- home
- Oncology
- Spleen tumor
Quite a lot of attention is paid to the morphology of focal lesions of the spleen. There are malignant and benign tumors of the spleen
.
The first include plasmacytoma and sarcomas, which, depending on the underlying tissue, can be of four forms: fibrosarcoma, lymphosarcoma, reticulosarcoma and angiosarcoma. Such lesions of the spleen are extremely rare. Among benign tumors,
lymphangiomas and hemangiomas are more common.
To determine the type of spleen tumor, its localization to the main structures of the organ and indications for surgery, as well as choosing the correct surgical treatment tactics, you must send me a complete description of the abdominal ultrasound, MSCT data of the spleen with contrast, and indicate your age and main complaints to your personal email address. Then I will be able to give a more accurate answer to your situation.
Symptoms, signs and clinical manifestations of a spleen tumor.
In patients with focal formations of the spleen, the most common complaints are the presence of a tumor-like formation in the upper floor of the abdominal cavity, a feeling of heaviness or fullness in the left hypochondrium and epigastrium, an enlarged abdomen and its asymmetry, decreased appetite, and weight loss.
Due to compression of neighboring organs, pain often appears, body weight decreases significantly, and weakness increases. Compression of the left renal artery sometimes leads to hypertension, dysuric disorders, edema of the lower extremities, symptoms of intoxication are often associated, and dyspeptic disorders may develop.
It is believed that for benign tumors and true cysts
clinical manifestations develop gradually, gradually. Patients cannot indicate the exact timing of the onset of signs and symptoms of the disease, which is due to the slow growth of benign formations.
Thus, the clinical picture of tumors and cysts of the spleen is extremely poor and nonspecific. Benign tumors and true cysts are particularly rare in their manifestations. More often, symptoms appear in formations with a complicated course. It becomes obvious that the leading place in the diagnosis of spleen tumors belongs to instrumental non-invasive research methods.
Medical educational literature
Hyalinosis is a vascular-stromal protein dystrophy, characterized by the deposition in tissues of homogeneous translucent dense masses of hyaline, reminiscent of the ground substance of hyaline cartilage. Hyalinosis should not be confused with hyaline-droplet dystrophy, which is an intracellular dysproteinosis.
Classification.
The following types of hyalinosis are distinguished:
- vascular hyalinosis;
- connective tissue hyalinosis;
- hyalinosis of serous membranes.
Hyalinosis can be widespread and local.
Occurrence.
Hyalinosis of the walls of blood vessels is very common and occurs in most elderly people, especially those suffering from hypertension, symptomatic hypertension, and diabetes mellitus. Hyalinosis of connective tissue is less common, and even less common is hyalinosis of the serous membranes.
Conditions of occurrence.
- For vascular hyalinosis - increased blood pressure and increased permeability of the walls of small arteries and arterioles.
- For hyalinosis of connective tissue - its previous disorganization in the form of mucoid swelling and fibrinoid changes.
- For hyalinosis of serous membranes - the presence of organizing fibrinous inflammatory exudate on the surface of the serous membrane.
Mechanisms of occurrence.
Hyalinosis of blood vessels - small arteries, arterioles and glomeruli of the kidney - occurs as a result of increased blood pressure, necrosis of smooth muscle cells of the tunica media, and infiltration of the vascular wall with blood plasma proteins. These proteins combine with the products of myocyte necrosis to form hyaline. Another mechanism for the development of vascular hyalinosis, for example, in vasculitis, as well as diabetes mellitus, is associated with an increase in the permeability of the vascular wall and its infiltration with plasma proteins, which are subsequently converted into hyaline.
Hyalinosis of connective tissue is preceded by destruction of its basic substance and, in particular, mucoproteins and collagen fibers. Acid mucopolysaccharides, which arise as a result of the breakdown of mucoproteins, lead to tissue swelling due to their pronounced hydrophilicity. At the same time, there is also an increase in the permeability of local microvessels, from which albumin, globulins and fibrinogen enter the tissue, which, when compacted, form hyaline. Hyalinosis of serous membranes is the result of the evolution of fibrinous inflammatory exudate on the surface of organs with a serous lining.
Macroscopic picture.
Vascular hyalinosis is not macroscopically determined, with the exception of hyalinosis of the fundus vessels, which is detected by ophthalmoscopy using a magnifying glass. The microvessels appear thickened and tortuous. Hyalipized connective tissue is dense, has lost elasticity, milky white or slightly creamy in color. This is especially noticeable in the leaflets of the heart valves, thickened and sharply deformed, and in the so-called keloid scars. With hyalinosis of the serous membranes, the tissue in this place is sharply thickened, milky white in color, and has a cartilaginous consistency. Organs with widespread hyalinosis of the membranes - the liver or spleen - look as if covered in sugar icing and are described as a glazed liver or spleen.
Microscopic picture.
The walls of hyalinized small arteries and arterioles are thickened due to the accumulation of homogeneous eosinophilic masses in them, their lumen is significantly narrowed or completely closed. The renal glomeruli are partially or completely replaced by such masses, in which the nuclei of single cells are occasionally found. With hyalinosis of connective tissue and serous membranes, among the few fibrocytes there are homogeneous eosinophilic masses, characterized by a positive PAS reaction, indicating the presence of blood glycoproteins in them. Colorful reactions to mucus observed with mucoid swelling with fibrinoid changes become negative.
Clinical significance.
A significant narrowing of the lumen of a hyalinized vessel leads to barotrauma, which is normally prevented by contraction of the arteriole, which has now lost its elasticity and ability to contract. Hydrodynamic shock leads to plasmatic impregnation of the distal areas of the blood-supplied tissue with loss of their functions. This is how glomerular hyalinosis develops in hypertension with a picture of increasing chronic renal failure. This is how diabetic retinopathy develops, which often results in complete blindness.
Hyalinosis of the connective tissue of the leaflets or valves of the heart valves leads to their deformation and incomplete closure, resulting in valve insufficiency.
Hyalinosis of the serous membranes in the vast majority of cases turns out to be an unexpected finding at surgery or autopsy and has no clinical significance. An exception may be cases of total or subtotal hyalinosis of the surface of the liver or spleen, which, under conditions of increased blood supply to the organs, interferes with the stretching of the capsule and may be accompanied by pain.
If you find an error, please select a piece of text and press Ctrl+Enter.
Diagnosis of spleen tumors
Currently, with the widespread introduction of non-invasive diagnostic methods, focal formations of the spleen are often detected accidentally during ultrasound or CT (NMRI) studies during a routine examination.
When a tumor-like formation is detected in the spleen, the specialists of instrumental research methods must set the following tasks for the surgeon:
- Clarification of the localization and size of the pathological focus in the spleen;
- Presumptive determination of the morphological nature of the formation (primarily, the likelihood of a malignant and parasitic nature);
- Determining the condition of adjacent organs (signs of compression, germination, primary lesion);
- Determination of the characteristics of the blood supply to the spleen (the level of division of the splenic artery into lobar branches, their number).
In recent years, spiral computed tomography (SCT) with intravenous bolus contrast enhancement using non-ionic contrast agents (Ultravist, Visipaque, Omnipaque) has become widespread. First, a non-contrast scan of the abdominal cavity is performed, and then a SCT study with BCU (intravenous administration of 100 ml of contrast agent at a rate of 3 ml/sec) with various scanning time delays (from 17 to 40-80 sec.).
The use of this technique makes it possible to clearly distinguish unchanged tissue zones that accumulate contrast agents well from areas of tissue decay and fluid accumulations. In addition, it is possible to obtain a more complete understanding of the angioarchitecture of the spleen itself and the adjacent great vessels, which largely contributes to a high differential diagnosis between cysts and tumor lesions. The diagnosis of SCT is usually confirmed morphologically in 95% of cases.
Thus, it is desirable to include ultrasound, duplex scanning, CT and MRI in a modern preoperative diagnostic program, and in this order, since each method, complementing the previous one, solves narrower, specific problems. Of course, none of them is absolute in identifying focal lesions of the spleen.
Noteworthy is the frequency of diagnostic errors when identifying spleen formations, which reaches 75-80% even when using modern research methods. Therefore, an integrated approach is needed here using all modern diagnostic methods.
Only a comprehensive examination in a specialized hospital makes it possible to determine the pathological focus, its topographic and anatomical characteristics and, ultimately, the optimal treatment tactics. Using this diagnostic scheme, in recent years we have been able not only to identify a lesion in the spleen, but also to accurately localize it in almost 94% of cases.
The final diagnosis is established only during surgery using histological examination.
Hyalinosis
Hyalinosis
(as a type of stromal vascular dystrophy).
(according to V.V. Serov, M.A. Paltsev)
Stromal-vascular (mesenchymal) dystrophies develop as a result of metabolic disorders in connective tissue and are detected in the stroma of organs and the walls of blood vessels.
- It is characterized by the accumulation in the tissues of translucent dense masses resembling hyaline cartilage.
- Occurs as a result of fibrinoid swelling, plasmorrhagia, sclerosis, necrosis.
- Hyaline is a complex fibrillar protein.
- The mechanism of hyaline formation consists of the destruction of fibrous structures and their impregnation with fibrin and other plasma components (globulins, beta-lipoproteins, immune complexes, etc.).
There are hyalinosis of the connective tissue itself and vascular hyalinosis; both of these types of hyalinosis can be widespread and local.
An example of local hyalinosis of the connective tissue itself, which developed as a result of mucoid swelling and fibrinoid changes, is hyalinosis of the heart valves in rheumatism (rheumatic heart disease).
Macroscopic picture: the heart is enlarged, the ventricular cavities are expanded. The mitral valve leaflets are dense, whitish in color, fused together and sharply deformed. The atrioventricular opening is narrowed. The chordal filaments are thickened and shortened.
There are 3 types of vascular hyaline:
a) simple hyaline - occurs as a result of plasmorrhagia of unchanged plasma components (more common in hypertension, atherosclerosis);
b) lipohyalin - contains lipids and beta-lipoproteins (most typical for diabetes mellitus);
c) complex hyaline - is built from immune complexes, fibrin and collapsing structures (characteristic of diseases with immunopathological disorders, for example, rheumatic diseases).
- Widespread hyalinosis of arterioles occurs in hypertension and diabetes mellitus as a result of plasmorrhagia.
- In hypertension, due to hyalinosis of arterioles, arteriolosclerotic nephrosclerosis, or primary wrinkled buds, develops: small dense buds with a fine-grained surface and a sharply thinned cortical layer.
• Widespread hyalinosis of small vessels (mainly arterioles) underlies diabetic microangiopathy.
| Rice. 1. In the thickness of the soft meninges of the brain, against the background of their pronounced edema, there is a small arterial vessel with moderate thickening and homogenization of the wall due to its plasmatic impregnation with elements of hyalinosis. Staining: hematoxylin-eosin. Magnification x250. |
| Rice. 2-5. Various degrees of hyalinosis of the walls of small vessels in the thickness of the adrenal capsule and peri-adrenal adipose tissue. Small vessels with moderately and significantly thickened walls, with their impregnation with translucent pink compacted masses, similar to hyaline cartilage. |
| Rice. 6, 7. Moderate and severe hyalinosis of the walls of the renal arterioles. Staining: hematoxylin-eosin. Magnification x250. | ||
| Rice. 8-10. Severe hyalinosis of the walls of the afferent arterioles of the renal glomeruli. Severe sclerosis and hyalinosis of the glomeruli (Fig. 9, 10). Staining: hematoxylin-eosin. Magnification x250. | ||
| Rice. 11-16. Moderate and severe hyalinosis of the walls of the central arteries of the lymphatic follicles of the spleen. In a number of them, atrophy of the lymphatic follicles and delymphatization of the white pulp. Hematoxylin-eosin. Magnification x250. | ||
| Rice. 17-20. Focal nephrosclerosis with large groups of glomeruli in a state of glomerulosclerosis with hyalinosis, hyalinosis of the afferent arterioles of the renal glomeruli, foci of productive interstitial inflammation, areas of the “thyroid kidney” (groups of compactly located tubules in a state of pronounced atrophy, with thread-like thinned epithelium, cystic dilation of the lumens, filling of the latter colloid-like pale pink homogeneous content). Hematoxylin-eosin. Magnification x100 and x250. |
There are no similar entries.
Indications for surgical treatment of splenic tumor
Based on the data obtained during the examination, the surgeon needs to decide on the nature and scope of the upcoming intervention.
The choice of treatment method, especially determining the indications for surgery for tumors and cysts of the spleen, is the most difficult issue. As already noted, a significant part of splenic formations with an uncomplicated course do not manifest symptoms and are often discovered by chance. Very often a dilemma arises here: to observe or operate. What should play a decisive role in solving this difficult issue?
In our opinion, when solving this problem, the presumptive morphological nature of the lesion and signs of a complicated course come first. Next, the answers to the challenges facing diagnostic methods fall into the balance in favor of the operation. These include the size and localization of the pathological focus, assessment of the blood supply to the spleen, and anatomical relationships with adjacent organs. But these data are more conducive to the choice of the upcoming volume and nature of the operation.
The size of the formation cannot serve as a guide for the choice of treatment tactics, but only determines the indications for a specific type of operation, especially if one of the methods of preoperative examination suspected the presence of an abscess or parasitic cyst. In practice, there are often cases when, after identifying a mass in the spleen, long-term follow-up is carried out, while in almost a third of cases, malignant tumors at the preoperative stage were interpreted as benign, and only a morphological study of the removed specimen made it possible to finally establish the diagnosis. Therefore, focal formations in the spleen, regardless of their size, serve as an indication for surgical treatment
.
It usually develops as a result of fibrinoid swelling, leading to the destruction of collagen and saturation of the tissue with plasma proteins and polysaccharides.
Microscopic examination. The connective tissue bundles become swollen, they lose their fibrillarity and merge into a homogeneous dense cartilage-like mass; cellular elements are compressed and undergo atrophy. This mechanism of development of systemic connective tissue hyalinosis is especially common in diseases with immune disorders (rheumatic diseases). Hyalinosis can complete fibrinoid changes in the bottom of a chronic gastric ulcer, in the appendix in appendicitis; it is similar to the mechanism of local hyalinosis in the focus of chronic inflammation.
Hyalinosis as an outcome of sclerosis is also mainly local in nature: it develops in scars, fibrous adhesions of serous cavities, the vascular wall in atherosclerosis, involutional sclerosis of the arteries, in the organization of a blood clot, in capsules, tumor stroma, etc. The basis of hyalinosis in these cases There are disorders of connective tissue metabolism. A similar mechanism has hyalinosis of necrotic tissues and fibrinous deposits.
Appearance.
With severe hyalinosis, the appearance of organs changes. Hyalinosis of small arteries and arterioles leads to atrophy, deformation and shrinkage of the organ (for example, the development of arteriolosclerotic nephrocirrhosis).
With hyalinosis of the connective tissue itself, it becomes dense, whitish, translucent (for example, hyalinosis of the heart valves with rheumatic disease).
Exodus.
In most cases, it is unfavorable, but resorption of hyaline masses is also possible. Thus, hyaline in scars - the so-called keloids - can undergo loosening and resorption. Let's reverse hyalinosis of the mammary gland, and the resorption of hyaline masses occurs in conditions of hyperfunction of the glands. Sometimes the hyalinized tissue becomes slimy.
Functional meaning.
Varies depending on the location, degree and prevalence of hyalinosis. Widespread hyalinosis of arterioles can lead to functional failure of the organ (renal failure in arteriolosclerotic nephrocirrhosis). Local hyalinosis (for example, heart valves with heart disease) can also cause functional failure of the organ. But in scars it may not cause any particular distress.
Amyloidosis
Amyloidosis (from the Latin amylum - starch), or amyloid dystrophy, is a stromal-vascular dysproteinosis, accompanied by a profound disruption of protein metabolism, the appearance of abnormal fibrillar protein and the formation of a complex substance - amyloid - in the interstitial tissue and walls of blood vessels.
In 1844, the Viennese pathologist K-Rokitansky described peculiar changes in parenchymal organs, which, in addition to sharp compaction, acquired a waxy, greasy appearance. He called the disease in which such changes in organs occurred “sebaceous disease.” A few years later, R. Virchow showed that these changes are associated with the appearance in the organs of a special substance, which, under the influence of iodine and sulfuric acid, turns blue. Therefore, he called it amyloid, and the “greasy disease” amyloidosis. The protein nature of amyloid was established by M. M. Rudnev together with Klone in 1865.
Chemical composition and physical properties of amyloid. Amyloid is a glycoprotein, the main component of which is fibrillar proteins (F-component). They form fibrils with a characteristic ultramicroscopic structure (Fig. 33). Fibrillar amyloid proteins are heterogeneous. There are 4 types of these proteins, characteristic of certain forms of amyloidosis:
1. AA protein (not associated with immunoglobulins), formed from its serum analogue - the SAA protein;
2. AL protein (associated with immunoglobulins), its precursor is the L-chains (light chains) of immunoglobulins;
3. AF protein, the formation of which mainly involves prealbumin;
4. ASC i-protein, the precursor of which is also prealbumin.
Proteins of amyloid fibrils can be identified using specific sera during immunohistochemical examination, as well as a number of chemical (reactions with potassium permanganate, alkaline guapidine) and physical (autoclaving) reactions.
Fibrillar amyloid proteins, which are produced by cells - amyloidoblasts, are included in complex compounds with blood plasma glucoproteins. This plasma component (P-component) of amyloid is represented by rod-shaped structures (“periodic rods” - see Fig. 33). The fibrillar and plasma components of amyloid have antigenic properties. Amyloid fibrils and the plasma component combine with tissue chondroitin sulfates, and so-called hematogenous additives are added to the resulting complex, among which fibrin and immune complexes are of primary importance. The bonds of proteins and polysaccharides in the amyloid substance are extremely strong, which explains the lack of effect when various enzymes of the body act on amyloid.
Characteristic of amyloid is its red staining with Congo red, methyl (or gentznan) violet; Specific luminescence with thioflavins S or T is characteristic. Amyloid is also detected using a polarizing microscope. It is characterized by dichroism and anisotropy (the birefringence spectrum lies in the range of 540 - 560 nm). These properties allow amyloid to be distinguished from other fibrillar proteins. For macroscopic diagnosis of amyloidosis, the tissue is exposed to a Lugol's solution, and then a 10% sulfuric acid solution; the amyloid becomes blue-violet or dirty green.
The colorful reactions of amyloid, associated with the characteristics of its chemical composition, may vary depending on the form, type and type of amyloidosis. In some cases they are absent, then they speak of achromatic amyloid, or achroamyloid.
The classification of amyloidosis takes into account the following characteristics:
1. possible reason;
2. specificity of amyloid fibril protein;
3. prevalence of amyloidosis;
4. the originality of clinical manifestations due to the predominant damage to certain organs and systems.
Based on the cause, primary (idiopathic), hereditary (genetic, family), secondary (acquired) and senile amyloidosis are distinguished. Primary, hereditary, senile amyloidoses are considered as nosological forms. Secondary amyloid dose, which occurs in certain diseases, is a complication of these diseases, a “second disease”.
Primary (idiopathic) amyloidosis is characterized by: the absence of a previous or concomitant “causal” disease; damage to predominantly mesodermal tissues - the cardiovascular system, striated and smooth muscles, nerves and skin (generalized amyloidosis); tendency to form nodular deposits, inconsistent color reactions of the amyloid substance (negative results are often obtained when staining with Congo red).
Methods of surgical treatment of tumors and cysts of the spleen
Watch a video of operations for a spleen tumor performed by Professor K.V. Puchkov. You can visit the website “Video of operations of the best surgeons in the world.”
All methods of surgical treatment can be reduced to the following:
- Organ removal – splenectomy (open or laparoscopic approach)
- Organ-preserving – resection of the spleen or splenectomy with autotransplantation of splenic tissue into the greater omentum (open or laparoscopic approach)
- Percutaneous punctures for cystic formations of the spleen.
Splenectomy
Indications for splenectomy (removal of the spleen) can be divided into two groups: surgical and hematological.
Surgical indications:
- Injuries to the spleen, open, closed (one- and two-stage ruptures)
- Spleen abscess
- Splenic cysts (non-parasitic, parasitic)
- Tumors of the spleen (benign - hemangiomas, lymphangiomas, endotheliomas, malignant - fibrosarcomas, lymphosarcomas, etc.)
- Portal hypertension with splenomegaly and hypersplenism
Hematological indications:
- ITP (Werlhof's disease)
- Aplastic anemia
- Microspherocytic anemia (Minkowski-Choffard disease)
- Acquired autoimmune hemolytic anemias
- Polycythemia (erythremia)
- Chronic leukemia
- Non-Hodgkin's lymphoma
- For the purpose of diagnosis and treatment of lymphogranulomatosis.
The essence of splenectomy is the ligation and intersection of the vessels leading to the spleen, and the removal of the organ itself. The key to the success of the operation is sufficient access to the vascular pedicle and control over it throughout the operation. These parameters are most adequately met by the laparoscopic approach.
Laparoscopic splenectomy is an alternative to open surgery and, with appropriate manual skills of the surgeon and sufficient material and technical support of the clinic, can significantly reduce the frequency of intra- and postoperative complications, reduce postoperative hospital stay and improve the quality of life of patients.
Spleen resection
In clinical practice, as a rule, atypical resection of the spleen is performed, in which the pathological formation is removed without taking into account the segmental structure of the organ. This operation should be performed only for benign diseases of the spleen. When performing atypical resections, it is possible to preserve a much larger volume of parenchyma than with anatomical resections. Organ-saving operations on the spleen should be carried out using modern devices for separating parenchyma (ultrasonic scissors, argon-enhanced plasma, dosed bipolar ligation, etc.), modern local hemostatic agents - PerClot (Italy) and highly qualified surgeons performing surgery.
Heterotopic autotransplantation of splenic tissue into the greater omentum during splenectomy.
Indications for surgical autotransplantation of splenic tissue:
- Impossibility of performing organ-preserving surgery for spleen injury.
- Forced splenectomy during operations on adjacent organs.
- Removal of the spleen due to benign formations.
Contraindications to autotransplantation of splenic tissue:
- A malignant process in the spleen or organ during which it was removed.
- Malignant blood disease.
- The presence of residual tissue foci (splenosis, accessory spleen) during splenectomy.
- Total damage to an organ by a pathological process.
- Age over 50 years.
- The critical condition of the patient and other reasons requiring a reduction in the scope of the operation.
Autotransplantation into the greater omentum is considered preferable due to the peculiarities of its blood supply and ease of manipulation; however, it is also possible to use the mesentery of the small intestine for this purpose. An experimental study demonstrated a similar course of engraftment of spleen fragments both in the greater omentum and in the mesentery of the small intestine. Scientific studies prove a significant degree of regeneration of transplanted spleen fragments, which is confirmed by histological methods.
It should be emphasized that autotransplantation of splenic tissue, which is often considered as an organ-preserving procedure, is a method of prosthetics of some organ functions, since it is necessarily preceded by splenectomy, and the structure of the graft differs in many respects from normal splenic tissue. As a rule, this tissue takes on up to 70% of the functions of the organ. In 20%, there is a lack of engraftment of the transplanted splenic tissue and its gradual resorption.
Percutaneous punctures for cystic formations of the spleen
Percutaneous therapeutic punctures and catheter drainage for fluid formations of the spleen, performed under local anesthesia, are especially justified in patients with severe concomitant diseases. In cases of suspected benign genesis of focal lesions, small sizes and “convenient” localization for puncture (subcapsular location), such manipulations are effective, low-traumatic organ-saving operations. As a rule, these interventions are carried out for small cysts up to 4-5 cm in diameter.
Stromal-vascular protein dystrophies (dysproteinoses)
Among connective tissue proteins, collagen is of primary importance, from the macromolecules of which collagen and reticular fibers are built. Collagen is an integral part of basement membranes (endothelium, epithelium) and elastic fibers, which, in addition to collagen, include elastin. Collagen is synthesized by connective tissue cells, among which fibroblasts play a major role. In addition to collagen, these cells synthesize glycosaminoglycans of the main substance of connective tissue, which also contains proteins and polysaccharides of the blood plasma.
Connective tissue fibers have a characteristic ultrastructure. They are well identified using a number of histological methods: collagen - by staining with a picrofuchsin mixture (according to van Gieson), elastic - by staining with fuchselin or orcein, reticular - by impregnation with silver salts (reticular fibers are argyrophilic).
In the connective tissue, in addition to its cells that synthesize collagen and glycosaminoglycans (fibroblast, reticular cell), as well as a number of biologically active substances (mast cell, or mast cell), there are cells of hematogenous origin that carry out phagocytosis (polymorphonuclear leukocytes, histiocytes, macrophages) and immune reactions (plasmoblasts and plasmacytes, lymphocytes, macrophages).
Stromal-vascular dysproteinoses include mucoid swelling, fibrinoid swelling (fibrinoid), hyalinosis, amyloidosis.
Often, mucoid swelling, fibrinoid swelling and hyalinosis are successive stages of connective tissue disorganization; This process is based on the accumulation of blood plasma products in the main substance as a result of increased tissue-vascular permeability (plasmorrhagia), destruction of connective tissue elements and the formation of protein (protein-polysaccharide) complexes. Amyloidosis differs from these processes in that the resulting protein-polysaccharide complexes include a fibrillar protein that is not usually found, synthesized by amyloidoblast cells .
Mucoid swelling
Mucoid swelling is a superficial and reversible disorganization of connective tissue. In this case, accumulation and redistribution of glycosaminoglycans occur in the main substance due to an increase in the content of primarily hyaluronic acid. Glycosaminoglycans have hydrophilic properties, their accumulation causes an increase in tissue and vascular permeability. As a result, plasma proteins (mainly globulins) and glycoproteins are mixed with glycosaminoglycans. Hydration and swelling of the main interstitial substance develop.
Microscopic examination. The main substance is basophilic, and when stained with toluidine blue it appears lilac or red. The phenomenon of metachromasia arises, which is based on a change in the state of the main interstitial substance with the accumulation of chromotropic substances. Collagen fibers usually retain their bundle structure, but swell and undergo fibrillar disintegration. They become less resistant to the action of collagenase and, when stained with picrofuchsin, appear yellow-orange rather than brick-red. Changes in the ground substance and collagen fibers during mucoid swelling can be accompanied by cellular reactions - the appearance of lymphocytic, plasma cell and histiocytic infiltrates.
Mucoid swelling occurs in various organs and tissues, but more often in the walls of arteries, heart valves, endocardium and epicardium, i.e. where chromotropic substances are found normally; at the same time, the amount of chromotropic substances increases sharply. It is most often observed in infectious and allergic diseases, rheumatic diseases, atherosclerosis, endocrinopathies, etc.
Appearance. With mucoid swelling, the tissue or organ is preserved; characteristic changes are established using histochemical reactions during microscopic examination.
Causes. Hypoxia, infection, especially streptococcal, and immunopathological reactions (hypersensitivity reactions) are of great importance in its development.
The outcome can be twofold: complete tissue restoration or transition to fibrinoid swelling. The function of the organ suffers (for example, impaired heart function due to the development of rheumatic endocarditis - valvulitis).
Fibrinoid swelling (fibrinoid)
Fibrinoid swelling is a deep and irreversible disorganization of connective tissue, which is based on the destruction of its basic substance and fibers, accompanied by a sharp increase in vascular permeability and the formation of fibrinoid.
Fibrinoid is a complex substance that includes proteins and polysaccharides of decomposing collagen fibers, the main substance and blood plasma, as well as cellular nucleoproteins. Histochemically, the fibrinoid is different in various diseases, but its obligatory component is fibrin (hence the terms “fibrinoid swelling”, “fibrinoid”).
Microscopic picture. With fibrinoid swelling, bundles of collagen fibers impregnated with plasma proteins become homogeneous, forming insoluble strong compounds with fibrin; they are eosinophilic, stained yellow with pyrofuchsin, sharply CHIC-positive and pyroninophilic during the Brachet reaction, and also argyrophilic when impregnated with silver salts. Metachromasia of the connective tissue is not expressed or is weakly expressed, which is explained by the depolymerization of glycosaminoglycans of the main substance.
As a result of fibrinoid swelling, fibrinoid necrosis sometimes develops, characterized by complete destruction of connective tissue. Around the foci of necrosis, the reaction of macrophages is usually pronounced.
Appearance. Various organs and tissues where fibrinoid swelling occurs change little in appearance; characteristic changes are usually detected only upon microscopic examination.
Causes. Most often, this is a manifestation of infectious-allergic (for example, fibrinoid of blood vessels in tuberculosis with hyperergic reactions), allergic and autoimmune (fibrinoid changes in connective tissue in rheumatic diseases, capillaries of the renal glomeruli in glomerulonephritis) and angioneurotic (fibrinoid of arterioles in hypertension and arterial hypertension) reactions . In such cases, fibrinoid swelling is widespread (systemic). Local fibrinoid swelling can occur during inflammation, especially chronic (fibrinoid in the appendix with appendicitis, in the bottom of a chronic gastric ulcer, trophic skin ulcers, etc.).
The outcome of fibrinoid changes is characterized by the development of necrosis, replacement of the focus of destruction with connective tissue (sclerosis) or hyalinosis. Fibrinoid swelling leads to disruption and often cessation of organ function (for example, acute renal failure in malignant hypertension, characterized by fibrinoid necrosis and changes in glomerular arterioles).
Hyalinosis
With hyalinosis (from the Greek hyalos - transparent, glassy), or hyaline dystrophy, homogeneous translucent dense masses (hyalin) are formed in the connective tissue, resembling hyaline cartilage. The tissue becomes denser, so hyalinosis is also considered a type of sclerosis.
Hyaline is a fibrillar protein. An immunohistochemical study reveals not only plasma proteins and fibrin, but also components of immune complexes (immunoglobulins, complement fractions), as well as lipids. Hyaline masses are resistant to acids, alkalis, enzymes, are CHIC-positive, accept acidic dyes (eosin, acid fuchsin) well, and are stained yellow or red with picrofuchsin.
The mechanism of hyalinosis is complex. The leading factors in its development are the destruction of fibrous structures and increased tissue-vascular permeability (plasmorrhagia) in connection with angioneurotic (dyscirculatory), metabolic and immunopathological processes. Plasmorrhagia is associated with the impregnation of tissue with plasma proteins and their adsorption on altered fibrous structures, followed by precipitation and the formation of protein - hyaline. Smooth muscle cells take part in the formation of vascular hyaline. Hyalinosis can develop as a result of various processes: plasma impregnation, fibrinoid swelling (fibrinoid), inflammation, necrosis, sclerosis.
Classification. A distinction is made between vascular hyalinosis and hyalinosis of the connective tissue itself. Each of them can be widespread (systemic) and local.
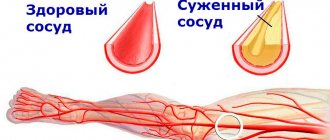
Vascular hyalinosis. Hyalinosis occurs mainly in small arteries and arterioles. It is preceded by damage to the endothelium, its membrane and smooth muscle cells of the wall and its saturation with blood plasma.
Microscopic examination. Hyaline is found in the subendothelial space, it pushes outward and destroys the elastic lamina, the middle membrane becomes thinner, and finally the arterioles turn into thickened glassy tubes with a sharply narrowed or completely closed lumen.
Hyalinosis of small arteries and arterioles is systemic in nature, but is most pronounced in the kidneys, brain, retina, pancreas, and skin. It is especially characteristic of hypertension and hypertensive conditions (hypertensive arteriolohyalinosis), diabetic microangiopathy (diabetic arteriolohyalinosis) and diseases with impaired immunity. As a physiological phenomenon, local arterial hyalinosis is observed in the spleen of adults and elderly people, reflecting the functional and morphological characteristics of the spleen as a blood deposition organ.
Vascular hyaline is a substance of predominantly hematogenous nature. Not only hemodynamic and metabolic, but also immune mechanisms play a role in its formation. Guided by the peculiarities of the pathogenesis of vascular hyalinosis, 3 types of vascular hyaline are distinguished: