Effect on blood pressure
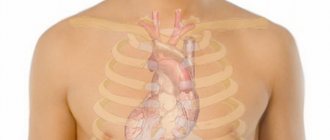
The active ingredient in Nitroglycerin is glycerol trinitrate. Its main purpose is to relieve an attack of angina and relieve pain accompanying colic and spasms. The drug is allowed to be used for diagnosed heart failure, in order to reduce the flow of venous blood to the myocardium.
An additional effect is the prevention of blood stagnation in the pulmonary circulation. When using the medicine, the myocardium relaxes, vascular spasms are relieved, which ultimately leads to a decrease in blood pressure. Therefore, if we talk about whether this drug increases or decreases blood pressure, then it reduces it.
Nitroglycerin can be taken - if really necessary - with low blood pressure, but it is important to carefully monitor the effect of the drug on the body and accompanying symptoms. If dizziness or nausea occurs, use should be stopped immediately.
Important! At what blood pressure should you not take Nitroglycerin? The product is prohibited for use at blood pressure of 100/60. Since the medicine reduces the indicators, it is possible that blood pressure will drop to a critical level.
When should nitroglycerin be used?
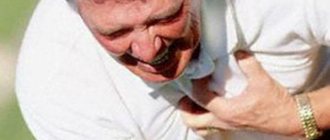
- During an attack of angina pectoris to relieve chest pain. If necessary, the drug can be taken again.
- To prevent a possible attack of angina before performing physical activity or emotional stress that causes chest pain.
- As a preventative measure, nitroglycerin can be taken before going uphill, 2-3 minutes before going outside, especially in frosty, windy weather.
- When severe shortness of breath appears (during an attack of cardiac asthma). In other cases, taking nitroglycerin is useless and even harmful.
Indications for use
Indications for the use of Nitroglycerin are not too numerous. The drug is used in the following cases:
- decrease in blood pressure;
- normalization of myocardial function;
- relief of an attack of angina of any strength;
- myocardial ischemia;
- swelling of lung tissue;
- hypertensive crisis;
- improving the patency of the gastrointestinal tract (gastrointestinal tract) and biliary tract;
- acute pancreatitis;
- reduction of pressure during neurosurgical operations.
Self-administration of the medication is unacceptable. The attending physician must decide whether Nitroglycerin can be taken or not.
How often can nitroglycerin be used?
Sometimes patients do not take nitroglycerin during an angina attack and prefer to endure the pain. This is wrong behavior. If an attack of angina pectoris does not go away at rest, but increases, it is necessary to urgently stop the attack. Prolonged pain is more difficult to stop. In addition, pain is only a symptom; it can hide serious complications.
During a severe attack, when the pain increases, some patients take up to 60 tablets of this drug per day. This is an acceptable norm, but unreasonable use of nitroglycerin should be avoided.
Scientists at Stanford University, in a study of nitroglycerin, concluded that its continuous use increases the severity of heart attacks.
Experts conducted experiments on mice. It turned out that a course of therapy for 16 hours almost doubles the consequences of a heart attack. Nitroglycerin, an overdose of this drug, leads to the fact that the enzyme that causes vasodilation and which is destroyed when taking the drug does not have time to be restored.
News on the topic By 2030, almost 8 million Ukrainians will die from cardiovascular diseases
Mode of application
There are specific instructions for using Nitroglycerin for high blood pressure. In order for the drug to provide the expected effect, the following rules must be observed:
- capsule - open it and place it under the tongue;
- tablet - place half under the tongue and wait for it to dissolve on its own;
- alcohol solution - pour 2 drops onto a piece of sugar or drop the same volume under the tongue;
- spray - spray the dose under the tongue.
Severe migraines are one of the possible reactions to starting to take the drug, caused by a sharp dilation of blood vessels
Before using the medicine, you need to sit down/lie down, as the medicine can cause severe dizziness. The permissible single dosage of tablets and capsules is 1 (for a single dose) and 5 (for a daily dose). Alcohol solution – 4 drops (one-time) and 16 drops (daily). After taking the medication, it is necessary to measure blood pressure every 15 minutes.
If its level remains unchanged, you can take another tablet/capsule or take 2 more drops. But usually the effect of Nitroglycerin begins one to two minutes after administration and lasts about half an hour. The maximum duration of use is a month. Subsequently, addiction develops, and the effectiveness of treatment decreases significantly.
Reception features
Nitroglycerin should be taken in a sitting or lying position, especially for older people, since the development of dizziness and fainting is possible. If the therapeutic effect does not occur, then after two minutes you must take the next dose. The intervals between them are 5 minutes. The total number of receptions is 3 times. The dosage at the first dose should not exceed ½ tablet.
This will allow you to assess the tolerability of the drug. For older people, the dosage is also ½ tablet. The recommendation is due to the possibility of developing fainting conditions. If nitroglycerin drops are used, it is best to apply a single dose (2 drops) to a piece of sugar and dissolve. If this is not possible, then drip the same amount of product under the tongue.
How to reduce blood pressure using folk remedies?
And you also need to remember the following recommendations:
- After the first dose of the drug, a person may develop migraines. The reason is the rapid expansion of the lumens of blood vessels and a drop in blood pressure levels. After several days of use, the headaches weaken and disappear completely. This is a normal reaction of the body to the drug, it continues to work actively, the body has simply adapted and does not react so painfully. The recommended dosage cannot be increased. In this case, getting rid of migraines will be very problematic.
- 5 hours after taking the dose, it is possible that pain in the heart area may develop. The reason is the removal of the drug from the myocardium. The muscle begins to work in the same mode, which provokes painful sensations. To stabilize your health, you need to take the next dose.
- If after taking the capsule the pain does not stop within the next 15 minutes, you can put another capsule under the tongue.
The red capsules contain an oil solution of nitroglycerin. After two months of regular use of the drug, the body begins to get used to it, so it becomes ineffective. To avoid this, you need to take a break after taking it for a month.
To prevent addiction to Nitroglycerin, doctors recommend taking any form of the drug no earlier than after 5 hours. This will allow the active substances to completely leave the blood, and the body will receive a “new” dose each time.
If you have low blood pressure, use the medicine with extreme caution. If you exceed the dosage, you can get severe migraines, dizziness and fainting. If you have glaucoma, use Nitroglycerin only after consultation with your doctor.
In rare cases, vision deterioration occurs during treatment. An increase in intraocular pressure is possible. In both cases, therapy must be stopped immediately. It is important to remember that a physician should select the dosage, taking into account the current clinical picture. An overdose can cause severe nausea, paleness of the skin and mucous membranes, and loss of consciousness.
When should you not take nitroglycerin?
The use of the drug in any pharmaceutical forms should be abandoned if the patient:
- Individual intolerance to the drug
- Circulatory disorders (vascular or cardiogenic collapse)
- Hypotension
- Intracranial hypertension
- Toxic pulmonary edema
- Severe anemia
- Acute myocardial infarction
- Angle-closure glaucoma
- If you are taking sildenafil
- Alcohol intoxication
In the latter case, you should know that you should not drink alcohol for at least 3 hours after taking nitroglycerin. Since it is impossible to understand whether an attack of pain is caused by angina pectoris or another pathology. And, in addition, a sharp drop in blood pressure, which can be caused by nitroglycerin, against the background of intoxication, is fraught with various consequences, including those requiring emergency medical intervention. The correct thing to do in this case would be to send the patient to the hospital by ambulance.
Links[edit]
- ^ ab "Occupational Safety and Health Guidelines for Nitroglycerin". Archived from the original on May 16, 2013. Retrieved October 19, 2021.
- NIOSH Pocket Guide to Chemical Hazards. "#0456" . National Institute of Occupational Safety and Health (NIOSH).
- "NFPA Fire Diamond Hazard Rating Information". Archived from the original on February 17, 2015.
- ^ ab Chen, Z.; Foster, MW; Zhang, J.; Mao, L.; Rockman, H. A.; Kawamoto, T.; Kitagawa, K.; Nakayama, Kentucky; Hess, D.T.; Stamler, J. S. (2005). "An important role of mitochondrial aldehyde dehydrogenase in the bioactivation of nitroglycerin". Proceedings of the National Academy of Sciences
.
102
(34):12159–12164. Bibcode: 2005PNAS..10212159C. DOI: 10.1073/pnas.0503723102. PMC 1189320. PMID 16103363. - "Unknown, behind paywall, archived". Archived from the original on May 10, 2021. Retrieved April 14, 2021.
- Sobrero, Ascan (1847). "Sur plusieur composés détonants produces avec l'acide nitrique et le sucre, la dextrine, la lactine, la mannite et la glycérine" [On certain detonating compounds produced with nitric acid and sugar, dextrin, lactose, mannitol and glycerin]. Comptes Rendus
.
24
: 247–248. - Sobrero, Ascanio (1849). "Sopra alcuni nuovi composti fulminanti ottenuti col mezzo dell'azione dell'acido nitrico sulle sostante organiche Vegetali" [On some new explosives obtained by the action of nitric acid on certain plant organic substances]. Memorie della Reale Accademia delle Scienze di Torino (2nd series)
.
10
: 195–201. On page 197, Sobrero calls nitroglycerin pyroglycerin: “Quelle gocciole costituiscono il corpo nuovo di cui descriverò ora le proprietà, e che chiamerò
Piroglicerina
.” (These drops constitute a new substance, the properties of which I will now describe and which I will call “pyroglycerin.”) - "Emil Nobel". NobelPrize.org
. Archived from the original on January 15, 2009. Retrieved October 6, 2008. - "Krümmel" NobelPrize.org
. Archived from the original on July 10, 2006. - "Transcontinental Railroad - People and Events: Nitroglycerin". American experience
. PBS. - ↑
North Wales Daily Post 14 October 2021. - Murrell, William (1879). "Nitroglycerin as a remedy for angina pectoris". Lancet
.
1
: 80–81, 113–115, 151–152, 225–227. DOI: 10.1016/s0140-6736(02)46032-1. - ^ ab Sneader, Walter (2005). Drug Discovery: A History
. John Wiley and Sons. ISBN 978-0-471-89980-8. - "History of TNG". yonddiscovery.org
. Archived from the original on November 1, 2010. Retrieved April 14, 2021. - "Tales of Destruction - Melting Can Be Hell".
- "Tales of Destruction - Nitroglycerin in it?" .
- ^ ab "Nitroglycerin". Encyclopedia Britannica
. Source +23 March 2005. - "Zusammensetzung der Zuckerasche" [Composition of sugar ash]. Annalen der Chemie und Pharmacie
.
64
(3):398–399. 1848. DOI: 10.1002/jlac.18480640364. - "Uber nitroglycerin." Annalen der Chemie und Pharmacie
.
92
(3):305–306. 1854. DOI: 10.1002/jlac.18540920309. - Jump up
↑ Di Carlo, F. J. (1975).
"Nitroglycerin Revisited: Chemistry, Biochemistry, Interactions." Drug Metabolism Reviews
.
4
(1): 1–38. DOI: 10.3109/03602537508993747. PMID 812687. - ↑
Bellis, Mary.
"Alfred Nobel and the History of Dynamite". About.com Money
. - Miller, J. S.; Johansen, R. T. (1976). "Breaking Oil Shale with Explosives for In-Situ Mining" (PDF). Shale oil, tar sand and related fuel sources
: 151. Retrieved March 27, 2015. - "Nitroglycerin".
- ^ abc Ogbru, Omudhom. "Nitroglycerin, Nitro-Bid: Drug Facts, Side Effects and Dosage". MedicineNet
. - Kaplan, K. J.; Taber, M.; Teegarden, Jr.; Parker, M.; Davison, R. (1985). "Association of methemoglobinemia and intravenous nitroglycerin." American Journal of Cardiology
.
55
(1): 181–183. DOI: 10.1016/0002-9149 (85) 90324-8. PMID 3917597. - "IntraMed - Bienvenido". www.intramed.net
. Retrieved April 14, 2021. - ↑
"Nitroglycerin for Angina, February 1997, Volume 7". Archived from the original on May 10, 2021. Retrieved November 9, 2009. - Smith, E.; Hart, F. D. (1971). "William Murrell, physician and general practitioner". British Medical Journal
.
3
(5775): 632–633. DOI: 10.1136/bmj.3.5775.632. PMC 1798737. PMID 4998847. - Amdur, Mary O.; Dull, John (1991). Casarette and Dull's Toxicology
(4th ed.). Elsevier. ISBN 978-0071052399. - Sullivan, John B., Jr.; Krieger, Gary R. (2001). Clinical environmental status and toxic effects: latex. Lippincott Williams and Wilkins. p. 264. ISBN 978-0-683-08027-8. Retrieved April 23, 2013.
- Marsh, N.; Marsh, A. (2000). "A Brief History of Nitroglycerin and Nitric Oxide in Pharmacology and Physiology". Clinical and experimental pharmacology and physiology
.
27
(4): 313–319. DOI: 10.1046/j.1440-1681.2000.03240.x. PMID 10779131. - Life Sciences Assembly (USA) Toxicology Advisory Center. Toxicology reports. National Academies. paragraph 115. NAP: 11288. Retrieved April 23, 2013.
- "Nitroglycerin". NIOSH Pocket Guide to Chemical Hazards
. CDC. Retrieved November 21, 2015.
First aid and treatment for poisoning
An overdose of nitroglycerin requires emergency assistance. It will be useful for everyone to know how to help a person who has suffered from intoxication of the body.
First aid for overdose:
- The patient is placed on a flat surface and his legs are raised to an elevated position.
- Open the windows or take him outside so that the victim can breathe fresh air.
- Considering the patient’s condition, the stomach is washed with warm water.
- Blood pressure and pulse are measured.
- After washing, the victim will benefit from a viscous drink - jelly. Acetylsalicylic acid will help cope with elevated body temperature. Activated carbon and validol are used for nausea.
The legs are elevated to improve blood circulation to the heart and brain, since reduced pressure leads to a collapsed state.
Treatment at home is impossible, the patient is unconditionally hospitalized for intensive care. With the help of special medications, the patient’s vascular tone is increased and the volume of blood supply is increased.
Medical help is needed if:
- A pregnant woman or a small child was injured;
- The patient lost consciousness;
- The skin, nails and palms turn blue;
- Blood during vomiting or bowel movements;
- Blood pressure drops sharply;
- Tachycardia, palpitations;
Deadly danger of the drug
Gastrointestinal bleeding: first aid, causes, symptoms, signs, treatment, consequences
Nitroglycerin taken in large quantities can be fatal
Exodus. Therefore, you must strictly adhere to the doctor’s recommendations.
But so far there is no recorded fatal amount of medication, since the reaction is individual for everyone: someone died after a single pill taken at the wrong time, and for some even a pack of nitroglycerin cannot be affected.
For sure, one thing can be said - alcohol and drugs are the only combination that can cause death. This applies to every pharmacological agent, since some medications lose their healing power under the influence of strong drinks, while others pose a threat to the life of any patient.
We usually associate the name of this drug with heart disease. Many people know that a nitroglycerin tablet should be placed under the tongue when the “motor” begins to tingle. However, heart disease is not the only area where nitroglycerin is used. People who use the drug sometimes have a question: what will happen if a person drank alcohol, and then he needed to take nitroglycerin? What could be the consequences? Let's find out in detail.
Physicochemical characteristics
A transparent, viscous, non-volatile liquid (like oil), prone to hypothermia. Miscible with organic solvents, almost insoluble in water (0.13% at 20 °C, 0.2% at 50 °C, 0.35% at 80 °C). When heated with water to 80 °C, it hydrolyzes. Quickly decomposes with alkalis.
Very sensitive to impact, friction, high temperatures, sudden heating, etc. Impact sensitivity for a load of 2 kg - 4 cm (mercury fulminate - 2 cm, TNT - 100 cm). Very dangerous to handle. When ignited carefully in small quantities, it burns unsteadily with a blue flame. Crystallization temperature - 13.5 °C (stable modification, labile modification crystallizes at 2.8 °C). Crystallizes with a significant increase in sensitivity to friction. When heated to 50 °C, it begins to slowly decompose and becomes even more explosive. Flash point is about 200 °C. The heat of explosion is 6.535 MJ/kg. The explosion temperature is 4110 °C. Despite the high sensitivity, the susceptibility to detonation is quite low - a detonator capsule No. 8 is required for a complete explosion. Detonation speed is 7650 m/s. 8000-8200 m/s - in a steel pipe with a diameter of 35 mm, initiated using detonator No. 8. Under normal conditions, liquid NGC often detonates in a low-speed mode of 1100-2000 m/s. Density - 1.595 g/cm³, in solid form - 1.735 g/cm³. Solid nitroglycerin is less sensitive to impact, but more sensitive to friction, and therefore very dangerous. The volume of explosion products is 715 l/kg. High explosiveness and brisance strongly depend on the method of initiation; when using a weak detonator, the power is relatively low. High explosiveness in sand - 390 ml, in water - 590 ml (crystalline is slightly higher), operability (high explosiveness) in a lead bomb - 550 cm³. It is used as a component of some liquid explosives, dynamites and mainly smokeless powders (for the plasticization of cellulose nitrates). In addition, it is used in medicine in small concentrations.
Receipt
In the laboratory it is obtained by esterification of glycerin with a mixture of concentrated nitric and sulfuric acids. Acids and glycerin must be free of impurities. To ensure process safety and good glycerol yield, the acid mixture must have a low water content. The process begins by mixing oleum (or laboratory 98% sulfuric acid) and melange. Mixing of acids is carried out while cooling to prevent thermal decomposition of concentrated nitric acid. Glycerin is added from a dropping funnel with vigorous stirring and constant cooling of the flask with ice (with the addition of table salt). Temperature control is carried out with a mercury or electronic thermometer. The process of mixing acids can be expressed in a simplified form by the following reaction:
The reaction is equilibrium with a strong shift of equilibrium to the left. Sulfuric acid is necessary for binding water into strong solvates and for protonating nitric acid molecules to form nitrosonium cations NO2+. The positive charge is delocalized over all electron orbitals of the cation, which ensures its stability.
Then the reaction mixture of acids and glycerin is kept for a short time under ice cooling. The liquid separates into two layers. Nitroglycerin is lighter than the nitrating mixture and floats as a cloudy layer. The esterification process is carried out at temperatures around 0˚C. At lower temperatures the speed of the process is low; at higher temperatures the process becomes dangerous and the product yield sharply decreases. Exceeding the temperature above 25 ° C threatens an explosion, so the synthesis must be carried out under strict temperature control. The equation for the esterification of glycerol with nitric acid in the presence of sulfuric acid can be simplified as follows:
The top layer from the reaction glass (flask) is immediately poured into a large volume of cold water with stirring. The water temperature should be 6–15 °C, the volume should be no less than 100–110 times the volume of the obtained NGC. Acids dissolve in water, and nitroglycerin settles to the bottom of the container in the form of cloudy beige drops. The water is drained and replaced with a new portion of cold water with the addition of a small amount of soda (1-3% by weight). The final washing is carried out with a small amount of soda solution until the aqueous phase reacts neutrally. To obtain the purest possible nitroglycerin (for example, for research purposes), a final purification is carried out by washing with water, which makes it possible to separate the remaining soda and sodium nitrate. The disadvantages of laboratory production of NGC are largely associated with the need to use a large volume of washing water, which sharply reduces the yield of the product due to irreversible losses of NGC due to solubility in water; in practice, these losses can reach 30-50% of the total product obtained. A large volume of rinsing water, on the contrary, allows you to rinse the NGC as quickly and safely as possible. Insufficient washing of NGC from acidic impurities and products of incomplete esterification leads to very low stability of products (gunpowder, TRT, BVV, etc.) and makes NGC extremely dangerous.
In industry, it is obtained by continuous nitration of glycerin with a nitrating mixture in special injectors. The resulting mixture is immediately separated in separators (mainly Biazzi systems). After washing, nitroglycerin is used in the form of an aqueous emulsion, which simplifies and makes its transportation between workshops easier and safer. Due to the possible risk of explosion, NGC is not stored, but is immediately processed into smokeless powder or explosives.
Most of the production premises of the enterprise producing NGC are occupied by workshops for the cleaning and processing of liquid waste and other production waste. The most promising technologies in this area are based on closed cycles of using circulating media (wash water, spent acid mixture, etc.).
Home conditions
Unfortunately, although, rather, fortunately, the synthesis of nitroglycerin at home is associated with too many difficulties, overcoming which is generally not worth the result.
The only possible method of synthesis at home is to obtain nitroglycerin from glycerin (as in the laboratory method). And here the main problem is sulfuric and nitric acids. The sale of these reagents is permitted only to certain legal entities and is strictly controlled by the state.
The obvious solution is to synthesize them yourself. Jules Verne in his novel “The Mysterious Island,” talking about the episode of the production of nitroglycerin by the main characters, omitted the final moment of the process, but described in great detail the process of obtaining sulfuric and nitric acids.
Those who are really interested can look into the book (the first part, chapter seventeen), however, there is a catch here - the uninhabited island was literally replete with the necessary reagents, so the heroes had at their disposal sulfur pyrites, algae, a lot of coal (for burning), potassium nitrate, and so on. Will the average addicted person have this? Hardly. Therefore, homemade nitroglycerin in the vast majority of cases remains just a dream.