Sofia Kasatskaya “Nature” No. 2, 2016
about the authorSofya Alekseevna Kasatskaya — Junior Researcher, Laboratory of Genomics of Adaptive Immunity, Institute of Bioorganic Chemistry named after. Academicians M. M. Shemyakin and Yu. A. Ovchinnikov RAS. Area of scientific interests: T-cell immunity, neuro- and oncoimmunology. Winner of the “Bio/mol/text” competition 2015 in the category “Best article on immunology.” |
The body’s adequate defense response to the invasion of viruses, bacteria and other pathogens is to destroy the affected cells, preventing the spread of infection and the death of a large number of its own cells. If a cell infected with a virus notices it, innate immune processes are launched: autophagy (utilization of internal cell components using lysosome enzymes) and apoptosis (programmed cell death). However, there are a lot of pathogenic viruses and bacteria, and they are constantly changing beyond recognition. To cope with them, the adaptive immune system and its main participants - lymphocytes - are activated. The pinnacle of the evolution of adaptive immunity was the cytotoxic T-lymphocyte, or killer T-lymphocyte. To recognize a fragment of the virus (antigen) on an infected cell, it uses the T cell receptor
, TCR), randomly and independently assembled on each T cell in the thymus gland. The mechanism of TCR assembly is unique and is characteristic only of the immune system of vertebrates. It is believed that these advantages were first obtained by primitive fish about 500 million years ago, when, as a result of a retroviral infection, genes encoding special proteins (recombinases) responsible for the recombination of TCR genes were introduced into their gametes.
Classical human immunology is based on the study of immune cells in the blood simply because a blood sample can be taken from any patient and examined in normal and pathological conditions. It is on blood cells that the classification of T-lymphocytes was built - division into T-killers and T-helpers, which check the antigenic specificity of T-killers, give them a “license to kill” and are able to control the entire course of the immune response through soluble signaling molecules and cytokines. Later, a group of regulatory T cells was isolated from the T-helper branch, suppressing excess adaptive immunity.
But as yogurt commercials remind us, a significant portion of the immune system's cells are concentrated around the lining of the digestive tract and in other tissues. While there are about 6–15 billion T-lymphocytes in 5–6 liters of blood in an adult, there are 20 billion T-cells in the epidermis and skin [], and another 4 billion in the liver []. Is studying blood samples enough to fully characterize T cell function if there are more T cells in peripheral organs than in the bloodstream? And are classical subpopulations sufficient to describe all the types of T cells found in the human body?
Killer T cells:
Killer T cells are the most famous subpopulation of lymphocytes. They have the ability to destroy defective cells of the body by coming into direct contact with them. They are also called cytotoxic lymphocytes: “cyto” in translation means “cell”, the meaning of the word “toxic” does not need to be explained.
Killer T cells, which strictly carry out immune surveillance, react aggressively to foreign proteins. They are the ones who cause graft rejection during organ transplantation. For this reason, when a person is transplanted with any organ, he is given special medications for some time that suppress the immune system: they reduce the increased content of lymphocytes and disrupt their interaction. Otherwise, any such operation would end in the rejection of a new organ or tissue, and perhaps even in the death of the patient undergoing such an intervention.
The mechanism of operation of these cells is interesting. Unlike phagocytes, which actively attack, devour and digest foreign particles, T-killers behave at first glance quite restrained. With their appendages they touch the object, and then break contact and “go about their business.” The cell that the lymphocyte touches dies after some time... Why?
The fact is that during their “kiss of death” T-killers leave particles of their membrane on the surface of the cell they destroy. At points of contact, the particles “corrode” the surface of the object of attack. As a result, a through hole is actually formed in a cell doomed to death. It loses potassium ions, sodium ions and water enter it - since the cell barrier is broken, its internal environment begins to communicate directly with the external one... As a result, the cell swells with water that has penetrated inside it, cytoplasmic proteins come out of it, organelles are destroyed... It dies, and then phagocytes approach it and devour its remains. This is the terrible punishment the body prepares for all cells that were recognized by the immune system as “wrong” or foreign.
The study includes the determination of absolute and relative values of the subpopulation composition of T-lymphocytes (CD3, CD4, CD8, CD45), the number of T-regulatory lymphocytes (T-reg. Cells), the ratio of T - helper/T - cytotoxic cells and T-cells, carrying activation markers CD38 and HLA-DR on their surface. It is recommended for use to monitor the dynamics of the cellular component of the immune system after a comprehensive immunological examination.
Synonyms Russian
Immunophenotyping, cellular immunity, multicolor cellular analysis by flow cytometry, T cells, T helper cells, T cytotoxic cells, T regulatory lymphocytes.
English synonyms
Human Immune System, Immunophenotyping, Multicolor Flow Cytometry Cell Analysis, Human Leukocyte Differentiation Antigens, Human T cells, T helper cells, Cytotoxic T cells, T-reg Cells, Activation markers.
Research method
Flow cytometry.
What biomaterial can be used for research?
Venous blood.
General information about the study
Assessment of the cellular composition (immunophenotyping) of human blood lymphocytes—the main component in assessing immune status—is performed by flow cytometry.
Immunophenotyping is the characterization of cells using monoclonal antibodies or any other probes that make it possible to judge their type and functional state by the presence of a particular set of cellular markers.
Immunophenotyping of leukocytes involves detecting differentiation markers, or CD antigens, on their surface. Leukocytes express a range of surface and cytoplasmic antigens unique to their subpopulation and developmental stage. CD antigens (cluster of differentiation antigens) are antigens on the surface of cells, markers that distinguish one cell type from another. The differentiations of these antigens have been studied and standardized, and they are assigned specific numbers. CDs can be recognized using appropriate monoclonal antibodies. Using fluorescently labeled monoclonal antibodies that bind to specific CDs, flow cytometry can be used to count the content of lymphocytes belonging to subpopulations of different functions or developmental stages.
Flow cytometry is based on photometric and fluorescent measurements of individual cells as they traverse a beam of monochromatic light, usually laser light, one after another along with a fluid stream.
C D 3
This marker allows you to identify mature resting (intact) T cells and count the total number of T lymphocytes. Quantitative assessment of the CD3+ lymphocyte subpopulation has diagnostic value in the following cases:
— primary and secondary immunodeficiencies;
— acute viral infections, including HIV;
- intracellular bacterial and parasitic infectious diseases (for example, tuberculosis, leprosy, leishmaniasis);
— malignant neoplasms;
— transplant rejection reactions and graft-versus-host disease;
— lymphoproliferative disorders (acute T-lymphoblastic leukemia).
In diabetes mellitus, a decrease in the percentage and absolute number of CD3+ lymphocytes is quite often observed in patients.
C D 4
The use of mAbs against the CD4 antigen makes it possible to quantitatively characterize a special clone of cells called T helper/inducer cells. CD4+ cells are functionally divided into two types of helper lymphocytes: 1st order T helpers (Th1 cells) and 2nd order (Th2 cells). Different CD4+ T cells produce different sets of cytokines. Th1 cells (they are also called delayed-type hypersensitivity cells - DTH) - cytokines for the cellular immune response: interleukin 2 (IL-2), IL-3, g-IFN, TNF-a, TNF-b, among which a discriminant cytokine is g-IFN. Th2 secrete a set of cytokines necessary for the humoral immune response: IL-3, 4, 5, 6, 10, 13, TNF-b, among which IL-4 is the discriminant cytokine.
Determining the number of CD4+ cells is important in the diagnosis of conditions associated with defects in antibody production and cell-mediated immunity reactions. The CD4+ cell count plays a decisive role in predicting the course of HIV infection.
The functional state of CD4+ lymphocytes is tested according to the cytokine profile: the functional usefulness of Th1 cells is confirmed by the secretion of g-IFN, and Th2 cells by the secretion of IL-4.
C D 8
The differentiation molecule CD8 is a glycoprotein found on the surface of thymocytes and T lymphocytes and is involved in the recognition of antigenic peptides in the context of major histocompatibility complex (MHC) class I molecules.
Clinical significance of determining the number of CD8+ lymphocytes:
- viral infections (with a certain modification, it is possible to quantify virus-specific cytolytic CD8+ T-lymphocytes);
- in a number of diseases, the ratio between the CD4 and CD8 subpopulations of T lymphocytes (immunoregulatory index CD4/CD8) is of great prognostic significance; for example, a progressive decrease in the immunoregulatory index in HIV-infected patients may indicate a transition to AIDS;
- malignant neoplasms;
- assessment of the effectiveness of vaccination (especially antiviral vaccines).
Until recently, the suppressor activity attributed to the subpopulation of CD8+ cells is now almost completely rejected. According to most experimental and clinical studies, it is believed that the existence of any separate population of T-suppressor cells, even without binding to the CD8 marker, is unlikely.
In autoimmune thyroiditis, in particular in diffuse toxic goiter (DTZ), the reactions of cellular immunity indicate a decrease in the subpopulation of CD8+ lymphocytes and a decrease in the functional activity of cytotoxic lymphocytes.
In diabetes mellitus, there is also a decrease in functional activity and the number of CD8+ lymphocytes.
A decrease in the CD8+ lymphocyte fraction is also observed in patients with primary chronic adrenal insufficiency (Addison's disease).
Anti- HLA - DR
The HLA-DR molecule is also an activation marker and belongs to MHC class II. It is a transmembrane glycoprotein consisting of a- and b-subunits with a molecular weight of 36 and 27 kDa. Anti-HLA-DR reacts only with the HLA-DR epitope and does not cross-react with the HLA-DQ and HLA-DP molecules. It is expressed on B lymphocytes, monocytes, macrophages, and activated T lymphocytes.
There is evidence that the HLA-DR molecule is expressed on approximately 10% of PC T lymphocytes, but when cells are activated by mitogen, the number and density of its expression increases sharply. It has been suggested that the HLA-DR molecule on T cells may act as a receptor involved in signal transduction by activated T lymphocytes. This suggests its possible role as a “professional” APC involved in the maintenance of immune memory.
HLA-DR may also be present on thymic epithelial cells, on cells of B-lymphocyte-dependent fields of the spleen and lymph nodes, and B-cell lymphoma. This antigen is co-expressed with CD1a antigen on Langerhans cells.
CD25
The CD25 antigen is known as a low-affinity IL2 receptor with a molecular weight of 55 kDa.
The CD25 molecule, associated with the b-chain (CD122) and the common g-chain (CD132), forms a high-affinity IL-2 receptor complex. During inflammation, a soluble form of IL-2R can be produced. The CD25 marker is present on subpopulations of T- and B-lymphocytes of peripheral blood, including activated macrophages, NK. Its expression increases sharply upon activation of PHA and ConA on the surface of CD3-activated T lymphocytes, on T cells from a mixed culture of lymphocytes, and on HTLV-infected T lymphocytes of the leukemic line in T-lymphocytic leukemia.
The method allows you to determine the quantitative ratio of the main populations of T-lymphocytes:
- T lymphocytes (CD3+CD19-) ;
- T helper/inducer cells (CD3+CD4+CD45+) ;
- T-cytotoxic lymphocytes (T-CTL) (CD3+CD8+CD45+) ;
- ratio of T-helpers/T-cytotoxic lymphocytes (CD3+CD4+/ CD3+CD8+) .
small cell populations, and also study their functional activity:
- activated T-lymphocytes (CD3+ HLA - DR + CD 45 +);
- regulatory T-helper cells ( CD 4+ CD 25 brig CD 45+), performing an immunosuppressive function
- activated cytotoxic T lymphocytes ( CD 3+ CD 8 bright CD 38+) (% of all T lymphocytes)
- activated T lymphocytes expressing the IL-2 receptor a-chain (CD3+ CD 25+ CD 45+) .
When is the study scheduled?
Being real suppressors, T-regulatory cells play a leading role in many immunological processes: they regulate T-cell homeostasis, prevent autoimmune diseases, allergies, hypersensitivity, graft-versus-host disease. At the same time, regulatory T cells reduce antitumor immunity and immunity to infections.
Of particular interest are studies related to the study of the ratio of autoactive clones of B cells and regulatory T cells in various pathologies of inflammatory origin. Thus, in the complicated course of a number of pathological inflammatory processes, the preservation of a high level of T-reg and B1 cells by the 30th day characterizes the persistence of the intensity of the inflammatory process and, possibly, the beginning of the formation of a defect in the functioning of T-reg cells, which can subsequently lead to chronic inflammation and to the development of an autoimmune process.
Thus, the presence and quantitative characteristics of this population serve as an important diagnostic feature.
Recommended for a comprehensive examination of patients at risk for the four main immunopathological syndromes.
With infectious syndrome:
- frequent acute respiratory viral infections, chronic infections of the ENT organs (purulent sinusitis, otitis, periodic lymphadenitis, pneumonia with a tendency to recur, bronchopleuropneumonia);
- bacterial infections of the skin and subcutaneous tissue (pyoderma, furunculosis, abscesses, cellulitis, septic granulomas, recurrent paraproctitis in adults);
- urogenital infections;
- fungal infections of the skin and mucous membranes, candidiasis, parasitic infestations;
- recurrent herpes of various localizations;
- gastroenteropathy with chronic diarrhea of unknown etiology, dysbacteriosis;
- prolonged low-grade fever, fever of unknown etiology;
- generalized infections (sepsis, purulent meningitis).
With allergic (atopic) syndrome:
- atopic dermatitis;
- neurodermatitis;
- eczema with an infectious component;
- severe atopic bronchial asthma, hay fever, chronic asthmatic bronchitis.
With autoimmune syndrome:
- rheumatoid arthritis;
- multiple sclerosis;
- diffuse connective tissue diseases (systemic lupus erythematosus, scleroderma, dermatomyositis);
- autoimmune thyroiditis;
- nonspecific ulcerative colitis;
With immunoproliferative syndrome:
- tumor processes in the immune system (lymphomas, Hodgkin's disease, acute and chronic lymphocytic leukemia, Kaposi's sarcoma).
What do the results mean?
Changes in various cell populations of lymphocytes, up or down, develop during various pathological processes in the body, such as infections, autoimmune and oncological diseases, immunodeficiencies, in the postoperative period, and during organ transplantation.
Below is a table with clinical situations that can lead to changes in the subpopulation composition of lymphocytes.
| Lymphocyte subpopulation | Increasing the indicator | Decrease in indicator |
| T lymphocytes (CD3+CD19-) | • Acute and chronic infections; • hormonal imbalance; • long-term use of medications (especially monotherapy); • taking dietary supplements; • intense sports activities; • pregnancy; • T-cell leukemias. | • Some types of infections; • immunodeficiency states; • alcoholic cirrhosis of the liver; • liver carcinoma; • autoimmune diseases; • taking immunosuppressive drugs. |
| T helper cells (CD3+CD4+CD45+) | • A number of autoimmune diseases; • hormonal imbalance; • some infections; • selected T-cell leukemias; • poisoning with beryllium salts. | • Immunodeficiency states (the main laboratory sign of secondary immunodeficiency); • alcoholic liver disease; • autoimmune diseases; • taking immunosuppressive drugs or steroids. |
| T-cytotoxic lymphocytes (CD3+CD8+CD45+) | • Some viral infections; • a number of T-cell leukemias; • anesthesia; • acute phase of allergy; • a number of autoimmune pathologies. | • Some types of autoimmune and allergic diseases; • immunosuppressive therapy. |
| T-reg. (regulatory T cells (CD4+CD25brightCD45+) | • Various neoplasms; • lymphoproliferative processes; • infectious diseases. | • Autoimmune pathology (diabetes mellitus type 1, multiple sclerosis, rheumatoid arthritis, autoimmune thyroiditis, ulcerative colitis, Crohn's disease, myasthenia gravis); • allergic diseases (bronchial asthma, atopic dermatitis, food allergies). |
| Activated T lymphocytes (CD3+HLA-DR+CD45+) | • Infections; • autoimmune pathology; • allergies; • oncological diseases; • alcoholic cirrhosis of the liver; • pregnancy. | They have no diagnostic value. |
In combination with clinical data, symptoms, and other laboratory research methods, the above changes are a diagnostic sign of the occurrence of these pathological processes in the human body.
Helper T cells:
The task of helpers is also quite obvious at first glance. These are helper cells (“help” means “to help”). Who or what do they help? They induce and stimulate an immune response: under their influence, cytotoxic lymphocytes enhance their work. Helpers also transmit information about the presence of a foreign protein in the body to B-lymphocytes, which secrete protective antibodies against them. Finally, helpers have a stimulating effect on the work of phagocytes, mainly monocytes.
T helper cells
Circulating immune complexes (CIC)
CECs are circulating immune complexes, the level of which increases during acute infections and autoimmune diseases. Circulating immune complexes (CICs) are present in many people with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), especially those with complications such as vasculitis. There is a positive correlation (* systematic and conditional relationship) between disease activity and the level of CEC in the blood. CEC formation is a physiological defense mechanism resulting in the rapid elimination of either endogenous or exogenous antigens (eg, microorganisms, viruses, parasites, plant antigens, fungal antigens, pollen or food antigens) through the reticuloendothelial system. High levels of CEC in serum and/or other biological fluids are observed in many inflammatory and malignant diseases, which can cause the development of pathology. Determination of serum CEC is an important marker for assessing disease activity, especially in autoimmune diseases.
Amplifier lymphocytes:
After an aggressor has entered the body, an increased content of lymphocytes is observed in the blood and tissues. Their number increases literally within a few hours and can more than double. Why does the increase in the number of cells occur so quickly? It’s just that the body has a certain supply of them.
Mature, full-fledged lymphocytes live in the spleen and thymus. Their difference from the others is only that they “have not decided” what type of lymphocyte they belong to. These are amplifier cells; if necessary, they participate in increasing the number of other T-lymphocytes.
References
- Litvinova, L.S., Gutsol, A.A., Sokhonevich N.A. and others. Basic surface markers of the functional activity of T-lymphocytes. Medical immunology, 2014. - No. 1.
- Histology (introduction to pathology) / ed. E.G. Ulumbekova, Yu.A. Chelysheva, 1997. - P. 15-17, 532 -546.
- Encyclopedia of clinical laboratory tests / ed. WELL. Titsa. - M.: Labinform, 1997. - P. 214-215.
- Saraiva, D., Jacinto, A., Borralho, P. et al. HLA-DR in Cytotoxic T Lymphocytes Predicts Breast Cancer Patients' Response to Neoadjuvant Chemotherapy. Front Immunol., 2021. - Vol. 9. - P. 2605.
Memory T cells:
Having coped with the next threat, lymphocytes remember it. A special clone of cells is formed in the human body, which store these “memories”. Each clone carries information about a certain type of threat. If some aggressor that the immune system has already encountered enters the body, the corresponding clone multiplies and quickly forms a secondary immune response.
The conversation about the types of lymphocytes and their functions is quite long. Here this topic was presented in the most acceptable and simple form, without loading it with specific terms and unclear names. Let's hope that any reader, even without a medical education, has roughly understood how different types of T-lymphocytes function in his body.
From all this we can draw an obvious conclusion: in order to live a full, healthy life, you must have a strong immune system. It is necessary that processes that many people do not think about, and even more people do not even know, happen as they should.
If nature has not rewarded you with stable immunity, you should think about strengthening it yourself. To do this, you can start taking Transfer Factor. It contains information molecules with the help of which lymphocytes normally communicate with each other, manage and coordinate various processes. Compensating for the lack of natural information molecules, the product is one of the most recommended and effective drugs for normalizing the immune system, improving health and preventing diseases.
Immune cells have memory and transmit information to each other
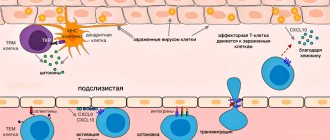
The work of resident T cells: do not confuse tourism with emigration
In a normal situation, mouse resident tissue cells hardly move within non-lymphoid tissue and are quite firmly attached by adhesion molecules to the stroma of the organ. When resident macrophages of the same tissue initiate an inflammatory response by secreting cytokines, TRMs become more motile and patrol the nearby epithelium in search of infected cells.
If the inflammatory reaction intensifies, then the cells understand this as a signal for reinforcement: TCM and TEM cells newly arriving from the blood are connected to the work of patrol TRM. These blood cells are much more mobile and move better in the epithelium. Does this mean that it is in the blood that killer T-cells are ready to act among TEM, and CD8+ TRMs perform helper and regulatory functions in the tissue?
On the one hand, T-helpers are more tissue-specific in the spectrum of T-cell receptors, i.e. There is very little overlap between the TCR repertoires of cells taken from different tissues, while cells of the same killer T cell clone are found in different tissues among TEMs []. The range of functions and repertoire of antigen specificity of TRM remains to be studied, but TRM killers definitely have the ability to destroy infected tissue cells. Moreover, in a model of murine polyomavirus infection in brain tissue, the affinity of virus-specific T-cell receptors of resident killer cells is higher than that of virus-specific central memory cells [].
However, the size of the T-cell population depends not only on the specificity of the TCR for infections that previously occurred in a given organ, but also on the homeostatic proliferation of T-cells - the proliferation of more successful cells to fill the organ's capacity according to the number of T-lymphocytes. By using the markers CD28 and CD127 on the cell surface, it is possible to distinguish cells recently and long ago activated through the TCR from those that received only a homeostatic signal to proliferation from the growth factor IL-7. As tissue ages, homeostatic cell proliferation begins to dominate over the proliferation of TCR-activated cells.
NKT cells, a type of liver-resident cell found in other tissues, often function independently of T-cell receptors. They can be activated by NK cell receptors through recognition not of individual antigens, but of general molecular patterns of danger and tissue stress. When activated, CD8+ NKT cells release cytotoxic granules and lyse suspicious tissue cells, for example, single tumor cells and virus-infected cells that express and display stress molecules on the outer membrane. With aging, the tendency of TRM to be activated without the T cell receptor, through NK cell receptors or cytokine signals, can lead to erroneous lysis of tissue cells, insufficient control of chronically infected or degenerating areas of the epithelium.
Pathological manifestations associated with the work of resident T cells include organ-specific autoimmune syndromes and chronic tissue inflammation syndromes. Examples of chronic inflammation maintained by resident T lymphocytes are contact dermatitis and psoriasis, and the mechanism is the release of inflammatory factors IL-17 by resident killer T cells and IL-22 by resident dermal helper T cells. CD8+ effector T killer cells located in the brain are similar in their set of membrane marker molecules to the TRM of the skin, intestines and lungs and are able to push the development of intermittent multiple sclerosis with periodic releases of inflammatory cytokines. It is unclear, however, whether there is a normal TRM population in the brain or whether these are T lymphocytes remaining in the tissue after a neurotropic viral infection [].
The functions of resident memory cells normally—in the absence of infection or chronic inflammation—may include cross-talk
(mutual regulation mainly through the secretion of cytokines and costimulatory molecules) with non-classical, poorly studied lymphoid cells.
They may be mucosal-associated γ/δ T cells, which carry an alternative variant of T-cell receptor assembly, or Innate Lymphoid
(ILCs), which share common features of the epigenetic landscape with T and B lymphocytes, but do not have T-/B- or NK-cell receptors [, ].
Proposed functions of tissue resident T-lymphocytes. Some functions can be performed in interaction with resident macrophages
TRM cells come into contact with antigen-presenting tissue cells—dendritic cells of the skin and resident tissue macrophages. Resident myeloid cells in different tissues are differentiated and slightly similar to each other. For example, marginal zone macrophages of the spleen, liver macrophages, and microglia (brain macrophages) will differ greatly in both morphology and range of functions. In addition to detecting antigens in tissue, resident macrophages are involved in regulating the processes of aging and tissue self-renewal, in particular, they secrete growth factors and cytokines that stimulate the division of tissue stem cells. In adipose tissue, for example, macrophages stimulate the differentiation of new fat cells, but when they enter an activated M1 state, they trigger inflammation and, instead of differentiating, cause existing fat cells to enlarge and swell. Concomitant changes in adipose tissue metabolism lead to the accumulation of fat mass and in recent years have been associated with the mechanisms of development of obesity and type II diabetes. In the skin, cytokines released by macrophages and resident γ/δ T cells stimulate stem cell division during epidermal and hair follicle stem cell regeneration [, ]. It can be assumed that helper TRM cells, when patrolling the epithelium and forming contacts with tissue macrophages, can modulate the spectrum and volume of growth factors secreted by the latter for stem cells, inflammatory cytokines and epithelial remodeling factors - and thereby participate in tissue renewal.
T cells are considered extremely important in the fight against autoimmune diseases
T cells are considered extremely important in the fight against autoimmune diseases, especially in the cases of human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS). Many of the treatments currently used for these viruses involve stimulating the production of these cells and their response to combat the harmful effects of these conditions. Naturally occurring "killer" T cells are unable to effectively fight HIV, so scientists have developed a variety of techniques to improve the cells and make the cells' receptors more sensitive to this deadly virus.
Because the study of cellular function is still relatively new, many scientists still do not understand what T cells are and how they work. As researchers continue to study these beneficial cells, many believe it may be possible to reduce cellular transplant rejection and improve the treatment of autoimmune diseases.
What else is prescribed with this study?
Activated lymphocytes (T-lymphocytes, T-helpers, T-cytotoxic cells, immunoregulatory index, T-activated, NK- and B-activated cells)
17.54. Ven. blood 3 days
5,540 ₽ Add to cart
Humoral immunity (immunoglobulins IgA, IgM, IgG, IgE, circulating immunocomplexes, complement components C3, C4)
17.51. Ven. blood 8 days
2 180 ₽ Add to cart
Immune status (screening) (Phagocytic activity of leukocytes, cellular immunity, total immunoglobulin IgE, immunoglobulins IgA, IgM, IgG)
27.960. Ven. blood 3 days
5 450 ₽ Add to cart
Cellular immunity (T-lymphocytes, T-helpers, T-cytotoxic cells, Immunoregulatory index, B-lymphocytes, NK-T cells, NK cells, Leukocyte formula)
17.50. Ven. blood 3 days
4 140 ₽ Add to cart