Anemia in diabetic nephropathy
Diabetes mellitus (DM) is a common disease, affecting about 5% of the European population. The prevalence of this disease is growing every year. It is expected that in the next few years the number of such patients in Europe will exceed 32 million people [1]. A characteristic complication of both type 1 and type 2 diabetes is nephropathy. In industrialized countries, diabetic nephropathy has now become the leading cause of end-stage chronic kidney disease (CKD) [2, 3]. As the number of patients with diabetes grows, we can expect a proportional increase in the role of diabetic nephropathy in the structure of patients with end-stage renal failure.
Approximately half of patients with CKD suffer from anemia [4, 5]. Accordingly, diabetes is one of the main causes of renal anemia. In diabetic nephropathy, anemia develops earlier and more often and is more severe than in patients with kidney diseases of other nature. For example, according to the epidemiological study NHANES III (National Health and Nutrition Examination Survey), conducted in the USA, the incidence of anemia in patients with CKD stages III–IV and diabetes was 2 times higher than in patients with comparable renal impairment who did not suffer from diabetes. [6].
Anemia has an undesirable effect on the quality of life of patients, causes a decrease in performance and exercise tolerance, deterioration of sexual and cognitive functions and is accompanied by various symptoms (shortness of breath, dizziness, poor appetite, etc.). Moreover, anemia in patients with diabetes predicts an increased risk of adverse outcomes (regardless of the severity of nephropathy) and, apparently, itself contributes to the progression of micro- and macroangiopathy. However, physicians usually do not attach much importance to anemia in such patients [7].
The leading role in the pathogenesis of renal anemia is played by a deficiency of erythropoietin produced by the kidneys. In this regard, it has been suggested that its earlier use in patients with diabetic nephropathy may lead to an improved prognosis for this condition.
Frequency of anemia in patients with diabetes mellitus
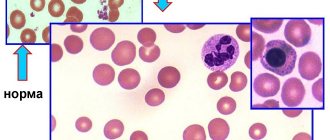
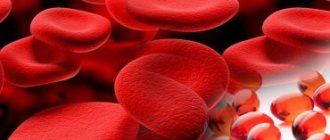
In accordance with WHO recommendations, the criterion for the diagnosis of anemia is a decrease in hemoglobin levels < 120 g/l in women and < 130 g/l in men. Similar criteria are used in the European guidelines for the treatment of anemia in patients with CKD (hemoglobin <115 g/L in women and <135 g/L in men aged <70 years and <120 g/L in men aged over 70 years) [8 ]. Using these criteria, approximately every fourth patient with type 1 or type 2 diabetes (about 23%) suffers from anemia [9–11]. A more pronounced decrease in hemoglobin levels (< 110 g/l) is observed in approximately 7–8% of patients.
The risk of developing anemia increases significantly when signs of diabetic nephropathy (decreased renal function and/or albuminuria) appear. For example, according to M. Thomas, 60% of patients with anemia diagnosed based on WHO criteria had a decrease in glomerular filtration rate <60 ml/min/1.73 m2 [12]. As kidney function deteriorates, the incidence of anemia increases exponentially in both men and women. Hemoglobin levels are most closely associated with glomerular filtration rate, including in patients with normal serum creatinine levels.
In diabetic nephropathy, anemia develops earlier than in patients with other kidney diseases. D. Bosman et al. [13] compared the prevalence of anemia in patients with type 1 diabetes complicated by nephropathy and patients with chronic glomerulonephritis. Anemia was detected in almost half of the patients with diabetic nephropathy and was absent in all patients in the comparison group. Moreover, an increased incidence of anemia is recorded in patients with diabetes who do not suffer from impaired renal function (glomerular filtration rate > 90 ml/min/1.73 m2), although this does not always indicate a real absence of nephropathy. Anemia is associated with microalbuminuria, which is an early marker of inflammation and microvascular damage and precedes deterioration of renal function.
Diabetes contributes to the development of more severe anemia. E. Ishimura et al. [14] compared hemoglobin levels in patients with type 2 diabetes and patients with nondiabetic kidney disease. The hemoglobin concentration in patients with diabetes was significantly lower than in patients in the control group (p < 0.01). According to the authors, diabetes, along with serum creatinine levels, is an independent risk factor for the development of anemia.
The role of erythropoietin deficiency in the development of anemia in patients with diabetes
Although renal anemia in patients with diabetes can be due to various reasons (iron deficiency, hidden blood loss, chronic hypoxia, etc.), the leading role in its development is played by the lack of erythropoietin, a glycoprotein that regulates the formation of red blood cells and is synthesized by peritubular fibroblasts of the renal cortex [ 15]. The gene encoding erythropoietin is located on chromosome 7q11–q22. The main signal for its transcription is hypoxia. It has been suggested that erythropoietin provides the coordination between plasma volume and red blood cell mass necessary for maximal oxygen delivery to tissues [16].
The direct cause of anemia in CKD is considered to be insufficient production of erythropoietin in response to a decrease in hemoglobin levels. In healthy people, there is an inverse relationship between the content of erythropoietin and hemoglobin. In non-renal anemia (iron deficiency, hemolytic), a compensatory increase in the secretion of erythropoietin occurs, which enhances erythropoiesis. At the same time, in patients with renal anemia, erythropoietin levels remain normal, reflecting its relative deficiency [17]. A. Symeonidis et al. [18] compared erythropoietin levels in patients with anemia of various origins, with and without diabetes. Hemoglobin concentrations were comparable in the two groups. The serum level of erythropoietin in patients with diabetes was significantly lower than in patients without diabetes (36.5 ± 61 and 69.4 ± 191 IU/ml, p < 0.0001), and this difference persisted in all forms of anemia, with the exception of myeloproliferative diseases and megaloblastic anemia. The authors found a negative correlation between the concentration of erythropoietin and the level of glycated hemoglobin (r = –0.446) and suggested that the reason for the decrease in erythropoietin secretion may be an increase in the level of glycated hemoglobin.
The cause of impaired erythropoietin secretion in patients with diabetes is probably damage to the renal tubulointerstitium, which, like anemia, precedes a decrease in glomerular filtration rate [12]. In the initial stages of diabetic nephropathy, even in the absence of microalbuminuria, thickening of the basement membrane of the tubules is detected [19]. It can be assumed that damage to the interstitial cells that produce erythropoietin, or disruption of the interaction between the tubules, peritubular fibroblasts and endothelium, necessary for normal hematopoiesis, contributes to the deterioration of erythropoietin secretion [12]. Some authors [20] suggest considering the production of this hormone as a marker of the severity of tubulointerstitial changes in diabetes. The development of anemia can probably be caused not only by damage to tubulointerstitial cells, but also by disruption of the feedback mechanisms between tissue oxygenation, erythropoietin and hemoglobin [12]. This is supported by the persistence of the response to acute hypoxia in patients with diabetes and anemia [21]. Inhibition of angiotensin-converting enzyme (ACE) leads to a sharp increase in the blood of stages III–V CKD patients of the physiological inhibitor of erythropoiesis, the tetrapeptide Ac-SDKP. Therefore, when treated with ACE inhibitors, patients require higher doses of erythropoietin.
In patients with persistent nephrotic syndrome, with the development of protein-energy deficiency, the mass of circulating erythrocytes can be significantly reduced due to a decrease in the production of erythropoietin, according to a decrease in basal metabolism. However, the true severity of anemia can be judged only after restoration of normal plasma albumin levels [22].
Anemia as a risk factor
Anemia leads to a number of symptoms that worsen the quality of life of patients, their physical and cognitive function, sleep, appetite, and exercise tolerance. Anemia is accompanied by an increase in heart rate and cardiac output, progressive myocardial hypertrophy and impaired diastolic function. It has been established that anemia is a risk factor for the development of heart failure and worsens its course [23]. P. Srivastava et al. [24] revealed cardiac dysfunction in 94% of patients with type 2 diabetes and anemia using echocardiography. Tissue hypoxia during anemia can contribute to the exacerbation of coronary heart disease. This problem is especially relevant for patients with type 2 diabetes, which is often combined with cardiovascular diseases. Renal failure itself increases the risk of their development [25].
Anemia is associated with an increased risk of developing vascular complications of diabetes, including nephropathy, retinopathy and neuropathy [26, 27]. In the RENAAL study, which examined the nephroprotective properties of losartan in patients with type 2 diabetes, low hemoglobin concentration predicted rapid progression of kidney damage [28]. In this study, patients who experienced a doubling of serum creatinine, development of stage V CKD, or death had lower baseline hemoglobin concentrations than other patients. It should be noted that anemia itself does not cause microangiopathy, but can be considered as its manifestation [29]. This allows us to understand why anemia acts as a risk marker for microvascular complications.
However, it cannot be excluded that anemia itself may contribute to the development or progression of microangiopathy [30]. Anemia is accompanied by tissue hypoxia, which produces mitogenic and fibrogenic effects, and also modifies the expression of genes regulating angiogenesis and capillary permeability, vasomotor response, glycolysis, cell apoptosis, etc. [31]. In addition, anemia causes activation of the sympathetic and renin-angiotensin systems, which contributes to the development of proteinuria and arterial hypertension in patients with CKD [32].
As stated above, anemia causes various undesirable changes in the cardiovascular system, in particular left ventricular hypertrophy and increased myocardial ischemia, and theoretically may be a risk factor for macrovascular complications. For example, in patients with heart failure, anemia has been associated with increased hospitalization and overall mortality [33]. In the ARIC (Atherosclerosis Risk in Communities) study, anemia in patients with CKD was a risk factor for cardiovascular complications [34]. However, in the NHANES II study, there was no significant association between anemia and mortality from coronary heart disease (CHD) [35].
P. Vlagopoulos et al. [36] studied the prognostic value of anemia in 3015 patients with diabetes who took part in four large population-based studies, including the Framingham study. Anemia was present in 8.1% of them, and CKD (glomerular filtration rate 15–60 ml/min/1.73 m2) was present in 13.8%. The frequency of the combined endpoint (myocardial infarction/death from ischemic heart disease/stroke/death) and its individual components was analyzed. In patients with nephropathy, anemia was associated with a 1.7-fold increase in the risk of the composite endpoint, a 1.6-fold increase in the risk of myocardial infarction/CHD death, a 1.8-fold increase in stroke, and a 1.9-fold increase in death from any cause. At the same time, in patients without nephropathy, the presence of anemia was not accompanied by an increased risk of any cardiovascular outcomes. According to the authors, the results obtained can be explained by various reasons. First, patients with diabetic nephropathy often have damage to other organs, including the heart, and may therefore be more susceptible to anemia-induced ischemia. Secondly, renal anemia is caused by a deficiency of erythropoietin, which in animal experiments had a beneficial effect on the cardiovascular system (limiting myocardial damage by suppressing apoptosis of endothelial cells and cardiomyocytes, as well as stimulating angiogenesis) [37]. Accordingly, the cause of a worsening prognosis may be not only anemia, but also a lack of erythropoietin. Thirdly, some factors that influence the prognosis may not have been taken into account, for example, the severity of arterial hypertension or the long duration of diabetes in patients with nephropathy.
Erythropoietin in the treatment of anemia in patients with diabetes mellitus
The basis for the treatment of renal anemia is the use of recombinant human erythropoietin, created using genetic engineering at the end of the 20th century. Modern erythropoietin preparations are highly purified glycoproteins consisting of polypeptide chains and a carbohydrate part (alpha or beta), at the ends of which there are sialic groups that prevent inactivation of the hormone. There are erythropoietin alpha and erythropoietin beta. Recombinant human erythropoietin preparations are administered intravenously or subcutaneously. The subcutaneous method of administering erythropoietin is not inferior to the intravenous method in terms of effectiveness, but at the same time is safer and more economical: correction of anemia is achieved in the same time frame as with the intravenous method, but due to the use of smaller (1.5–2 times) cumulative and maintenance doses [8]. The most common treatment for renal anemia is erythropoietin beta (Recormon), which is administered subcutaneously or intravenously three times a week. In recent years, the possibility of subcutaneous use of these drugs once a week has also been shown (the total weekly dose corresponds to that for three injections), which greatly facilitates the practical use of the drug. Another drug, darbepoetin alfa (Aranesp), has not yet been registered in Russia. European guidelines for the treatment of renal anemia recommend that erythropoietin be prescribed to all patients with chronic kidney disease whose hemoglobin level is < 110 g/l on two consecutive measurements and other possible causes of anemia (primarily iron deficiency) have been excluded [8, 39]. Preference should be given to subcutaneous administration, as it reduces the required dose of erythropoietin and, accordingly, the cost of treatment.
In recent years, early use of erythropoietin in the predialysis period has attracted great interest among researchers. It has been suggested that such therapy may delay the progression of kidney disease or reduce the risk of cardiovascular complications, although the beneficial effects of erythropoietin may be offset by its ability to induce hypertension. J. Cody et al. [40] performed a meta-analysis of 15 controlled studies of recombinant human erythropoietin in 461 patients with renal anemia not receiving dialysis treatment. Therapy with this drug resulted in a significant increase in hemoglobin levels (by an average of 1.82 g/dL) and hematocrit (by 9.85%) and a decrease in the need for blood transfusions, as well as improvements in quality of life and exercise tolerance. There was no significant increase in the incidence of adverse events or signs of deterioration in renal function. Some authors have been able to demonstrate the beneficial effects of early use of erythropoietin on renal function. For example, S. Gouva et al. [41] studied this drug in a randomized trial in 88 patients with serum creatinine levels of 2–6 mg/dL and hemoglobin concentrations of 90–116 g/L. Treatment with erythropoietin also had a beneficial effect on cardiac function in patients with renal anemia, in particular causing a decrease in myocardial hypertrophy.
In a study conducted in our clinic [42], in all 34 patients with CKD stages III–IV, echocardiography and 24-hour monitoring over time demonstrated a beneficial effect of erythropoietin beta on cardiac function. 5 months after the start of treatment, normalization of cardiac output and diastolic function was revealed, as well as a clear tendency towards the reverse development of myocardial hypertrophy. None of the 34 patients had developed eccentric hypertrophy of the left ventricle at the time of admission to hemodialysis.
J. Ayus et al. [43] treated 40 patients with diabetic and nondiabetic nephropathy and anemia (hemoglobin < 100 g/l) with erythropoietin. After 6 months, the left ventricular myocardial mass index significantly decreased (p = 0.007), and the hemoglobin level increased (p = 0.001). In the control group, no significant changes in these indicators were detected.
Convincing evidence has been obtained indicating that the effects of erythropoietin beta in CKD are not limited to stimulation of hematopoiesis. Thus, Van der Meer P. et al. [44] using a model of myocardial infarction in rats showed that this drug reduces the area of damage to cardiomyocytes by 56% (p < 0.05) and suppresses their apoptosis by 15% (p < 0.05). It has been established that erythropoietin beta accelerates the proliferation of cardiomyocytes [45]. In this regard, it can be assumed that the effect of erythropoietin beta on the infarction area, on the one hand, is due to the suppression of apoptosis of endothelial cells and cardiomyocytes, and on the other, to an increase in the proliferation of cardiomyocytes.
Currently, several large controlled studies have been launched to study the safety of early erythropoietin therapy and its effectiveness in preventing cardiovascular complications and progression of renal failure in patients with CKD. For example, in the CREATE (Cardiovascular Risk reduction by Early Anaemia Treatment with Epoetin beta) study, 600 patients with a creatinine clearance of 15–35 ml/min and anemia (110–125 g/l) were randomized [46]. The purpose of the study was to compare the long-term results of early (target hemoglobin level 130–150 g/l) and delayed (with a decrease in hemoglobin level < 105 g/l, target concentration 105–115 g/l) therapy with erythropoietin beta. The ACORD (Anaemia CORrection in Diabetes) study will evaluate the effectiveness of early correction of anemia with erythropoietin beta in 160 patients with diabetic nephropathy. Patients in the main group will begin treatment with erythropoietin beta immediately after inclusion in the study (target hemoglobin level 130–150 g/l), and patients in the control group are planned to begin standard therapy with a hemoglobin level less than 105 g/l. The TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy) study, which has a similar design, plans to study the effectiveness of early therapy with darbepoetin alfa.
Selection of dose and frequency of administration of erythropoietin and iron supplements
At the pre-dialysis stage of CKD (III–IV stages of CKD), erythropoietin beta is administered to patients subcutaneously at a dose of 20 IU/kg 3 times a week or 60 IU/kg once a week. In this case, the target hemoglobin level (120 g/l), corresponding to its complete correction, is reached within 4 months. The iron balance in patients with stage III CKD and diabetes can be maintained by taking oral iron supplements, for example Maltofer, which is prescribed once a day 2 hours before meals, while the dose of elemental iron should be at least 200 mg/day. During treatment, constant monitoring of residual renal function (dynamics of glomerular filtration rate and blood creatinine level), blood pressure (including 24-hour monitoring), circulating blood volume, and cardiac hemodynamics is necessary. Therefore, the combination of erythropoietin with antihypertensive therapy, adherence to a low-protein diet (0.6 g of protein per 1 kg of body weight) and limiting sodium intake is of great importance.
Currently, a system for subcutaneous administration of Recormon is being produced - the Reko-pen syringe pen and cartridges with erythropoietin beta of 10,000 IU and 20,000 IU. Using this system allows patients to self-dose the drug, and the very thin needle minimizes discomfort associated with local reactions. The use of Reko-pen is not only well tolerated by patients, but also reduces treatment costs.
A slow increase in hemoglobin levels (less than 10 g/L per month or hematocrit less than 0.5% per month) is a sign of decreased effectiveness of erythropoietin. The most common cause of decreased response to erythropoietin is iron deficiency. Achieving and maintaining the target hemoglobin level is ensured by maintaining a normal iron balance: ferritin level 200–500 μg/L, transferrin saturation (TSAT) 30–40%, hypochromic red blood cell count < 2.5%. If a patient with chronic renal failure with a reduced response to erythropoietin does not have signs of iron deficiency, it is necessary to look for other reasons for the reduced response to erythropoietin. Among them: bacterial infections (including tuberculosis), chronic bleeding, severe uremic hyperparathyroidism, cancer (myeloma), the influence of drugs (ACE inhibitors, angiotensin II receptor blockers, cytostatics, histamine H2 receptor blockers, theophylline, vitamin A). An indicator of a current active inflammatory process is an increase in the level of C-reactive protein (CRP) in the blood of more than 50 mg/ml. If occult gastrointestinal bleeding and elevated CRP levels are not detected in a patient with resistance to erythropoietin, the dose of erythropoietin is increased by 50%.
Conclusion
Anemia is one of the most common manifestations of CKD. The main reason for its development is insufficient production of erythropoietin in the kidney tissue. With diabetic nephropathy, anemia develops more often and earlier and is more severe than with CKD of other etiologies. Renal anemia worsens the quality of life of patients with type 1 and type 2 diabetes, their physical and cognitive function, sleep, appetite, and exercise tolerance. Moreover, it is associated with an increased risk of microvascular and macrovascular complications of diabetes. For example, anemia several times increases the risk of progression of diabetic nephropathy to end-stage CKD in both type 1 and type 2 diabetes.
An effective method for correcting anemia in patients with chronic kidney disease, including diabetic nephropathy, is the subcutaneous use of erythropoietin. It is believed that early administration may lead to a reduction in the risk of cardiovascular disease. This hypothesis is currently being studied in randomized controlled trials.
Literature
- Amos A., McCarty D., Zimmet P. The rising global burden of diabetes and its complications: estimates and projections of the year 2010 // Diabetic Med. 1997; 14(5):S7–S85.
- Van Ypersele de Strihou C. Should anemia in subtypes of CRF patients be managed differently? // Nephrol. Dial. Transplant. 1999; 14 (2): 37–45.
- Management of diabetic patients with end-stage renal failure on dialysis. Guidelines, ed. I. I. Dedova and N. A. Tomilina. 2004. 62 p.
- McClellan W., Aronoff S., Bolton W. et al. The prevalence of anemia in patients with chronic kidney disease // Curr. Med. Res. Opin. 2004; 20: 1501–1510.
- Dobronravov V. A., Smirnov A. V. Anemia and chronic kidney disease // Anemia. 2005; 2:2–8.
- Astor B., Muntner P., Levin A. et al. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994) // Arch. Intern. Med. 2002; 162:1401–1408.
- Stevens P., O'Donogue D., Lameire N. Anemia in patients with diabetes: unrecognized, undetected and untreated? //Curr. Med. Res. Opin. 2003; 19: 395–401.
- Locatelli F., Aljama P., Barany P. et al. Revised European best practice guidelines for the management of anemia in patients with chronic renal failure // Nephrol. Dial. Transplant. 2004; 19 (2): ii1–ii47.
- Thomas M., MacIsaac R., Tsalamandris C. et al. Unrecognized anemia in patients with diabetes: a cross-sectional survey // Diabetes Care. 2003; 26:1164–1169.
- Thomas M., MacIsaac R., Tsalamandris C. et al. The burden of anemia in type 2 diabetes and the role of nephropathy: a cross-sectional audit // Nephrol. Dial. Transplant. 2004; 19: 1792–1797.
- Thomas M., MacIsaac R., Tsalamandris C. et al. Anemia in patients with type 1 diabetes // J. Clin. Endocrinol. Metab. 2004; 89:4359–4363.
- Thomas M., Cooper M., Rossing K. et al. Anaemia in diabetes: is there a rationale to TREAT? // Diabetologia. 2006; Apr. 4 .
- Bosman D., Winkler A., Marsden J. et al. Anemia with erythropoietin deficiency occurs early in diabetic nephropathy // Diabetes Care. 2001; 24:495–499.
- Ishimura E., Nishizawa Y., Okuno S. et al. Diabetes mellitus increases the severity of anemia in non-dialyzed patients with renal failure // J. Nephrol. 1998; 11:83–86.
- Maxwell P., Osmond M., Pugh C. et al. Identification of the renal erythropoietinproducing cells using transgenic mice // Kidney Int. 1993; 44:1149–1162.
- Donnelly S. Why is erythropoietin made in the kidney? The kidney functions as a critmeter // Am. J. Kidney Dis. 2001; 38:415–425.
- Thomas M., Cooper M., Tsalamandris C. et al. Anemia with impaired erythropoietin response in diabetic patients // Arch. Intern. Med. 2005; 165:466–469.
- Symeonidis A., Kouraklis-Symeonidis A., Psiroyiannis A. et al. Inappropriately low erythropoietin response for the degree of anemia in patients with noninsulin-dependent diabetes mellitus // Ann. Hematol. 2006; 85 (2): 79–85.
- Brito P., Fioretto P., Drummond K. et al. Proximal tubular basement membrane width in insulin-dependent diabetes mellitus // Kidney Int. 1998; 53:754–761.
- Inomata S., Itoh M., Imai H., Sato T. Serum levels of erythropoietin as a novel marker reflecting the severity of diabetic nephropathy // Nephron. 1997; 75:426–430.
- Bosman D., Osborne C., Marsden J. et al. Erythropoietin response to hypoxia in patients with diabetic autonomic neuropathy and non-diabetic chronic renal failure // Diabet Med. 2002; 19: 65–66.
- Milovanov Yu. S., Kozlovskaya L. V., Milovanova L. Yu. Treatment of anemia in patients with chronic renal failure at the pre-dialysis stage // Treating Physician. 2006; 7:12–23.
- Dries D., Sweitzer N., Drazner M. et al. Prognostic impact of diabetes mellitus in patients with heart failure according to the etiology of left ventricular systolic dysfunction // J. Am. Coll. Cardiol. 2001; 38:421–428.
- Srivastava P., Thomas M., Calafiore P. et al. Diastolic dysfunction is associated with anemia in patients with Type II diabetes // Clin. Sci. (Lond.). 2006; 110: 109–116.
- Tomilina N. A., Volgina G. V., Bikbov B. T., Kim I. G. The problem of cardiovascular diseases in chronic renal failure // Nephrology and dialysis. 2003; 5 (1): 15–23.
- Ueda H., Ishimura E., Shoji T. et al. Factors affecting progression of renal failure in patients with type 2 diabetes // Diabetes Care. 2003; 26:1530–1534.
- Qiao Q., Keinanen-Kiukaanniemi S., Laara E. The relationship between hemoglobin levels and diabetic retinopathy // J. Clin. Epidemiol. 1997; 50: 153–158.
- Keane W., Brenner B., de Zeeuw D. et al. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study // Kidney Int. 2003; 63:1499–1507.
- Thomas M., Tsalamandris C., MacIssaac R., Jerums G. Anemia in diabetes; an emerging complication of microvascular disease. Current Diabetes Rev. 2005; 1: 107–126.
- Shestakova M.V., Koshel L.V., Vagodin V.A., Dedov I.I. Risk factors for the progression of diabetic nephropathy in patients with long-term diabetes mellitus according to a retrospective analysis // Ter. archive. 2006; 6:34–39.
- Deicher R., Horl W. Anaemia as a risk factor for the progression of chronic kidney disease // Curr. Opin. Nephrol. Hypertens. 2003; 12: 139–143.
- Fine L., Bandyopadhay D., Norman J. Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic hypoxia // Kidney Int. Suppl. 2000; 75:S22–S28.
- Anand I., McMurray J., Whitmore J. et al. Anemia and its relationship to clinical outcome in heart failure // Circulation. 2004; 110: 149–154.
- Sarnak M., Tighiouart H., Manjunath G. et al. Anemia as a risk factor for cardiovascular disease in the Atherosclerosis Risk in Communities (ARIC) study // J. Am. Coll. Cardiol. 2002; 40: 27–33.
- Brown D., Giles W., Croft J. Hematocrit and the risk of coronary heart disease mortality // Am. Heart J. 2001; 142:657–663.
- Vlagopoulos P., Tighiouart H., Weiner D. et al. Anemia as a risk factor for cardiovascular disease and all-cause mortality in diabetes: the impact of chronic kidney disease // J. Am. Soc. Nephrol. 2005; 16:3403–3410.
- Van der Meer P., Voors A., Lipsic E. et al. Erythropoietin in cardiovascular diseases // Eur. Heart J. 2004; 25: 285–291.
- Milovanov Yu. S., Kozlovskaya L. V., Nikolaev A. Yu., Milovanova L. Yu. Anemia in patients with chronic renal failure: principles of therapy // Attending Physician. 2005; 10: 18–24.
- Ermolenko V.M., Khasabov N.N., Mikhailova N.A. Recommendations for the use of iron supplements in patients with chronic renal failure // Anemia. 2005; 2:9–25.
- Cody J., Daly C., Campbell M. et al. Recombinant human erythropoietin for chronic renal failure anemia in pre-dialysis patients // Cochrane Database Syst. Rev. 2005; (3): CD003266.
- Gouva C., Nikolopoulos P., Ionnidis J., Siamopoulos K. Treating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trial // Kidney Int. 2004; 66:753–760.
- Kozlovskaya L.V., Milovanov Yu.S., Fomin V.V., Milovanova L.Yu. Cardiovascular effects of erythropoietin in patients with the conservative stage of chronic renal failure // Doctor. 2004; 10:57–59.
- Ayus J., Go A., Valderrabano F., Verde E. Effects of erythropoietin on left ventricular hypertrophy in adults with severe chronic renal failure and hemoglobin 10 g/dL // Kidney Int. 2005; 68(2):788–795.
- Van der Meer P., Lipsic E., Boer et al. A functional erythropoetin receptor in the rat heart is linked to anti-apoptotic effects // J. Am. Coll. Cardiol. 2003; 41: 330A.
- Saraste A., Pulkki K., Kallajoki M. et al. Apoptosis in human acute myocardial infarction // Circulation. 1997; 95: 320–322.
- Eckardt K. The CREATE trial-building the evidence // Nephrol. Dial. Transplant. 2001; 16 (2): 16–18.
Yu. S. Milovanov , Doctor of Medical Sciences S. Yu. Milovanova , Candidate of Medical Sciences MMA named after. I. M. Sechenova , Moscow
What is the normal saturation level for a healthy person?
The norm for a healthy person is SpO2 = 95-99 (or 100)%. The rate of blood oxygen saturation depends on the individual characteristics of the human body, for example, on the presence or absence of anemia, apnea, chronic diseases of the respiratory and cardiovascular systems, bad habits, and age. At night, each person's saturation decreases, and the differences can be significant. For example, in people with chronic diseases of the respiratory system (COPD, apnea), who have adapted to a constant lack of oxygen, the rate can drop to 90% (in the deep sleep phase).*
According to the observations of doctors working in hospitals with seriously ill patients who are on oxygen, the most dangerous time is from 3 to 7 am. At this time, the largest number of deaths are recorded due to decreased saturation, or more precisely due to oxygen hypoxemia.
RE Gries, LJ Brooks, Normal oxyhemoglobin saturation during sleep. How low does it go? K. Szabó, F. Ihász, The effect of reduced oxygen saturation during sleep on depression, 2020
What foods increase hemoglobin
To improve your well-being and normalize metabolic processes, you need to know how to increase hemoglobin in diabetes mellitus. To do this, you need to include the following products in your diet:
- Beef and chicken liver.
- Veal and beef.
- Turkey.
- Egg yolk.
- Squid, mussels.
- Legumes – beans, green peas
- Parsley, spinach.
- Sesame, sunflower and pumpkin seeds.
- Walnuts.
- Blueberry.
- Apricots and plums.
- Dried fruits
- Raspberries.
- Buckwheat and wheat bran.
All of these foods contain a lot of iron, but it is best absorbed from animal products. Ascorbic acid from rosehip decoction, apple or blackcurrant juice enhances its absorption, and coffee, tea and dairy products inhibit it.
Legumes are rich in iron and protein, but for better absorption, you need to soak them overnight and then rinse them. This removes phytic acid, which inhibits iron absorption.
You can prepare a mixture of dried fruits and walnuts, chopped in a blender, and lemons. Everything must be taken in equal parts. Take a tablespoon in the morning on an empty stomach, washed down with rosehip decoction.
Prevention of changes in the blood
Once faced with anemia, you should adhere to general recommendations throughout your life:
- Have a medical examination twice a year
- Form a balanced, varied diet.
- Monitor your work and rest schedule, take walks in the fresh air.
- Eliminate bad habits
- Maintain normal body weight.
Walking is good prevention
Study design
The population-based cohort study used data from the Swedish National Diabetes Registry (1998–2017).
Study participants included 10,398 children and adults with type 1 diabetes who were followed from diagnosis until the end of 2021.
As the basis of the endpoint
The relative risk (odds ratio) of retinopathy and nephropathy for different levels of glycated hemoglobin was selected.
Test results
Only a doctor can interpret test results, since he evaluates them in conjunction with other symptoms, weight, heredity, and condition. So in pregnant women, blood sugar levels may increase and the doctor decides whether adjustments are required or not. If a diagnosis of diabetes has been avoided, many may find themselves with prediabetes, impaired glucose tolerance and metabolic syndrome.
One of the clear ways to correct your diet and physical activity level is to use a Contour Plus glucometer with test strips included. A small drop of blood - an accurate measurement method will allow you to see the results of your efforts on the display and instill new healthy lifestyle habits. This can help avoid many problems in the future.