatory cytokines, interleukins, interferons, complement components).
Monocytes/macrophages capable of amoeboid movement exhibit pronounced phagocytic and bactericidal activity. One macrophage can absorb up to 100 microorganisms, while a neutrophil can only absorb 20-30. They appear at the site of inflammation after neutrophils and show maximum activity in an acidic environment, in which neutrophils lose their activity. At the site of inflammation, macrophages phagocytize microorganisms, as well as dead leukocytes and damaged cells of inflamed tissue, cleaning the site of inflammation and preparing it for regeneration. Macrophages are more effective than neutrophils in phagocytosis of mycobacteria, fungi and macromolecules. In the spleen, macrophages ensure the disposal of sensitized and aging red blood cells. Monocytosis (an increase in the absolute number of monocytes by more than 10xNU/l) is observed in patients with chronic infections or inflammatory processes.
ESR (Erythrocyte Sedimentation Rate, ESR)
Nonspecific indicator of inflammation.
ESR is an indicator of the rate of separation of blood stabilized by an anticoagulant in a capillary into two layers: the upper (transparent blood plasma) and the lower (settled red blood cells and other blood cells). ESR is estimated by the height of the formed layer of blood plasma (in mm) in 1 hour. The specific gravity of erythrocytes is higher than the specific gravity of plasma, therefore, under the influence of gravity, erythrocytes settle to the bottom. The process of erythrocyte sedimentation can be divided into 3 phases, which occur at different rates. At first, red blood cells slowly settle into individual cells. Then they form aggregates - “coin columns”, and subsidence occurs faster. In the third phase, a lot of red blood cell aggregates are formed, their sedimentation first slows down and then gradually stops. The main factor influencing the formation of “coin columns” and the erythrocyte sedimentation rate is the protein composition of the blood plasma. Proteins of the acute phase of inflammation, adsorbed on the surface of erythrocytes, reduce their charge and repulsion from each other, contribute to the formation of “coin columns” and accelerated sedimentation of erythrocytes. An increase in the content of acute-phase proteins, for example, C-reactive protein, haptoglobin, alpha-1-antitrypsin, during acute inflammation leads to an increase in ESR. In acute inflammatory and infectious processes, a change in the erythrocyte sedimentation rate is observed 24 hours after an increase in temperature and an increase in the number of leukocytes. In chronic inflammation, an increase in ESR is caused by an increase in the concentration of fibrinogen and immunoglobulins. A decrease in the content of erythrocytes in the blood (anemia) leads to an acceleration of ESR, and, on the contrary, an increase in the content of erythrocytes in the blood slows down the rate of their sedimentation. Determination of ESR is used in screening examinations, as well as in monitoring the course and monitoring the effectiveness of treatment of inflammatory and infectious diseases, usually in combination with a general blood test.
The ESR level varies depending on many physiological factors. ESR values in women are slightly higher than in men. Changes in the protein composition of the blood during pregnancy lead to an increase in ESR. The values may fluctuate during the day; the maximum level is observed during the daytime.
Do unnaturally large red blood cells appear in the blood?
The appearance of abnormally large formed elements of the erythrocyte series and polysegmented neutrophils in a general blood test indicates, first of all, a disorder of hematopoiesis in the early stages, in the bone marrow (megaloblastic type of hematopoiesis). As a result of megaloblastic hematopoiesis in the bone marrow:
- Cell division is disrupted, at the stage of these processes the cells' nuclear structure, diameter and volume change, and as a result, they unnaturally increase in size (megaloblasts);
- Cells mature at different times - asynchronously: some are already close to mature forms, others have made little progress in their development and remain at the level of promyeloblasts;
- Megaloblasts begin to be prematurely saturated with chromoprotein - hemoglobin, fortunately, the red blood pigment of their increased volume is more than enough, there is somewhere to be located (provided that hemoglobin synthesis is not impaired);
- However, degenerative changes in the nuclei make many cells defective, unable to survive to a mature state.
Thus, ineffective erythropoiesis, “giving life” to giant cells, does not provide them with good “health”. Most of the elements that are destined by nature to become full-fledged red blood cells and perform important functions (transport oxygen and carbon dioxide, take part in metabolic processes, play the role of suppressors in immune reactions, etc.) simply die, leaving behind only fragments that will then circulate in the blood (schizocytosis), along with other formed elements.
Of course, not all cells will die at the maturation stage; some of them (the most persistent) will remain and enter the bloodstream - in a general blood test they will be represented by abnormally enlarged, mainly hyperchromic, erythrocytes-macrocytes (erythrocyte macrocytosis). In addition, fragments of “former” (dead) red blood cells (schizocytes) will be detected in the erythrogram, creating a picture of poikilocytosis and suggesting the development of hyperchromic macrocytic anemia. However, for a more accurate diagnosis, additional research will be needed.
In addition to macrocytosis of erythrocytes in a general blood test, macrocytic morphological changes in bone marrow aspirate and the level of vitamins in the blood should be taken into account.
To prevent the diagnostic search from going down the wrong path, other possible prerequisites for the disease must be studied and taken into account:
- Medical history and previous pathology;
- Family, social, professional, medicinal history;
- Examinations of the head and neck, cardiovascular and nervous system, respiratory system and abdominal cavity.
All these diagnostic criteria are necessary to establish the etiology and form of macrocytic anemia.
Reasons for their appearance
In general, macrocytosis of erythrocytes is not an independent nosological entity, it is a laboratory symptom indicating another pathology. The cause of such metamorphoses that occur with red blood cells may be conditions that disrupt the normal course of events in the process of maturation of red blood cells in the bone marrow or their increased destruction accompanying some pathological condition:
- Vitamin B12 deficiency is a hereditary or acquired deficiency of cyanocobalamin (vitamin B12), which causes the formation of hyperchromic megaloblastic (B12-deficiency) anemia;
- Folate deficiency (vitamin B9) – a lack of folic acid in the body threatens the development of folate deficiency anemia (this is also hyperchromic macrocytic anemia);
- Combined variant - B12-folate deficiency anemia (medicine encounters this form most often);
- Anemic syndrome, the development of which is caused by acute blood loss (the compensatory mechanism is activated, but the red cells do not have time to become full-fledged red blood cells with normal diameter and volume);
- Certain types of hemolytic anemia - increased breakdown of red cells in blood vessels or tissues triggers intense but ineffective erythropoiesis in the bone marrow, as a result - in a general blood test, poikilocytosis (schizocytosis), macrocytosis of erythrocytes, and other signs of breakdown of blood cells can be observed;
- Myelodysplastic syndrome, in which various changes in the general blood test are generally possible;
- Some forms of leukemia;
- Pre-leukemic conditions (idiopathic sideroblastic anemia, Di Guglielmo syndrome - acute malignant erythromyelosis);
- Decreased functional capacity of the thyroid gland (hypothyroidism);
- Severe long-term intoxication in chronic alcoholism;
- Damage to the liver parenchyma, which creates reserves of various vitamins (including cyanocobalamin and folic acid), which are subsequently assigned to participate in the synthesis of purine and pyrimidine bases, and they are known to be necessary for the production of complete DNA;
- The use of certain medications: inhibitors of dehydrofolate reductase (triamterene, chloridine, methotrexate), pharmaceuticals that affect the metabolism of purines and pyrimidines (allopurinol, febuxostat), as well as antiepileptic drugs and oral contraceptives - all of them can disrupt DNA synthesis and cause the development of macrocytic anemia ;
- Malignant neoplasms that disrupt cell division and DNA formation;
- Diseases of the gastrointestinal tract and surgical interventions on the digestive organs;
- Pregnancy (during this period, hematopoiesis occurs in an active mode, vitamins received and accumulated are also consumed intensively, which is why folate deficiency anemia often forms during this period).
Due to the fact that B12 and folate deficiency conditions are first on the list of causes, I would like to draw the reader’s attention to the complex functional relationship between these vitamins, which creates conditions for disruption of the entire process of DNA formation. A lack of vitamin B9 in food very quickly reduces the ability to demethylate and bind to cyanocobalamin individual folates that accumulate in the liver, resulting in impaired DNA production. With a lack of vitamin B12 in food, similar disorders occur and the result is also similar - DNA synthesis suffers.
An extensive study of the qualitative and quantitative composition of blood, which provides characteristics of red blood cells and their specific indicators (MCV, MCH, MCHC, RDW), leukocytes and their varieties in percentage (leukocyte formula) and platelets.
Microscopy of the leukocyte formula is always performed as part of the analysis.
English synonyms
Complete blood count (CBC) with differential.
Research method
- SLS (sodium lauryl sulfate) - method
- Conductometric method
- Microscopy
- Flow cytometry
What biomaterial can be used for research?
Venous, capillary blood.
How to properly prepare for research?
- Eliminate alcohol from your diet for 24 hours before the test.
- Children under 1 year of age should not eat for 30-40 minutes before the test.
- Children aged 1 to 5 years should not eat for 2-3 hours before the test.
- Do not eat for 8 hours before the test; you can drink clean still water.
- Avoid physical and emotional stress for 30 minutes before the test.
- Do not smoke for 30 minutes before the test.
General information about the study
A clinical blood test with a leukocyte formula is one of the most frequently performed tests in medical practice. Today, this study is automated and allows you to obtain detailed information about the quantity and quality of blood cells: red blood cells, leukocytes and platelets. From a practical point of view, the doctor should primarily focus on the following parameters of this analysis:
- Hb (hemoglobin) – hemoglobin;
- MCV (mean corpuscular volume) – average volume of an erythrocyte;
- RDW (RBC distribution width) – distribution of red blood cells by volume;
- total number of red blood cells;
- total platelet count;
- total leukocyte count;
- leukocyte formula - the percentage of different leukocytes: neutrophils, lymphocytes, monocytes, eosinophils and basophils.
Determining these parameters makes it possible to diagnose conditions such as anemia/polycythemia, thrombocytopenia/thrombocytosis and leukopenia/leukocytosis, which can either be symptoms of a disease or act as independent pathologies.
When interpreting the analysis, the following features should be taken into account:
- In 5% of healthy people, blood test results deviate from the accepted reference values. On the other hand, the patient may show a significant deviation from his usual indicators, which at the same time remain within the accepted norms. For this reason, test results must be interpreted in the context of each individual's individual normal performance.
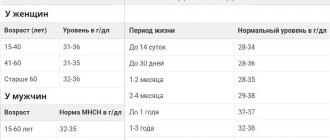
- Blood counts vary by race and gender. Thus, in women the number and quality characteristics of red blood cells are lower, and the number of platelets is higher than in men. For comparison: men – Hb 12.7-17.0 g/dl, erythrocytes 4.0-5.6×1012/l, platelets 143-332×109/l, women – Hb 11.6-15.6 g /dl, red blood cells 3.8-5.2×1012/l, platelets 169-358×109/l. In addition, hemoglobin, neutrophils and platelets are lower in dark-skinned people than in white people.
What is the research used for?
- For diagnosis and control of treatment of many diseases.
When is the study scheduled?
- During a preventive examination;
- if the patient has complaints or symptoms of any disease.
What do the results mean?
Reference values
| Component | Floor | Age | Reference values |
| White blood cells (WBC), *10^9/l | 0-1 year | 6.0 — 17.5 | |
| 1-2 years | 6.0 — 17.0 | ||
| 2-4 years | 5.5 — 15.5 | ||
| 4-6 years | 5.0 — 14.5 | ||
| 6-10 years | 4.5 — 13.5 | ||
| 10-16 years | 4.5 — 13.0 | ||
| More than 16 years | 4.0 — 10.0 | ||
| Red blood cells (RBC), *10^12/l | female | 0-14 days | 3.9 — 5.9 |
| male | 0-14 days | 3.9 — 5.9 | |
| male | 1-4 months | 3.5 — 5.1 | |
| female | 1-4 months | 3.5 — 5.1 | |
| male | 4-6 months | 3.9 — 5.5 | |
| female | 4-6 months | 3.9 — 5.5 | |
| male | 6-9 months | 4.0 — 5.3 | |
| female | 6-9 months | 4.0 — 5.3 | |
| male | 9-12 months | 4.1 — 5.3 | |
| female | 9-12 months | 4.1 — 5.3 | |
| male | 1-3 years | 3.8 — 4.8 | |
| female | 1-3 years | 3.8 — 4.8 | |
| male | 3-6 years | 3.7 — 4.9 | |
| female | 3-6 years | 3.7 — 4.9 | |
| male | 6-9 years | 3.8 — 4.9 | |
| female | 6-9 years | 3.8 — 4.9 | |
| male | 9-12 years | 3.9 — 5.1 | |
| female | 9-12 years | 3.9 — 5.1 | |
| male | 12-15 years | 4.1 — 5.2 | |
| female | 12-15 years | 3.8 — 5.0 | |
| male | 15-18 years old | 4.2 — 5.6 | |
| female | 15-18 years old | 3.9 — 5.1 | |
| male | 18-45 years old | 4.3 — 5.7 | |
| female | 18-45 years old | 3.8 — 5.1 | |
| male | 45-65 years | 4.2 — 5.6 | |
| female | 45-65 years | 3.8 — 5.3 | |
| male | More than 65 years | 3.8 — 5.8 | |
| female | More than 65 years | 3.8 — 5.2 | |
| female | 14-30 days | 3.3 — 5.3 | |
| male | 14-30 days | 3.3 — 5.3 | |
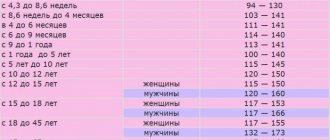
| Hemoglobin (HGB), g/l | male | 0-14 days | 134 — 198 |
| female | 0-14 days | 134 — 198 | |
| male | 14-30 days | 107 — 171 | |
| female | 14-30 days | 107 — 171 | |
| male | 1-2 months | 94 — 130 | |
| female | 1-2 months | 94 — 130 | |
| male | 2-4 months | 103 — 141 | |
| female | 2-4 months | 103 — 141 | |
| male | 4-6 months | 111 — 141 | |
| female | 4-6 months | 111 — 141 | |
| male | 6-9 months | 114 — 140 | |
| female | 6-9 months | 114 — 140 | |
| male | 9-12 months | 113 — 141 | |
| female | 9-12 months | 113 — 141 | |
| male | 1-5 years | 110 — 140 | |
| female | 1-5 years | 110 — 140 | |
| male | 5-10 years | 115 — 145 | |
| female | 5-10 years | 115 — 145 | |
| male | 10-12 years | 120 — 150 | |
| female | 10-12 years | 120 — 150 | |
| male | 12-15 years | 120 — 160 | |
| female | 12-15 years | 115 — 150 | |
| male | 15-18 years old | 117 — 166 | |
| female | 15-18 years old | 117 — 153 | |
| male | 18-45 years old | 132 — 173 | |
| female | 18-45 years old | 117 — 155 | |
| male | 45-65 years | 131 — 172 | |
| female | 45-65 years | 117 — 160 | |
| male | More than 65 years | 126 — 174 | |
| female | More than 65 years | 117 — 161 | |
| Hematocrit (HCT), % | male | 0-14 days | 41 — 65 |
| female | 0-14 days | 41 — 65 | |
| male | 14-30 days | 33 — 55 | |
| female | 14-30 days | 33 — 55 | |
| male | 1-2 months | 28 — 42 | |
| female | 1-2 months | 28 — 42 | |
| male | 2-4 months | 32 — 44 | |
| female | 2-4 months | 32 — 44 | |
| male | 4-6 months | 31 — 41 | |
| female | 4-6 months | 31 — 41 | |
| male | 6-9 months | 32 — 40 | |
| female | 6-9 months | 32 — 40 | |
| male | 9-12 months | 33 — 41 | |
| female | 9-12 months | 33 — 41 | |
| male | 1-3 years | 32 — 40 | |
| female | 1-3 years | 32 — 40 | |
| male | 3-6 years | 32 — 42 | |
| female | 3-6 years | 32 — 42 | |
| male | 6-9 years | 33 — 41 | |
| female | 6-9 years | 33 — 41 | |
| male | 9-12 years | 34 — 43 | |
| female | 9-12 years | 34 — 43 | |
| male | 12-15 years | 35 — 45 | |
| female | 12-15 years | 34 — 44 | |
| male | 15-18 years old | 37 — 48 | |
| female | 15-18 years old | 34 — 44 | |
| male | 18-45 years old | 39 — 49 | |
| female | 18-45 years old | 35 — 45 | |
| male | 45-65 years | 39 — 50 | |
| female | 45-65 years | 35 — 47 | |
| male | More than 65 years | 37 — 51 | |
| female | More than 65 years | 35 — 47 | |
| Mean erythrocyte volume (MCV), fL | female | 0-12 months | 71 — 112 |
| male | 0-12 months | 71 — 112 | |
| female | 1-5 years | 73 — 85 | |
| male | 1-5 years | 73 — 85 | |
| female | 5-10 years | 75 — 87 | |
| male | 5-10 years | 75 — 87 | |
| female | 10-12 years | 76 — 94 | |
| male | 10-12 years | 76 — 94 | |
| female | 12-15 years | 73 — 95 | |
| female | 15-18 years old | 78 — 98 | |
| female | 18-45 years old | 81 — 100 | |
| female | 45-65 years | 81 — 101 | |
| female | More than 65 years | 81 — 102 | |
| male | 12-15 years | 77 — 94 | |
| male | 15-18 years old | 79 — 95 | |
| male | 18-45 years old | 80 — 99 | |
| male | 45-65 years | 81 — 101 | |
| male | More than 65 years | 81 — 102 | |
| Avg. sod. hemoglobin in er-te (MCH), pg | female | 0-14 days | 30 — 37 |
| male | 0-14 days | 30 — 37 | |
| female | 14-30 days | 29 — 36 | |
| male | 14-30 days | 29 — 36 | |
| female | 1-2 months | 27 — 34 | |
| male | 1-2 months | 27 — 34 | |
| female | 2-4 months | 25 — 32 | |
| male | 2-4 months | 25 — 32 | |
| female | 4-6 months | 24 — 30 | |
| male | 4-6 months | 24 — 30 | |
| female | 6-9 months | 25 — 30 | |
| male | 6-9 months | 25 — 30 | |
| female | 9 months — 1 g. | 24 — 30 | |
| male | 9 months — 1 g. | 24 — 30 | |
| female | 1-3 years | 22 — 30 | |
| male | 1-3 years | 22 — 30 | |
| female | 3-6 years | 25 — 31 | |
| male | 3-6 years | 25 — 31 | |
| female | 6-9 years | 25 — 31 | |
| male | 6-9 years | 25 — 31 | |
| female | 9-15 years | 26 — 32 | |
| male | 9-15 years | 26 — 32 | |
| female | 15-18 years old | 26 — 34 | |
| male | 15-18 years old | 27 — 32 | |
| female | 18-45 years old | 27 — 34 | |
| male | 18-45 years old | 27 — 34 | |
| female | 45-65 years | 27 — 34 | |
| male | 45-65 years | 27 — 35 | |
| female | More than 65 years | 27 — 35 | |
| male | More than 65 years | 27 — 34 | |
| Avg. conc. hemoglobin in air (MCHC), g/l | 0-1 years | 290 — 370 | |
| 1-3 years | 280 — 380 | ||
| 3-12 years | 280 — 360 | ||
| 12-19 years old | 330 — 340 | ||
| More than 19 years | 300 — 380 | ||
| Distribution erith. along V - standard deviation (RDW-SD), fL | 37 — 54 | ||
| Distribution erith. according to V - coefficient. Variance (RDW-CV), % | More than 6 months | 11.6 — 14.8 | |
| 0-6 months | 14.9 — 18.7 | ||
| female | 0-10 days | 99 — 421 | |
| male | 0-10 days | 99 — 421 | |
| female | 10-30 days | 150 — 400 | |
| male | 10-30 days | 150 — 400 | |
| female | 1-6 months | 180 — 400 | |
| male | 1-6 months | 180 — 400 | |
| female | 6 months — 1 g. | 160 — 390 | |
| male | 6 months — 1 g. | 160 — 390 | |
| female | 1-5 years | 150 — 400 | |
| male | 1-5 years | 150 — 400 | |
| female | 5-10 years | 180 — 450 | |
| male | 5-10 years | 180 — 450 | |
| female | 10-15 years | 150 — 450 | |
| male | 10-15 years | 150 — 400 | |
| female | More than 15 years | 150 — 400 | |
| male | More than 15 years | 150 — 400 | |
| Distribution platelet count by volume (PDW), fL | 10 — 20 | ||
| Mean platelet volume (MPV), fL | 9.4 — 12.4 | ||
| Large platelet ratio (P-LCR), % | 13 — 43 | ||
| Neutrophils (NE), 10^9/l | 0-4 years | 1.5 — 8.5 | |
| 4-8 years | 1.5 — 8.0 | ||
| 8-16 years | 1.8 — 8.0 | ||
| More than 16 years | 1.8 — 7.7 | ||
| Lymphocytes (LY), *10^9/l | 0-1 years | 2.0 — 11.0 | |
| 1-2 years | 3.0 — 9.5 | ||
| 2-4 years | 2.0 — 8.0 | ||
| 4-6 years | 1.5 — 7.0 | ||
| 6-8 years | 1.5 — 6.8 | ||
| 8-10 years | 1.5 — 6.5 | ||
| 10-16 years | 1.2 — 5.2 | ||
| More than 16 years | 1.0 — 4.8 | ||
| Monocytes (MO), *10^9/l | 0-1 years | 0.05 — 1.1 | |
| 1-2 years | 0.05 — 0.6 | ||
| 2-4 years | 0.05 — 0.5 | ||
| 4-16 years | 0.05 — 0.4 | ||
| More than 16 years | 0.05 — 0.82 | ||
| Eosinophils (EO), *10^9/l | 0-1 years | 0.05 — 0.4 | |
| 1-6 years | 0.02 — 0.3 | ||
| More than 6 years | 0.02 — 0.5 | ||
| Basophils (BA), *10^9/l | 0 — 0.08 | ||
| Neutrophils, % (NE%), *10^9/l | 0-1 years | 16 — 45 | |
| 1-2 years | 28 — 48 | ||
| 2-4 years | 32 — 55 | ||
| 4-6 years | 32 — 58 | ||
| 6-8 years | 38 — 60 | ||
| 8-10 years | 41 — 60 | ||
| 10-16 years | 43 — 60 | ||
| More than 16 years | 47 — 72 | ||
| Lymphocytes, % (LY%) | 0-1 years | 45 — 75 | |
| 1-2 years | 37 — 60 | ||
| 2-4 years | 33 — 55 | ||
| 4-6 years | 33 — 50 | ||
| 6-8 years | 30 — 50 | ||
| 8-10 years | 30 — 46 | ||
| 10-16 years | 30 — 45 | ||
| More than 16 years | 19 — 37 | ||
| Monocytes, % (MO%) | 0-1 years | 4 — 10 | |
| 1-2 years | 3 — 10 | ||
| More than 2 years | 3 — 12 | ||
| Eosinophils, % (EO%) | 0-1 years | 1 — 6 | |
| 1-2 years | 1 — 7 | ||
| 2-4 years | 1 — 6 | ||
| More than 4 years | 1 — 5 | ||
| Basophils,% (BA%) | female | 0 — 1.2 | |
| male | 0 — 1.2 | ||
| Neutrophils, 10^9/l | 0-4 years | 1.5 — 8.5 | |
| 4-8 years | 1.5 — 8.0 | ||
| 8-16 years | 1.8 — 8.0 | ||
| More than 16 years | 1.8 — 7.7 | ||
| Lymphocytes, *10^9/l | 0-1 years | 2.0 — 11.0 | |
| 1-2 years | 3.0 — 9.5 | ||
| 2-4 years | 2.0 — 8.0 | ||
| 4-6 years | 1.5 — 7.0 | ||
| 6-8 years | 1.5 — 6.8 | ||
| 8-10 years | 1.5 — 6.5 | ||
| 10-16 years | 1.2 — 5.2 | ||
| More than 16 years | 1.0 — 4.8 | ||
| Monocytes, *10^9/l | 0-1 years | 0.05 — 1.1 | |
| 1-2 years | 0.05 — 0.6 | ||
| 2-4 years | 0.05 — 0.5 | ||
| 4-16 years | 0.05 — 0.4 | ||
| More than 16 years | 0.05 — 0.82 | ||
| Eosinophils, *10^9/l | 0-1 years | 0.05 — 0.4 | |
| 1-6 years | 0.02 — 0.3 | ||
| More than 6 years | 0.02 — 0.5 | ||
| Basophils, *10^9/l | 0 — 0.08 | ||
| Neutrophils: rods. (%) | 0 — 5 | ||
| Neutrophils: segment. (SEG%) | 0-1 years | 16 — 45 | |
| 1-2 years | 28 — 48 | ||
| 2-5 years | 32 — 55 | ||
| 5-7 years | 38 — 58 | ||
| 7-8 years | 41 — 60 | ||
| 8-12 years | 43 — 60 | ||
| 12-16 years old | 45 — 60 | ||
| More than 16 years | 47 — 72 | ||
| Lymphocytes, % (LY%) | 0-1 years | 45 — 75 | |
| 1-2 years | 37 — 60 | ||
| 2-4 years | 33 — 55 | ||
| 4-6 years | 33 — 50 | ||
| 6-8 years | 30 — 50 | ||
| 8-10 years | 30 — 46 | ||
| 10-16 years | 30 — 45 | ||
| More than 16 years | 19 — 37 | ||
| Monocytes, % (MO%) | 0-1 years | 4.0 — 10 | |
| 1-2 years | 3.0 — 10 | ||
| More than 2 years | 3.0 — 12 | ||
| Eosinophils, % (EO%) | 0-1 years | 1 — 6 | |
| 1-2 years | 1 — 7 | ||
| 2-4 years | 1 — 6 | ||
| More than 4 years | 1 — 5 | ||
| Basophils,% (BA%) | female | 0 — 1 | |
| male | 0 — 1 |
Interpretation of the analysis:
Anemia
A decrease in hemoglobin and/or red blood cells indicates the presence of anemia. Using the MCV indicator, you can perform a primary differential diagnosis of anemia:
- MCV less than 80 fl (microcytic anemia). Causes: iron deficiency anemia;
- thalassemia;
- anemia of chronic disease;
- sideroblastic anemia.
Considering that the most common cause of microcytic anemia is iron deficiency, when identifying microcytic anemia, it is recommended to determine the concentration of ferritin, as well as serum iron and total serum iron-binding capacity. It is recommended to pay attention to the RDW indicator (increased only in iron deficiency anemia) and platelet count (often increased in iron deficiency anemia).
- MCV 80-100 fl (normocytic anemia). Causes:
- bleeding;
- anemia in chronic renal failure - chronic renal failure;
- hemolysis;
- anemia due to iron or vitamin B12 deficiency.
To exclude hidden bleeding, a stool test for occult blood is recommended. To exclude iron or vitamin B12 deficiency as a cause of normocytic anemia, determine iron and vitamin B12 in the blood. To exclude chronic renal failure, tests for creatinine and blood urea are performed. To exclude hemolytic anemia - tests for haptoglobin, LDH, indirect bilirubin and reticulocytes.
- MCV more than 100 fl (macrocytic anemia). Causes:
- alcohol abuse;
- medications (hydroxyurea, zidovudine);
- deficiency of vitamin B12 and folic acid.
Marked macrocytosis (MCV greater than 110 fl) usually indicates a primary bone marrow disease.
To exclude vitamin B12 deficiency as a cause of macrocytic anemia, homocysteine and vitamin B12 tests are performed.
Thrombocytopenia
Causes:
- thrombocytopenic purpura/hemolytic-uremic syndrome;
- DIC syndrome (disseminated intravascular coagulation);
- drug thrombocytopenia (co-trimoxazole, procainamide, thiazide diuretics, heparin);
- hypersplenism;
- idiopathic thrombocytopenic purpura.
For the differential diagnosis of diseases occurring with thrombocytopenia, a coagulogram, an analysis for HIV infection, antinuclear antibodies and blood protein electrophoresis may also be required.
It should be remembered that in pregnant women, platelets can normally decrease to 75-150×109/l.
Leukopenia
For the differential diagnosis of leukopenia, both the absolute number of each of the 5 main lineages of leukocytes and their percentage (leukocyte formula) are important.
Neutropenia. A decrease in neutrophils of less than 0.5 × 109/l is severe neutropenia. Causes:
- congenital agranulocytosis (Kostmann syndrome);
- drug neutropenia (carbamazepine, penicillins, clozapine and others);
- infections (sepsis, viral infection);
- autoimmune neutropenia (SLE, Felty's syndrome).
Lymphopenia. Causes:
- tuberculosis.
- chronic renal failure;
- autoimmune lymphopenia (SLE, rheumatoid arthritis, sarcoidosis);
- viral infection (HIV);
- drug-induced lymphopenia (glucocorticosteroids, monoclonal antibodies);
- acquired variable immunodeficiency;
- congenital lymphopenia (Bruton agammaglobulinemia, severe combined immunodeficiency, DiGeorge syndrome);
Polycythemia
Increase in Hb and/or Ht concentration and/or red blood cell count:
- polycythemia vera is a myeloproliferative disease; in the blood test, in addition to erythrocytosis, thrombocytosis and leukocytosis are observed;
- relative polycythemia (bone marrow compensatory response to hypoxia in COPD or ischemic heart disease; excess erythropoietin in renal cell carcinoma).
For the differential diagnosis of polycythemia, a study of erythropoietin levels is recommended.
Thrombocytosis
Causes:
- primary thrombocytosis (malignant disease of the myeloid lineage of the bone marrow, including essential thrombocytosis and chronic myeloid leukemia);
- secondary thrombocytosis after removal of the spleen, during an infectious process, iron deficiency anemia, hemolysis, trauma and malignant diseases (reactive thrombocytosis).
An increase in Hb, MCV, or total leukocyte count suggests primary thrombocytosis. The degree of thrombocytosis, as a rule, does not allow differential diagnosis of primary and secondary thrombocytosis, since even a very significant increase in platelets (more than 1000 × 109 l) can also be observed with reactive thrombocytosis. Additional tests may also be recommended: blood ferritin and C-reactive protein.
Leukocytosis
The first step in interpreting leukocytosis is to evaluate the leukocyte count. Leukocytosis can be caused by an excess of immature leukocytes (blasts) in acute leukemia or mature, differentiated leukocytes (granulocytosis, monocytosis, lymphocytosis).
Granulocytosis - neutrophilia. Causes:
- leukemoid reaction (reactive neutrophilia in the presence of infection, inflammation, use of certain medications);
- myeloproliferative disease (eg, chronic myelogenous leukemia).
An increase in the percentage of band neutrophils of more than 6% indicates the presence of infection, but can also be observed in chronic myeloid leukemia and other myeloproliferative diseases.
Granulocytosis - eosinophilia. Causes:
- primary eosinophilia (hypereosinophilic myeloproliferative syndrome);
- secondary eosinophilia (parasitosis, helminthiasis, allergies, vasculitis, lymphoma).
Additional tests will depend on your medical history, but should at a minimum include a stool test for helminth eggs.
Granulocytosis - basophilia. Causes:
- chronic basophilic leukemia.
Monocytosis. Causes:
- myeloproliferative disease, such as CML;
- reactive monocytosis (chronic infections, granulomatous inflammation, radiation therapy, lymphoma).
Lymphocytosis. Causes:
- reactive lymphocytosis (viral infection); Virus-specific laboratory tests are recommended;
- lymphocytic leukemia (acute and chronic).
A clinical blood test with a leukocyte formula is a screening method that can be used to preliminarily assess the state of bone marrow function and suspect or exclude many diseases. This analysis, however, does not always make it possible to establish the cause of the changes, the identification of which, as a rule, requires additional laboratory tests, including pathomorphological and histochemical studies. The most accurate information can be obtained by dynamic monitoring of changes in blood parameters.
Prevalence
The prevalence of macrocytic (megaloblastic) anemia may depend on various factors:
- Nutritional characteristics of certain categories of people;
- Gastrointestinal pathology, common in a particular human population, and the number of surgical interventions on the digestive organs undergone;
- The use of drugs that interfere with the metabolism of folic acid, purine and pyrimidine bases and thus affect the quality of DNA synthesis;
- Prevalence of neoplastic processes in certain geographic areas.
At the same time, the leading prerequisites for the formation of macrocytic anemia in Russia and Western European countries primarily include hereditary or acquired disorders associated with deficiency of vitamin B12 and folic acid.
As statistics show, very often acquired macrocytic anemia develops in conditions that seem to be mutually exclusive - due to alcoholism or during pregnancy. In this regard, I would like to inform the reader of some interesting (in our opinion) facts.
Alcoholics don't always take risks
In alcoholism, megaloblastic anemia develops during a long binge, spanning several weeks. This happens because in most cases, alcoholics, carried away by the search for alcohol, completely forget about food, and this leads to depletion of vitamin B9 reserves in the body (true deficiency). Alcohol negatively affects metabolic processes designed to provide tissues with folate, and constant drunkenness, or rather chronic alcoholism, does not allow folic acid to take part in the intestinal-hepatic cycle, which reduces its entry into the bone marrow. As a result, bone marrow hematopoiesis is disrupted, and hyperchromic megaloblastic anemia develops. However, there is one “but”...
If a person suffering from alcoholism does not neglect a normal “snack” and consumes vitamins in sufficient quantities with food (including folic acid and cyanocobalamin), then bone marrow hematopoiesis continues to proceed normally and anemic syndrome does not develop.
The fetus has more privileges
And regarding pregnancy...
It should be noted that the child has more privileges than the mother regarding the consumption of vitamins involved in DNA synthesis. The fact is that the placenta, protecting the baby, extracts the incoming amount of folate and takes it upon itself, without “sharing” much with the mother’s body.
A lack of folic acid in the food of a pregnant woman will most likely not affect the development of the baby - the placenta will “take care” and extract the required amount to the detriment of the mother’s body, but the mother herself must think about herself and calculate her diet so that it contains enough benefits for her substances.
Vitamin B12 deficiency, on the contrary, is unlikely to threaten a woman during pregnancy, much less a fetus. If the reserves of folic acid contained in the liver are enough for 2-3 months, then cyanocobalamin can be consumed for 2-3 years painlessly, the main thing is that in both the first and second cases the liver parenchyma must be healthy.
Start by eliminating the cause
Macrocytosis of erythrocytes itself, of course, is not treated, because it is just a symptom identified in a general blood test. Treatment begins with eliminating the cause that created the formation of large cells. Meanwhile, it is not always possible to quickly eliminate the culprits of the misfortunes that have befallen them. Then long-term therapy begins for the doctor and the patient, aimed at the underlying pathology that caused the development of macrocytic anemia.
With vitamin deficiency conditions, everything is more or less clear; they are treated with appropriate medications (medicinal forms of cyanocobalamin and folic acid). However, if acquired deficiency (lack of food, malabsorption in the intestines, the influence of alcohol, competitive absorption by helminths, etc.) is easily amenable to therapeutic influence, then congenital anomalies react poorly to treatment, moreover, they are often accompanied by mental retardation and other abnormalities (megaloblastic anemia in children). Here we have our own tactics.
As for the entire list of diseases that gave rise to the formation of abnormally enlarged red blood cells in the bone marrow or to their premature and intense breakdown in the blood, in each case there is a separate approach: in some cases blood transfusions (blood transfusions) will help, in others they will be required surgical intervention.
Unfortunately, it is not only quite difficult, but also impossible, to describe all the options for the treatment process, however, readers can find answers to their questions regarding certain types of macrocytic anemia in the relevant publications posted on the pages of our website.
Hypochromic anemia - symptoms and treatment
Treatment of iron deficiency anemia
IDA therapy includes:
- treatment of the disease that caused the anemia;
- diet therapy;
- prescribing iron supplements: oral (in the form of tablets) or parenteral (in the form of intravenous or intramuscular injections);
- blood transfusion - transfusion of red blood cells.
Goals of treatment for IDA:
- relieve signs of anemia (applies to acute anemia);
- normalize hemoglobin levels;
- restore iron reserves in the body and maintain their normal level.
The course of therapy is prescribed individually by the attending physician [2].
Diet therapy
It is impossible to cure anemia with diet alone, but a diet high in animal protein and iron will still be helpful to maintain hemoglobin levels [37].
For normal body function, adult men require 65–117 g of protein per day, women – 58–87 g per day. Proteins are found in plant foods (legumes, grains, vegetables and fruits) and in animal products (milk, dairy products, eggs, meat, fish and seafood). Protein and iron are better absorbed from animal products [35]:
- 15–35% of iron is absorbed from meat and fish;
- from plant products - 1–5% [24].
Apples, pomegranates, red juice (beets or tomatoes) cannot compensate for the lack of iron.
Meals should be 4-6 times a day with a small amount of food at one time. For normal digestion, food must be at room temperature: too cold or too hot food irritates the gastric mucosa, which prevents the absorption of nutrients. It is also recommended to give up alcohol and smoking [20].
Treatment with oral iron supplements
Two groups of drugs are used:
- containing the divalent form of iron (II): iron sulfate (Sorbifer Durules, Tardiferon, Aktiferrin, Fenyuls, Maxifer), iron fumarate (Ferretab, Ranferon-12), iron gluconate (Totema);
- containing the trivalent form of iron (III): iron hydroxide polymaltosate (Maltofer, Ferrum Lek), iron hydroxide sucrose complex (Venofer), iron hydroxide dextran (Cosmofer, Ferkail).
Difference between groups of iron preparations
| Main characteristics | Ferrous form of iron | Ferric form of iron |
| Route of administration | Orally (by mouth) | Orally (by mouth) Parenterally (injection) |
| Side effects | Occurs frequently with long-term use | Rarely occur |
| Effect of food on absorption | Dairy products, tea, coffee impair iron absorption. Products with vitamin C improve absorption | Does not affect |
| Suction effect | Well absorbed | Absorbed worse |
Iron supplements are not retained in the body and are quickly excreted by the kidneys, so it is not possible to quickly replenish iron deficiency. To replenish iron stores, the dose should be 100 to 300 mg of ferrous iron per day in tablet form. It makes no sense to increase the dosage, since iron absorption does not increase; the excess will be excreted in the feces.
On the 3rd–5th day from the start of treatment, the number of reticulocytes (immature red blood cells) usually increases in the CBC, which indicates the effectiveness of therapy. If the patient regularly takes iron supplements in a therapeutic dosage, the hemoglobin level normalizes after about 3-4 weeks from the start of therapy. Treatment should not be stopped at this stage. Maintenance therapy must be continued to replenish the iron depot. Maintenance therapy continues for about 6 months at a dose of 100 mg per day.
Possible side effects. All preparations containing iron salts can cause irritation of the gastric mucosa. Therefore, side effects such as vomiting, diarrhea or heartburn sometimes occur. To get rid of them, you need to:
- reduce the dosage of the drug;
- reduce treatment time;
- take the drug before bedtime;
- Do not combine with incompatible products.
If these measures do not help, the drug is discontinued [13].
What affects iron absorption? Vitamin C helps iron to be better absorbed: the acid prevents the oxidation of iron and maintains it in its divalent form. Therefore, it is recommended to add foods containing this vitamin to your diet. Additional vitamin C supplementation is not required [36].
Pregnant women are recommended to take iron supplements together with folic acid (vitamin B9), as it is involved in the process of hematopoiesis [28].
The presence of phytic acid in food (found in cereals, legumes, seeds, nuts, etc.), caffeine and tannin (in tea, coffee), phosphates, oxalates (in plant products) impairs iron absorption by 4–6 times, since they form insoluble complexes with ferric iron and are excreted in feces [23]. It is recommended to limit their consumption or consume them 6 hours before taking iron supplements.
Calcium, iodine, magnesium, zinc, chromium and selenium found in foods and supplements also interfere with iron absorption, so should not be taken together.
Treatment with parenteral iron supplements
Indications for parenteral administration of iron supplements:
- Intolerance to iron supplements in tablet form.
- Impaired absorption of iron in malabsorption syndrome, resection of the small intestine, enteritis, celiac disease and other gastrointestinal diseases.
- Exacerbation of chronic gastrointestinal diseases: gastric and duodenal ulcers, Crohn's disease, ulcerative colitis.
- Severe anemia (hemoglobin 70 g/l or less).
Preparations for parenteral administration: Ferinject, Ferractin, Ferrum Lek, Ferbitol, Ectofer. Drugs are prescribed only by the attending physician after an examination and clarification of the type of anemia. An excess of iron in some cases is even more dangerous than its deficiency: the enamel deteriorates, severe toxic hepatitis can develop before transforming into cirrhosis, etc.
Blood transfusion (transfusion of red blood cells)
Red blood cell transfusion may be required according to individual indications determined by the attending physician. Indications may be:
- Severe IDA;
- concomitant cardiovascular pathologies (for example, coronary heart disease), if there is a risk of decompensation of the condition due to anemia [38].
Treatment of thalassemia
Treatment tactics for different forms of the disease differ. β-thalassemia minor does not require treatment. For β-thalassemia major, treatment must begin in the first months of life: blood transfusion and chelating drugs that remove excess iron from the body (Deferasirox).
For all forms of thalassemia, taking B vitamins is indicated. With the development of hypersplenism, in which the spleen enlarges and actively destroys blood cells, it is necessary to remove the spleen.
One of the treatment methods for thalassemia is bone marrow transplantation [24].