Tests and diagnostics
To identify the true cause of changes in the myocardium, it is recommended to undergo a full examination, which includes:
- General analysis and biochemical blood test. Based on the results, it will be possible to talk about the presence of an inflammatory process in the body, the functioning of the renal system and kidneys, and the level of cholesterol , which forms plaques in the coronary arteries.
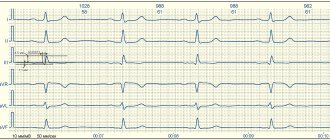
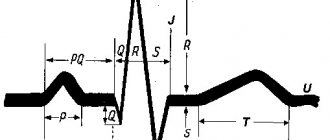
- ECG. Characteristic changes during the examination make it possible to determine further examination tactics. In some cases, it is recommended to perform an ECG with stress, or organize daily ECG monitoring.
- EchoCG. Ultrasound examination of the heart allows you to assess the condition of the valve apparatus of the heart, identify areas of damage, and evaluate the pumping function of the heart.
- Myocardial scintigraphy. The radioisotope research method shows areas of accumulation of a special substance to identify areas of damage and determine their nature.
Changes in the myocardium on the ECG make it possible to determine further tactics for examining the patient to establish an accurate diagnosis and select the correct therapy.
Treatment
If acute coronary syndrome is detected on the ECG, which indicates acute myocardial damage as a result of muscle necrosis, the patient is urgently hospitalized in the cardiology department with the possibility of performing coronary angiography and determining further treatment tactics.
When diffuse and dystrophic changes are detected, the underlying disease that could provoke them is treated. Additionally, metabolic therapy is carried out aimed at improving the nutrition of the myocardium for its speedy recovery.
Causes
Dystrophic changes in the myocardium
Such changes in the ECG are formed due to insufficient nutrition of cardiomyocytes, which inevitably leads to a decrease in the contractility of the left ventricle. Diffuse-dystrophic changes in the myocardium are observed with:
- pathologies of the endocrine system: diabetes mellitus , adrenal dysfunction, disorders of the thyroid gland;
- pathologies of the renal system and liver: excessive amounts of toxic metabolic products negatively affect the functioning of the heart;
- chronic diseases of infectious origin: changes can be observed with tuberculosis , influenza , malaria , etc.;
- chronic iron deficiency anemia : constant oxygen starvation affects the functioning of cardiomyocytes;
- with an unbalanced diet, with vitamin deficiency in the diet;
- with excessive nervous and physical overload;
- with fever and concomitant dehydration;
- in case of poisoning with alcohol, medications or chemical components.
Metabolic changes in the myocardium
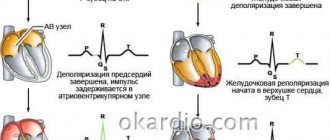
What it is? Characteristic nonspecific changes on the ECG are formed as a result of disturbances in intracellular metabolic processes associated with potassium and sodium ions.
Metabolic changes are associated with dystrophy of the heart muscle and appear when:
- ischemia , which is reflected on the cardiogram in the form of deviations of the T wave. Its polarity and shape changes in the leads corresponding to the damaged areas;
- myocardial infarction : the location of the ST segment changes on the ECG, which is located either above or below the isoline;
- death, necrosis of the myocardium, which is characterized by the appearance of an abnormal Q wave.
Scar changes
Areas of scar tissue form at the site of a former inflammatory process, necrosis, as a result of which normal, healthy cardiomyocytes lost their contractility and were replaced by connective tissue that does not have elasticity. Focal cicatricial changes on the ECG indicate a previous myocardial infarction .
- The lower wall of the left ventricle is characterized by changes in leads: II, III and a VF (indicates damage to the right, less often the left circumflex coronary artery).
- The anterior septal region is characterized by changes in leads: V1 and V2 (the left descending septal branch is damaged), or V2-V4 (the left descending coronary artery or its branches is involved).
- The anterior-lateral region is characterized by changes in leads: I, aVL, V4-V6 (the circumflex artery or the left descending artery is damaged).
- Anterior widespread infarction is characterized by changes in leads: I, aVL, V1-V6 (the left descending coronary branch is damaged).
Moderate inflammatory changes in the myocardium
Characteristic changes are observed in myocarditis, in which the voltage of the waves in all leads decreases and rhythm disturbances are recorded. Moderate left ventricular changes may occur after:
- typhus , diphtheria ;
- rheumatism , after streptococcal infection ( tonsillitis , tonsillitis , scarlet fever );
- infections caused by the Coxsackie virus, rubella , influenza , measles ;
- exacerbation of an autoimmune disease ( systemic lupus erythematosus , scleroderma ).
Brown myocardial atrophy
This is what a macropreparation is called during histological examination. Characteristic pathological changes in the myocardium are formed as a result of a long-term lack of blood supply, which is observed in debilitating diseases, cachexia , drug abuse, increased physical activity, and also in old age. lipofuscin, is deposited . Its granules are a product of impaired metabolism in cardiac muscle cells, weakened by insufficient nutrition and blood supply.
At the beginning of the era of surgical treatment of coronary heart disease, the main indication for reconstruction of the left ventricle was an aneurysm of its wall. Later, the concepts of “ischemic cardiomyopathy” and “ischemic congestive heart failure” appeared, in which reconstruction of the left ventricle (LV) began to be performed. Traditionally, assessment of the size and topography of the LV aneurysm has been performed using radiocontrast ventriculography. Then they began to use radioisotope, ultrasound methods, magnetic resonance and computed tomography.
In 2009, the results of the STICH study were published [1], which contradicted such tactics. Among other conclusions of this work was the following: “We agree that for some patients, LV reconstruction can significantly improve their functional status and indicators of intracardiac hemodynamics, but the results of our study do not allow us to define this group of patients.”
In general, the opponents' claims to the STICH study can be summarized in the words of G. Buckberg, S. Athanasuleas: “A mathematical model based on an incorrect clinical data base leads to errors in clinical conclusions” [2]. The opponents' claims regarding the choice of criteria for LV reconstruction in patients with post-infarction heart failure were the following: 1) undifferentiated selection of patients for such a study; 2) inadequate choice of methods for assessing viable myocardium and LV dimensions. Even when planning the STICH study, G. Buckberg [3] called for special attention to be paid to the assessment of viable myocardium in this work. J. Ghali et al. [4] note the lack of information about large, dyskinetic aneurysms [4]. M. Antunes et al. [5] emphasized that the 5-year survival rate in patients with large aneurysms without surgical reconstruction of the LV ranges from 47 to 70%. S. Athanasuleas et al. [8] note that when performing surgical reconstruction of the LV, there is no data on viability confirming regional myocardial necrosis, and 13% of patients did not have a history of myocardial infarction. Only in 50% of cases are akinesia or dyskinesia noted. According to S. Athanasuleas et al. [8], credible studies advocate the use of end-systolic volume assessment using magnetic resonance imaging (MRI). However, in the STICH study [7], these measurements were performed using echocardiography (EchoCG) and only in 38% of patients. Patients in the STICH trial who underwent surgical LV reconstruction in addition to coronary artery bypass grafting (CABG) experienced a 19% reduction in indexed end-systolic volume, compared with a 40% reduction in the same index in most studies in which surgical reconstruction was performed [8–10]. ]. S. Athanasuleas et al. [8–10] note that the results of the examination of patients undergoing surgical LV reconstruction in the STICH study are not comparable with the results of an international database consisting of 5000 patients in whom regional myocardial necrosis was 35% or more, indexed to final body surface area LV systolic volume was at least 60 ml/m2, and LV ejection fraction (EF) was 35% or less.
The STICH protocol itself, writes S. Athanasuleas, does not comply with the methodological recommendations for such studies, primarily due to improper selection of patients. LV function and the course of chronic heart failure improve when LV systolic volume can be significantly reduced as a result of cardiac surgery, so reliable studies of surgical ventricular reconstruction necessitate the selection of patients with necrosis of at least 35% of the anterior wall, verified by tomoscintigraphy, with an index final systolic volume equal to at least 60 ml/m2 and its reduction as a result of surgery by 30%. [7]. It is the increase in LV volume, and not the decrease in EF, that becomes an important prognostic factor for mortality [11]. LV reconstruction removes the scar, which causes overstretching of the contralateral compensatory functioning muscle [12], and allows the size of the LV to be restored. The essence of the problem of congestive heart failure of ischemic nature lies in the sharply increased oxygen demand of the myocardium, which remains viable in the LV [13]. If fibrosis occupies 20–25% of the LV surface, the degree of shortening required from the remaining functioning myocardium exceeds the physiological limit [14]. Thus, determining the size of the area of cardiosclerosis would be an integral part of the STICH study, especially if the surgeon decided to perform ventricular reconstruction.
After revascularization, a decrease in the size of the LV cavity occurs due to zones of the myocardium, including subendocardial changes up to half the thickness of the ventricular wall. Deeper subendocardial damage does not always lead to real restoration of ventricular wall function, especially if we are talking about transmural damage with a zone of continuous necrosis [15]. During ventriculotomy in patients with heart failure, the decisive prognostic significance of assessing the degree and size of cardiac fibrosis was noted back in 1998 [16], and later confirmed in an experiment [17].
In response to critics, the STICH authors write that the study used MRI in some patients, ECG-synchronized tomoscintigraphy in others, and echocardiography in all other patients [18]. In patients who underwent more than one study, the results were comparable. The authors refer to modern publications that support the use of ultrasound to assess the geometry and function of the LV before and after its reconstruction. M. Donato et al. [19] also used echocardiography to assess LV volumes and LVEF before and after reconstruction.
However, assessment of the size of the scar and the actual degree of scar changes in the wall is not available even when performing echocardiography with a dobutamine test. Only myocardial scintigraphy and MRI can differentiate the “contents” of the LV wall. If MRI allows you to most accurately assess the topography and size of the zone of cardiosclerosis, then scintigraphy makes it possible to assess the distribution of perfusion and the amount of myocardium remaining viable. Since these two methods still evaluate “two sides” of “the same coin,” there may be real differences in the interpretation of the results obtained.
We decided to compare the initial conclusions of imaging methods about the presence of anterior LV aneurysm with the type of cardiac surgery performed during the period from 2010 to 2011 in the center named after. acad. IN AND. Shumakova. The results of the study were isolated only from those patients (more than 200 in total) for whom perfusion tomoscintigraphy synchronized with ECG (PTS with ECG) was performed not only before, but also after surgery. All these patients underwent simultaneous echocardiography, and many underwent radiocontrast ventriculography and myocardial MRI with the introduction of a contrast agent. The results were divided into groups depending on the size of the transmural scar, calculated from the results of PTS with ECG. The following groups were formed:
Group 1 - patients whose transmural scar size is ≥30% of the myocardium (Table 1).
All patients in this group underwent aneurysm resection in combination with CABG;
Group 2 - patients with transmural scar size ≤30% (Table 2).
All patients in this group underwent either aneurysmography or (2 cases) resection of apical aneurysms in combination with CABG;
Group 3 - patients who underwent CABG only (Table 3).
The tables show that the most complete agreement between the conclusions of various methods about the presence of an aneurysm of the anterior wall of the LV is observed in patients with a transmural scar size that occupies more than 30% of the area of the entire LV.
As the size of the scar decreases and the living myocardium is included in the scar wall (fibromuscular changes), the frequency of agreement between the conclusions of different methods about the presence of an aneurysm decreases. The differences in conclusions in the group of patients who underwent only CABG without any LV reconstruction become maximum.
Here is an example of such a difference.
Patient V., 65 years old. Date of admission: 01/17/11.
Clinical diagnosis: IHD. Angina pectoris. III FC. Post-infarction cardiosclerosis (1995, 2005). Chronic fibromuscular aneurysm of the left ventricle. Stage III hypertension. Circulatory failure of the 1st degree. Cerebrovascular disease. Discirculatory encephalopathy of the 1st-2nd degree. Anxiety-depressive syndrome. Autoimmune thyroiditis, subclinical hypothyroidism. Chronic gastritis.
Complaints upon admission: discomfort behind the sternum, pain in the left shoulder, a feeling of a lump in the throat with slight physical and psycho-emotional stress, relieved when the load is stopped, an increase in blood pressure up to 200/110 mm Hg.
History of the disease: for many years (since the age of 35) he has suffered from arterial hypertension up to 200/110 mm Hg. Antihypertensive therapy is symptomatic. In 1995, she suffered an acute myocardial infarction of the posterior wall, in 2005, a myocardial infarction of the anterior wall, after which the above complaints appeared and began to progress. In December 2010, she was examined with a diagnosis of unstable angina, coronary angiography was performed, which revealed multivessel stenotic lesions of the coronary arteries, and surgical treatment was recommended.
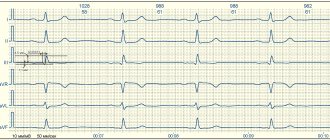
ECG: sinus rhythm, heart rate 56 beats/min (Fig. 1).
Figure 1. Electrocardiogram of patient B. Scar changes in the anteroseptal region with transition to the apex. Reduced blood supply to the anteroseptal and apical region of the left ventricle.
EchoCG: aorta 2.7 cm, left atrium 4.0 cm, right ventricle 2.5 cm, interventricular septum 1.4 cm, LV posterior wall thickness 1.25 cm, LV end-diastolic dimension 5.0 cm, end-systolic dimension 2.7 cm, stroke volume 88 ml, end-diastolic volume 115 ml, end-systolic volume 27 ml, EF 63%. Akinesia of the lower third of the interventricular septum and apex is noted. The presence of a thrombus in the apical region cannot be excluded. The aortic and mitral valves are atherosclerotically sealed. The average estimated pulmonary artery pressure is 28 mm Hg.
Coronary angiography: right type of coronary blood supply. Diffuse changes are noted in the form of uneven contours and tortuosity of the distal channel. The trunk of the left coronary artery has stenosis of the 2nd degree in the distal third. The anterior interventricular artery is occluded in the proximal third with retrograde filling along the interarterial anastomoses. The circumflex branch is represented by a large branch with a blunt edge - stenosis of the 2nd-3rd degree at the mouth. Right coronary artery - 3rd degree stenosis at the mouth, critical stenosis in the middle third, 3rd degree stenosis in the distal third.
Myocardial perfusion tomoscintigraphy: the myocardium of a non-enlarged LV is visualized with a deep point reduction in perfusion at the apex with moderate hypoperfusion spreading to the anterior wall. Impaired diastolic function. Akinesia with phenomena of paradoxical pulsation in the area of decreased perfusion. LVEF 46%. Signs of subendocardial changes and myocardial ischemia in the anterior apical region with a maximum at the apex.
MRI of the heart - signs of LV fibromuscular aneurysm of anteroseptal-apical localization. No thrombi were detected in the LV cavity. EF 50%, end-diastolic volume 109 ml, end-systolic volume 54 ml, stroke volume 55 ml.
MRI images show a large area of whitish inclusion of the indicator in the area of the anterior septal wall—the zone of cardiosclerosis (see Fig. 2, a and further in the color plate).
Figure 2. Images of the left ventricular myocardium obtained from patient V. A - magnetic resonance imaging; B, C - perfusion tomoscintigram synchronized with electrocardiography (B - before surgery, C - after surgery). The sections are presented in identical projections. Zones of cardiosclerosis and viable myocardium are indicated by arrows. Below is a scale for assessing myocardial viability based on perfusion scintigrams. In the same place, ECG sections of the PTS show a decrease in perfusion, but the remaining myocardium is sufficient to consider this wall viable (Fig. 2, b). This conclusion is confirmed by images of the same sections after revascularization (Fig. 2, c).
On January 24, 2011, the patient underwent surgery: mammarocoronary anastomosis with the anterior interventricular branch with endarterectomy, autovenous CABG of the right coronary artery and obtuse edge branches, Y-shaped bypass of the diagonal branch under conditions of cardiopulmonary bypass and pharmacocold cardioplegia. The postoperative period proceeded smoothly, without complications. On 02/01/11 the patient was discharged in satisfactory condition.
A quantitative assessment of the changes observed after surgery is presented in Fig. 3.
Figure 3. Quantitative assessment of the dynamics of myocardial perfusion after its revascularization in patient V. A - before vascularization; B — after revascularization. Presents midline slices in three standard planes, a standard three-dimensional map of perfusion of the left ventricular myocardium ("bull's eye") with quantitative assessment of 17 segments and a three-dimensional image of the ventricle. After revascularization, a clear decrease in the depth and extent of perfusion disturbances in the anterior wall of the LV, accompanied by an increase in LVEF, was noted.
Therefore, the diagnostician’s answer always depends on how the clinician’s question is formulated. However, the question can be formulated in different ways. The following example illustrates the discrepancy between the question and the results.
Clinical case 2.
Patient K., 52 years old, was treated from 02.02.10 to 01.03.10.
Diagnosis on admission: IHD. Unstable angina. Post-infarction cardiosclerosis (2005). Fibromuscular aneurysm of the left ventricle. Stage III hypertension. Chronical bronchitis. Chronic gastritis.
Complaints upon admission: burning, pressing pain behind the sternum with irradiation to the left shoulder and left half of the chest, discomfort in the heart, attacks of suffocation during physical exertion, palpitations that pass at rest, increased blood pressure to 220/140 mm Hg.
History of the disease: from the age of 40 - arterial hypertension up to 220/140 mm Hg. at working blood pressure 150/90 mm Hg. Since 2004, attacks of angina pectoris appeared during physical activity. She was repeatedly hospitalized in the hospital. Acute myocardial infarction in 2005. In September 2009, according to the examination, a LV aneurysm was detected, EF 45%. He constantly takes Accupro, hypothiazide, Concor, ThromboAss. Outpatient and inpatient treatment without effect. Entered the Federal Scientific Center for Technical and Instructional Science named after. acad. IN AND. Shumakov to conduct coronary angiography and decide on further treatment tactics.
ECG: sinus bradycardia, heart rate 55 beats/min. Subendocardial focal changes in the myocardium in the posterior and anterior apical region with signs of an aneurysm.
EchoCG: aorta 2.6 cm, left atrium 5.1 cm, right ventricle 2.8 cm, interventricular septum 1.8 cm, posterior wall thickness of the left ventricle 1.4 cm, end-diastolic volume 228 ml, end-systolic volume 130 ml , stroke volume 98 ml, EF 44%. Diastolic function is impaired according to the first type. Akinesia of the anterior apical and anteroseptal segments, hypokinesia of the lower third of the interventricular septum, posterobasal segments. The presence of a parietal apical thrombus cannot be excluded. The valve apparatus is without any special features. There is no fluid in the pericardial cavity. The average estimated pulmonary artery pressure is 27 mmHg.
Coronary angiography: right type of coronary blood supply. Diffuse changes are noted in the form of uneven contours, tortuosity of the distal channel, and slower passage of the contrast agent. Trunk of the left coronary artery: no stenotic lesion was detected. Anterior interventricular artery: extensive stenosis of the 2nd degree in the proximal third. Diagonal branch: 3rd degree stenosis at the orifice. The circumflex branch has 3rd degree stenosis in the proximal third, 2nd degree stenosis in the middle third. Right coronary artery: 3rd degree stenosis in the proximal third.
Myocardial perfusion tomoscintigraphy synchronized with ECG (Fig. 4, a):
Figure 4. Study of left ventricular myocardial perfusion in patient K. A - before surgery; B — after plication of a left ventricular aneurysm. Arrows indicate a perfusion defect in the apex of the left ventricle. the myocardium of a non-enlarged LV is clearly visualized (end-diastolic volume 180 ml) with a moderate decrease in perfusion along the posterolateral wall of the LV. The level of perfusion reduction is moderate (less than 50%). Perfusion of the anterior wall is satisfactory, moderate decrease in perfusion of the apical-septal segment. Hypokinesia and decreased systolic thickening in the anteroseptal region are noted. We can assume a moderate focal change along the posterolateral wall and apical parts of the interventricular septum. The entire myocardium is potentially viable. There was no evidence of LV aneurysm.
Cardiac MRI: LV myocardial hypertrophy. There was no data confirming LV aneurysm and thrombus in the LV cavity.
X-ray of the chest organs: pulmonary fields without pathological shadows. The roots are expanded, structural. Sinuses are free. The diaphragm is movable. The heart is enlarged to the left.
On 02/12/10 the patient underwent surgery: plication of the LV aneurysm, mammarocoronary anastomosis with the anterior interventricular artery, CABG of the right coronary artery, obtuse edge branch and diagonal branch under artificial circulation. The postoperative period proceeded quite smoothly. Postoperative sutures without signs of inflammation, healed by primary intention, consolidation of the sternum was satisfactory. In relatively satisfactory condition, the patient was discharged under the supervision of a cardiologist at her place of residence.
It would seem that both methods, capable of differentiating the contents of the LV wall, said “no” to LV aneurysm. Moreover, MRI results excluded the presence of a thrombus inside the ventricle. But the surgeons saw the “retraction” of the wall and performed its plication.
What has changed in the images of the myocardium? It can be seen that initially the myocardium is absolutely preserved (see Fig. 4, a). After plication of intact myocardium, perfusion at the apex worsens, and a perfusion defect occurs (indicated by an arrow) (see Fig. 4, b).
Case 2 illustrates another point made by STICH opponent G. Buckberg [20]: “The surgeon cannot know when to perform reconstruction without accurate information about viability and volume.”
Of the 1212 patients in the STICH study, myocardial viability was assessed in only 774 patients [21]. The main method of assessing viability was echocardiography with dobutamine. However, this method does not allow determining the size of the transmural scar. In 1996, R. Medrano et al. [22], studying hearts explanted during transplantation, compared the results of preoperative echocardiography and tomoscintigraphy to assess the size and thickness of scar zones. It turned out that 40% (!) of the zones of akinesis and dyskinesis contained unchanged myocardium. In this light, the selection criterion for myocardial resection used by the STICH authors: “...patients who had akinesis or dyskinesis of the anterior wall of the left ventricle were considered as requiring ventricular reconstruction...” looks more than doubtful. Tomoscintigraphy, on the contrary, made it possible to accurately diagnose and assess the size of lesions of cardiosclerosis, which was confirmed by microscopy results [22]. It must be recalled that even after complete revascularization, not all of the myocardium immediately restores its function. Sometimes this takes months and even years [23]. In a study [24], which compared the assessment of cardiosclerosis using dobutamine echocardiography and perfusion scintigraphy, it was shown that contractile reserve may be impaired in the absence of significant cardiosclerosis, whereas with significant cardiosclerosis, wall thickening may persist. Therefore, there is a high possibility that the STICH results could be confounded, for example by cases of viable myocardial resection.
When formulating the diagnosis of patients who underwent LV reconstruction, the STICH authors use the term “ischemic congestive heart failure.” But this diagnosis is clinical and cannot be an indication for LV reconstruction. In modern guidelines for cardiac surgery, the concept of left ventricular aneurysm has also begun to be replaced by “post-infarction remodeling” [25]. To a greater extent, instrumental “post-infarction remodeling” is a very broad concept and also cannot be an indication for LV reconstruction. Along with the disappearance of the concept of “LV aneurysm,” a clear rationale for indications for LV reconstruction based on the volume and depth of myocardial damage also disappeared. As a result, any bulging of the wall becomes a reason for reconstruction of the ventricle. Why then talk about viability?
The simplification of the concept of viable myocardium has been facilitated by the emergence of many methods that visualize the myocardium, which requires literacy on the part of clinicians making decisions about treatment tactics [25]. MRI itself, despite the highest image resolution, visualizes the myocardial wall without differentiating its contents. With the additional use of a contrast agent (gadolinium), which is included in areas of formed cardiosclerosis, it becomes possible to visualize scars. In intact cardiomyocytes, gadolinium is not incorporated and therefore cannot reveal their metabolism. Radionuclide agents are involved in the metabolism of cardiomyocytes and, therefore, indicate the degree of safety, viability and level of myocardial perfusion. Of course, MRI with gadolinium can visualize such small foci of cardiosclerosis that are not visualized by scintigraphy. But what does this mean in practical terms? From a practical point of view, this means that there is incomparably more “living” myocardium in this wall than areas of cardiosclerosis. Such myocardium must be revascularized, but in no case “reconstructed.” Therefore, while inferior in accuracy to determining zones of cardiosclerosis, scintigraphy may be more sensitive in assessing the myocardium that remains viable [26, 27].
What did the replacement of ideas about post-infarction aneurysm and simplifications in indications for reconstruction of the dysfunctional LV wall lead to? To the conclusion of the authors of STICH [1]: “we agree that in some patients, reconstruction of the left ventricle can significantly improve their functional status and indicators of intracardiac hemodynamics, but the results of our study do not allow us to determine this group of patients.” According to these conclusions, one can only “congratulate” cardiac surgeons on the fact that there are currently no indications for surgical reconstruction of the LV. An evidence-based medicine document shows that surgical reconstruction of the LV in patients with coronary artery disease is not effective.
Substitutions and simplifications led to paradoxical results and conclusions. Insufficient attention to instrumental criteria, in particular to the assessment of myocardial viability when planning LV reconstruction, and doubts about the reproducibility of STICH results do not inspire confidence in the results of even the most modern statistics. Accordingly, there is no confidence in the conclusions made by the STICH authors.
Dystrophic disorders
These changes in the myocardium of the left ventricle are provoked by a lack of nutrients, without which the heart muscle will not function normally. In medicine, this condition is referred to as cardiac dystrophy, and it appears due to the following factors:
- disruptions in the functioning of the kidneys and liver, because these organs are responsible for metabolic processes;
- diabetes;
- diseases of the endocrine system;
- shocks affecting the central nervous system;
- serious loads;
- low hemoglobin level;
- infectious pathologies of a chronic nature;
- intoxication;
- unhealthy diet, causing vitamin deficiency;
- frequent consumption of alcoholic beverages.
- long-term use of drugs.
- Features of the ECG during myocardial infarction - the procedure and signs of the disease
Often this condition is detected in adolescents during exams, when they are mentally overstrained. But in young children, changes in the myocardium are considered normal, and all because metabolic processes are not yet perfect. Also, older people may suffer from such changes, because metabolic processes in their bodies slow down.
Description of the pathology
Changes in the myocardium of the left ventricle can provoke various diseases or metabolic disorders in the heart muscle. Moderate cardiac dysfunction can be diffuse or focal. The first type is characterized by failure of the myocytes of the left ventricle, as a result of which they contract incorrectly. That is, the electrical impulse is carried out incorrectly through these cells.
The second type is focal changes. In this case, scars form on the wall of the left ventricle. They consist of connective tissue that is not capable of conducting electrical impulses.
Moderate metabolic disorders can return to normal on their own, but if such disruptions occur frequently, the myocardium cannot recover.
Pathogenesis
Changes in the ECG are not a disease, but only a manifestation of some pathological processes occurring in the myocardium. With shifts in the biochemical activity in the heart cells, their contractility changes, which is reflected in the cardiogram when recording the conduction of impulses. Cardiomyocyte function can be impaired during inflammatory processes, for example, with myocarditis . Taking certain medications also affects the functioning of the heart muscle.
Long-term diabetes mellitus can gradually lead to atherosclerosis . Not only large vessels are affected, but also the coronary arteries that supply the myocardium. With inflammatory pathology in the gastrointestinal tract, the absorption of nutrients is impaired, which also negatively affects metabolic processes in cardiomyocytes.
Symptoms
Moderate and minor changes in the ECG may not manifest themselves clinically and may be found on the cardiogram during routine medical examinations and medical examinations. With pronounced changes, special symptoms appear:
- chest pain of a pressing, burning nature, indicating an attack of angina pectoris ;
- swelling of the lower extremities, shortness of breath with minimal physical activity indicate the progression of heart failure ;
- a feeling of interruptions in the work of the heart, arrhythmias ;
- fatigue, pale skin;
- sticky sweat;
- tremors , weight loss in a short time.
Is there a danger with changes in the myocardium?
Moderate changes in the myocardium are a fairly common pathology that gradually affects healthy cells of the heart muscle. Doctors say that this leads to the development and progression of heart failure. Let's consider whether it is worth showing concern when diagnosing such a pathology, what it is: moderate disturbances in the myocardium.
Such changes may not always be dangerous. Especially if it does not manifest itself with pronounced symptoms. Often pathology is discovered during a regular medical examination. If a person does not feel any problems with the functioning of the heart, then most likely there is no need to show concern. The reason for a visit to a cardiologist should be:
- pain in the heart area;
- heart rhythm disturbances;
- dyspnea;
- high or low blood pressure;
- weakness, drowsiness.
Symptoms
Quite often, these changes are asymptomatic for several years, or appear slightly.
One of the most common signs of pathological changes in the heart muscle is angina pectoris. Because when the wall of the left ventricle thickens, compression of the vessels that feed the muscle occurs.
Atrial fibrillation and ventricular fibrillation can be the causes of the development of myocardial changes, as well as their consequence.
Another symptom of myocardial changes is “heart stopping.” In this case, the person feels that the heart does not beat for several seconds. As a result, he may lose consciousness.
Additionally, the following symptoms may occur:
- persistent increase in blood pressure, frequent changes;
- headache;
- pain in the heart area;
- weakness, fatigue;
- sleep disorders.
Diffuse changes
What are “diffuse-type changes in the left ventricular myocardium”? This type is the most common. In this case, not only the left ventricle is affected, but also the entire myocardium, since diffuse changes are characterized by uniform damage.
Diffuse disorders appear both in moderate pathological processes and in acute situations, such as myocardial infarction. In the latter case, there are changes in the structure of tissues and disruption of metabolic processes. Diffuse changes are an accumulation of myocytes in the left ventricle, which, under the influence of certain factors, have changed and do not conduct impulses.
With diffuse disorders of the left ventricular myocardium, swelling of the legs, tachycardia, and even fluid accumulation in the lungs are added to the general symptoms.
Diffuse changes in the myocardium of the left ventricle can provoke a deterioration in the circulatory process, myocardial hypoxia and the appearance of necrotic foci. The most dangerous consequence of these disorders is myocardial infarction.
Etiology
The reasons for the progression of myocardial dystrophy are quite varied. They are divided into factors that directly affect the functioning of the heart and causes that do not directly affect the organ (act through external factors).
The first group of reasons include:
- decreased oxygen uptake by the heart;
- increased levels of calcium in the ventricles of the heart;
- damage to the myocardium by fat cells;
- destruction of the organ structure by pathogenic bacteria;
- reduction of working cells in the heart due to the effects of other pathogenic processes.
The second group includes:
- the effect of hormones on the muscle layer of the heart;
- all kinds of acute poisoning of the body (drugs, alcoholic drinks, nicotine, medications);
- the effect of a large dose of radiation on the body;
- prolonged stress, depression, apathy;
- greater physical activity leads to the detection of such a disease in athletes;
- unhealthy diet, which mostly consists of very fatty and salty foods;
- abnormal functioning of the organs of the endocrine and digestive systems.
Left Ventricular Dysfunction and Treatment
Systolic dysfunction of the left ventricle is a decrease in its ability to eject blood into the aorta from its cavity. This is the most common cause of heart failure. Systolic dysfunction is usually caused by a decrease in contractility, leading to a decrease in stroke volume.
Diastolic dysfunction of the left ventricle is a decrease in its ability to pump blood into its cavity from the pulmonary artery system (in other words, to ensure diastolic filling). Diastolic dysfunction can lead to the development of pulmonary secondary venous and arterial hypertension, which manifests itself as:
- Cough;
- Dyspnea;
- Paroxysmal nocturnal dyspnea.