Article for the "bio/mol/text" competition: Cells of the immune system travel through the lymph and bloodstream in search of an antigen that can be recognized and begin a protective immune response. But a significant portion of T-lymphocytes are not found in the blood or lymph nodes, but in organs that are not related to the immune system. This article explains what tissue-resident T cells do, how they get there, and the medical benefits of studying them.
Note!
This work took first place in the “Best Article on Immunology” category of the “bio/mol/text” competition 2015.
The sponsor of the nomination “Best article on the mechanisms of aging and longevity” is the Science for Life Extension Foundation. The audience award was sponsored by Helicon.
Competition sponsors: Biotechnology Research Laboratory 3D Bioprinting Solutions and Scientific Graphics, Animation and Modeling Studio Visual Science.
An adequate protective reaction when infected with a pathogenic virus is to destroy the infected cells, preventing the infection from spreading throughout the body and killing more cells. A cell infected with a virus can notice the virus in itself and begin autophagy or apoptosis - or receive instructions for programmed cell death from a T-killer cell.
The cytotoxic T-lymphocyte, or killer T-lymphocyte, is the pinnacle of the evolution of adaptive immunity, because to recognize a fragment of the virus (antigen) on an infected cell, it uses a T-cell receptor, which randomly and independently assembles on each T-cell in the thymus. The T-cell receptor assembly mechanism, which has no analogues outside the adaptive immune system of vertebrates, uses the advantages obtained by vertebrates during genome duplications in the process of evolution and proceeds with the participation of special recombinase proteins, once borrowed from DNA transposons (see more in Chudakov’s article “ Analysis of individual repertoires of T-cell receptors").
Classical human immunology is based on the study of immune cells in the blood simply due to the fact that a blood test can be taken from any patient and examined in normal and pathological conditions. It is on blood cells that the classification of T-lymphocytes was built: division into T-killers and T-helpers, which check the antigen specificity of T-killers, give them a “license to kill” and are able to control the entire course of the immune response through soluble signaling molecules - cytokines. As well as the later isolation from the T-helper branch of a group of regulatory T cells that suppress excess adaptive immunity.
But as yogurt commercials remind us, a significant portion of the immune system's cells are concentrated around the lining of the digestive tract and in other tissues. While in 5–6 liters of blood of an adult there are about 6–15 billion lymphocytes, the number of T cells found in the epidermis and skin is estimated at 20 billion [1], and in the liver of an adult male there is another 4 billion [2 ]. Is studying blood cells enough to fully describe the functions of T cells if there are more T cells in peripheral organs than in the bloodstream? And are classical subpopulations sufficient to describe all the types of T cells found in the human body?
Life cycle of a T lymphocyte
Each T cell, after assembling a T-cell receptor, is tested for the functionality of the randomly assembled receptor (positive selection) and the lack of specificity for the body's own antigens (negative selection), that is, for the absence of an obvious autoimmune threat. The stages of selection occur in the thymus gland, thymus; in this case, more than 90% of precursor cells die, failing to correctly assemble the receptor or undergo selective selection. Surviving T cells proliferate and exit the thymus into the bloodstream - these are naïve T cells that have not encountered the antigen. The naive T cell circulates through the blood and periodically enters the lymph nodes, where in the T-cell zone it contacts specialized antigen-presenting cells.
After meeting the antigen in the lymph node, the T cell acquires the ability to divide again - it becomes the precursor of memory T cells (TSCM, stem cell memory T cells). Among the clone of its descendants, central memory cells (TCM), short-lived effector cells that carry out the immune response (SLEC or TEMRA cells), and effector memory precursor cells TEM, which in turn produce TEMRA when dividing, appear [3]. All these cells leave the lymph node and travel through the blood. Effector cells can then leave the bloodstream to carry out an immune response in the peripheral tissue of the organ where the pathogen resides. What then - another journey through the blood and lymph nodes?
Figure 1. Effector T cell emigration into tissue during viral infection. Inflammatory signals from infected epithelial cells with the participation of resident cells are transmitted to the vascular endothelium; endothelial cells attract effector T cells with chemokines CXCL9, CXCL10. Rolling: When moving along a postcapillary venule in tissue, the effector cell slows down, forming temporary contacts between E-selectins and P-selectins on endothelial cells. Arrest: The effector cell adheres tightly to the endothelium when LFA-1 and other alpha integrins interact with ICAM-1/VCAM-1/MAdCAM-1 (on the endothelium). Transmigration: The effector T cell binds endothelial JAM-1 with molecules PECAM, CD99, LFA-1 and penetrates through endothelial cells into the submucosa. Figure from [3].
The process of leukocyte transmigration.
The cells of the stroma, that is, the base of the lymph node, secrete signaling substances in order to call the T cell to the lymph node - chemokines. Lymph node chemokines are recognized by homing receptors CCR7 and CD62L. But effector cells lack both of these receptors. Because of this, it has long been a mystery how effector cells can get from peripheral tissue back to the secondary lymphoid organs - the spleen and lymph nodes.
At the same time, evidence began to accumulate of differences in membrane marker repertoires and transcriptional profiles between memory T cells in the blood (TEM) and memory T cells in other organs, which did not fit into the concept of constant migration of T cells between tissues and blood . It was decided to isolate a new subpopulation: resident memory cells that inhabit a specific organ and do not recycle - TRM cells [4].
Figure 2. Scheme of the transition of descendants of activated T lymphocytes between populations. Figure from [14].
When lymphocytes are low
The pathogenesis of a decrease in the number of these cells (lymphopenia) can occur in two ways:
- Cells are destroyed in the fight against an infectious pathogen, and new ones have not yet formed. A similar situation arises in the midst of viral diseases, when the patient does not receive treatment, the body does not have outside support and is forced to cope on its own.
- The organs responsible for the “reproduction” of cells are affected. Here the causes of damage can be different and depend on the specific disease.
Lymphopenia is typical for:
- development of anemia (anemia);
- oncological diseases (leukemia, lymphogranulomatosis, lymphosarcoma);
- consequences of radiation therapy;
- Itsenko-Cushing's disease (impaired production of pituitary and adrenal hormones);
- as a result of long-term treatment with corticosteroid hormones;
- with congenital anomalies of the lymphatic system;
- renal failure;
- systemic lupus erythematosus;
- AIDS.
Origin of tissue resident T cells
Where do tissue resident cells first appear? These are the descendants of effector cells that have lost their ability to recycle. Some tissues peripheral to the immune system, for example, the mucosa of the small intestine, the abdominal cavity, allow effector T lymphocytes to penetrate freely; others - very limited, a large flow of effector T cells into these tissues is observed only during an inflammatory reaction. The second type of tissue includes those separated by a barrier from the immune system, for example, the brain and spinal cord, as well as many others: peripheral ganglia, mucous membranes of the genital organs, lungs, epidermis, eyes. The difference between the two tissue types is the expression of additional homing molecules for effector T cells, for example, the epithelial infiltration adhesion molecule MadCAM-1 [3].
Figure 3. “To home or not to home?” - difficult choice of effector cell. To home is the process of homing, or migration of T cells, for example, to the place most familiar to naive cells - the lymph node. The alternative is to not travel through the body and become a resident tissue cell.
What else is prescribed with this study?
Activated lymphocytes (T-lymphocytes, T-helpers, T-cytotoxic cells, immunoregulatory index, T-activated, NK- and B-activated cells)
17.54. Ven. blood 3 days
6,510 ₽ Add to cart
Humoral immunity (immunoglobulins IgA, IgM, IgG, IgE, circulating immunocomplexes, complement components C3, C4)
17.51. Ven. blood 8 days
3,930 ₽ Add to cart
Immune status (screening) (Phagocytic activity of leukocytes, cellular immunity, total immunoglobulin IgE, immunoglobulins IgA, IgM, IgG)
27.960. Ven. blood 3 days
7,700 ₽ Add to cart
Cellular immunity (T-lymphocytes, T-helpers, T-cytotoxic cells, Immunoregulatory index, B-lymphocytes, NK-T cells, NK cells, Leukocyte formula)
17.50. Ven. blood 3 days
5 210 ₽ Add to cart
Resident T cells in aging human tissues
A map of the relationships between the presence of individual subpopulations of T cells in different human organs, oddly enough, was compiled only in 2014. Donna Farber's team at Columbia University Medical Center in New York compared the phenotypes of T cells isolated from the blood and tissue of organ donors of all age groups from 3 to 73 years, from a total of 56 donors [5]. Analysis of T cell subpopulations using flow cytometry confirmed many data obtained using methods with lower resolution and lower statistics, and some features of the description of the immune system transferred from mouse immunology to humans, for example, a decrease in the content of naïve T lymphocytes with aging in all organs .
The decrease in the number of naïve T cells with age is associated with rapid aging of the thymus gland, in which future T cells undergo the stages of T-cell receptor assembly, testing the functionality of the receptor, and selection for a lack of autoimmune potential. It is important not only to reduce the absolute number of naïve T cells, but also to reduce the diversity of the T-cell receptor repertoire, and therefore the ability to form an adaptive immune response to a previously unfamiliar infection [6]. For naive killer T cells, a progressive decline in abundance in the blood and lymph nodes was confirmed, although for naive helper T cells the negative correlation of abundance with age in this study was significant only in secondary lymphoid organs, but not in the blood.
Isolation of memory T lymphocytes, memory effector cells and short-lived effector cells from the mucous membranes of the lungs, small and large intestines, inguinal and mesenteric lymph nodes of organ donors made it possible for the first time to assess the dynamics of these populations in human tissues during aging. The proportion of central memory cells expectedly increases over the course of life, in accordance with the increase in the number of infections that the body has encountered and entered the memory library of the immune system. The percentage of terminally differentiated effector T-killer cells (TEMRA) also increases, but only in the lymph nodes and spleen; in nonlymphoid tissues, TEMRA abundance decreases. TEM effector memory cells rapidly fill the niche for T cells in the child's tissues, quickly displacing naïve T cells by about 12 years of age. Short-lived terminally differentiated killer T cells are most often found in the blood, spleen and mucous membranes of the lungs at any age, but among T helper cells this subpopulation is represented by a vanishingly small number of cells. Similarly, there are few central memory cells among T-killers; they are predominantly located in the mucous membranes of two barrier tissues: the lungs and intestines.
In broad strokes, the distribution map of human T-lymphocytes can be outlined as follows: naïve T-cells travel through the blood and periodically enter secondary lymphoid organs; TEMRA killers are found in the blood, spleen and lungs. Apparently, central memory cells are characterized by a more individual distribution across tissues than other subpopulations: in any case, patterns of dynamics during aging of different tissues could not be identified. Effector memory cells, including the TRM subpopulation, dominate among T cells of mucosal barrier tissues. In general, with aging of T-cell immunity in non-lymphoid tissues, there is a greater age-related change in T-cell types [5]. The stability of tissue cells is easier to explain if we understand which of the TEM effector cells remain in the tissue, become resident TRMs, and what events their life consists of after they stop traveling throughout the body.
Figure 4. Circulation pathways of T lymphocytes of different subpopulations. Tnaive - naive T cells, together with the TCM subpopulation, move through the blood and enter the T-cell zone of various lymph nodes; they are found in tissue capillaries, but do not exit into the tissue (red trajectory). Effector T cells (blue) move through the lymph and bloodstream; when they enter a lymph node, they do not enter the T-cell zones (center of the lymph node) - the trajectory is purple. Resident T-cells of tissues (shown in green in the skin and in different colors in mucous membranes) move only within the tissue - green trajectory. Figure from [9], with modifications.
Helper T cells:
The task of helpers is also quite obvious at first glance. These are helper cells (“help” means “to help”). Who or what do they help? They induce and stimulate an immune response: under their influence, cytotoxic lymphocytes enhance their work. Helpers also transmit information about the presence of a foreign protein in the body to B-lymphocytes, which secrete protective antibodies against them. Finally, helpers have a stimulating effect on the work of phagocytes, mainly monocytes.
T helper cells
How to distinguish resident tissue cells from admixtures of blood cells?
Resident T cells can be correctly, but inconveniently, determined each time by the ability of an individual cell to migrate to lymph nodes, so it is necessary to compile a list of characteristic features that can be used to determine membership in this subpopulation. Resident T lymphocytes in the body's natural barrier tissues (for example, in the lungs and small intestinal mucosa) are somewhat similar to classical effector blood cells: they express the activated cell marker CD69, and the expression is stable throughout life during adulthood and aging and is characteristic of all non-lymphoid tissues . But in addition, CD69 colocalizes with the marker CD103, which denotes a group of adhesion molecules - integrins, which promote the attachment of resident T cells to the epithelium and to fibroblasts in the submucosa of the selected organ. For effector T cells in secondary lymphoid organs, the expression of CD103 integrins is completely uncharacteristic: TEM cells constantly maintain a motile phenotype.
The map compiled by Donna Farber's team has a major flaw: it is unclear how cleanly T-lymphocytes can be isolated from an organ, and what proportion of the analyzed cells are actually blood T-lymphocytes from capillaries inside the organ.
The issue of contamination with blood cells is especially acute for the lungs; it is no coincidence that the subpopulation composition of T cells in the lungs is unexpectedly similar to T cells in the blood and lymph nodes. The issue of blood cell contamination was elegantly solved for mouse T cells: experimental mice were infected with lymphocytic choriomeningitis virus after transplantation of a transgenic P14 T cell clone specific for this virus. As a result, during infection, the majority of circulating cells were represented by the virus-specific P14 clone, and its presence in tissues could be monitored by immunofluorescence with a P14-specific antibody. Before the mice were killed, they were injected with an antibody to the killer T-cell marker anti-CD8, which quickly spread through the bloodstream and bound to all killer T-cells in the blood (but not in the tissues). By microscopy of organ sections, it was easy to distinguish resident killer TRMs from cells only recently released from the blood into the organ, labeled with an anti-CD8 antibody [7]. The number of resident cells calculated by this method was 70 times higher than the numbers determined by flow cytometry; a difference of less than twofold was observed only for resident cells of the lymph nodes and spleen: it turns out that standard methods for isolating lymphocytes from organs are poorly suited for the analysis of killer resident cells and significantly underestimate the population size.
Normal in humans
The lymphocyte count in a blood test is examined for any disease. It is part of the leukocyte formula, has an absolute value and makes up a certain part of the total content of leukocytes.
The norm of lymphocytes in the blood depends on age.
| Age (years) | Specific gravity in % | Abs. quantity (x109/l) |
| up to a year | 45-70 | 2-11 |
| from one to two years | 37-60 | 3-9 |
| from two to four | 33-50 | 2-8 |
| by the age of ten | 30-50 | 1,5-6,8 |
| in teenagers | 30-45 | 1,2-5,2 |
| in adults | 19-37 | 1-4,8 |
The number of lymphocytes in the blood of women does not differ from men.
The work of resident T cells: do not confuse tourism with emigration
In a normal situation, mouse resident tissue cells hardly move within non-lymphoid tissue and are quite firmly attached by adhesion molecules to the stroma of the organ. When resident macrophages of the same tissue initiate an inflammatory response by secreting cytokines, TRMs become more motile and patrol the nearby epithelium in search of infected cells.
If the inflammatory reaction intensifies, then the cells understand this as a signal for reinforcement: TCM and TEM cells newly arriving from the blood are connected to the work of patrol TRM. These blood cells are much more mobile and move better in the epithelium: does this mean that it is in the blood that T-killer T cells are ready to act among TEM, and CD8+ TRMs perform helper and regulatory functions in the tissue?
On the one hand, T-helpers are more tissue-specific in the spectrum of T-cell receptors, that is, there is very little overlap between the repertoires of T-cell receptors of cells taken from different tissues, while cells of the same T-killer clone are found in different tissues among TEM[ 5]. The range of functions and repertoire of antigen specificity of TRM remains to be studied, but TRM killers definitely have the ability to destroy infected tissue cells. Moreover, the affinity of virus-specific T-cell receptors (TCRs) of resident killer cells is higher than that of virus-specific central memory cells in a mouse model of polyomavirus infection in brain tissue [8].
However, the size of the T-cell population depends not only on the specificity of T-cell receptors for infections that previously occurred in a given organ, but also on the homeostatic proliferation of T-cells - the proliferation of more successful cells to fill the organ's capacity according to the number of T-lymphocytes. By using the markers CD28 and CD127 on the cell surface, it is possible to distinguish cells recently and long ago activated through the T-cell receptor from those that received only a homeostatic signal to proliferation from the growth factor IL-7. With tissue aging, homeostatic cell proliferation begins to prevail over the proliferation of TCR-activated cells.
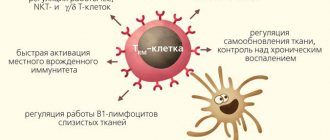
NKT cells, a large type of liver resident cell that is also found in other tissues, often function independently of T-cell receptors. They can be activated by NK cell receptors through recognition not of individual antigens, but of general molecular patterns of danger and tissue stress. When activated, CD8+ NKT cells release cytotoxic granules and lyse suspicious tissue cells, for example, single tumor cells and virus-infected cells that express and display MHC-like stress molecules on the outer membrane. With aging, the tendency of TRM to be activated without the T cell receptor through NK cell receptors or cytokine signals can lead to erroneous lysis of tissue cells, insufficient control of chronically infected or degenerating areas of the epithelium.
Pathological manifestations associated with the work of resident T cells include organ-specific autoimmune syndromes and chronic tissue inflammation syndromes. Examples of chronic inflammation supported by resident T lymphocytes are contact dermatitis and psoriasis, and the mechanism is the release of inflammatory factors IL-17 by resident killer T cells and IL-22 by resident T helper cells of the dermis. CD8+ effector killer T cells located in the brain are similar in their set of membrane marker molecules to the TRM of the skin, intestines and lungs and are able to push the development of intermittent multiple sclerosis with periodic releases of inflammatory cytokines; It is unclear, however, whether there is a normal TRM population in the brain or whether these are T lymphocytes remaining in the tissue after a neurotropic viral infection [9].
The functions of resident memory cells normally, in the absence of infection or chronic inflammation, may include cross-talk (mutual regulation primarily through the secretion of cytokines and costimulatory molecules) with non-classical, poorly understood lymphoid cells such as mucosal-associated gamma/delta T cells, carrying an alternative version of the T-cell receptor assembly; or innate lymphoid cells (ILC), which share common features of the epigenetic landscape with T and B lymphocytes, but do not have T/B or NK cell receptors [10, 11].
TRM cells come into contact with antigen-presenting cells of tissues - these are dendritic cells of the skin and resident tissue macrophages. Resident myeloid cells in different tissues are differentiated and slightly similar to each other. For example, marginal zone macrophages of the spleen, liver macrophages, and microglia (brain macrophages) will differ greatly in both morphology and range of functions. In addition to detecting antigens in tissue, resident macrophages are involved in regulating the processes of aging and tissue self-renewal, in particular, they secrete growth factors and cytokines that stimulate the division of tissue stem cells. In adipose tissue, for example, macrophages stimulate the differentiation of new fat cells, but when they enter an activated M1 state, they trigger inflammation and, instead of differentiating, cause existing fat cells to enlarge and swell. Concomitant changes in adipose tissue metabolism lead to the accumulation of fat mass and in recent years have been associated with the mechanisms of development of obesity and type II diabetes. In the skin, cytokines released by macrophages and resident gamma/delta T cells stimulate stem cell division during the regeneration of the epidermis and hair follicle stem cells [12, 13]. It can be assumed that TRM helper cells, when patrolling the epithelium and forming contacts with tissue macrophages, can modulate the spectrum and volume of growth factors secreted by the latter for stem cells, inflammatory cytokines and epithelial remodeling factors, and thereby participate in tissue renewal.
Figure 5. Proposed functions of tissue resident T lymphocytes. Some functions can be performed in interaction with resident macrophages (see explanations in the text).
Results
No matter how young a person looks, the parameters of his immune system will objectively reflect his age. Parameters such as the number of naïve T cells and TCR diversity decline almost linearly with aging. If you're the lucky recipient of increased TCR diversity, you can look forward to a few bonus years of life. In the future, humanity can expect bold new approaches to extending lifespan using our own naïve T-lymphocytes, collected and frozen many years ago.
What can the study of Trm give to medicine?
Understanding how resident T cells work is absolutely essential for fighting infections that do not enter the bloodstream directly, but enter the body through barrier tissues—that is, for the vast majority of infections. Rational design of vaccines for protection against this group of infections can be aimed specifically at enhancing the first stage of protection with the help of resident cells: a situation in which optimally activated antigen-specific cells eliminate the pathogen in the barrier tissue is much more beneficial than triggering acute inflammation to call T lymphocytes from the blood, since there is less tissue damage.
The T-cell receptor repertoire of cells associated with mucosal barrier tissues is considered to be partially degenerate and public, that is, identical for many individuals in a population. However, biases in the isolation of T cells from organs, data bias resulting from the selection of only certain Caucasian donors into cohorts, and the overall small amount of accumulated sequencing data do not provide confidence in the public availability of T-cell receptor repertoires of TRM cells. While this would be convenient, vaccine design could be reduced to finding and modifying the most affinity and immunogenic peptides from a pathogen to interact with one of the publicly available TCR variants in the pathogen's barrier tissue.
Of course, understanding what T-cell receptors TRM cells carry on their surface is not enough to effectively manipulate immune responses in tissue. It is necessary to study in detail the factors influencing the colonization of tissues by certain T-cell clones and to understand the mechanisms of activation of local tissue immunity and induction of TRM tolerance. How are the niches of T-lymphocytes populated in the mucous membranes of a child before encountering a large number of pathogens and, accordingly, before the formation of a significant pool of effector memory T-cells - precursors of resident cells and central memory cells? Why and how, instead of the classical activation of lymphocytes, ignoring and tolerance reaction to microbes of non-pathogenic mucosal flora is formed? These questions are on the agenda in the study of resident cells of the immune system.
Determining the patterns of T-lymphocyte homing into specific tissues may provide an advantage in cellular immunotherapy of tumor diseases. Theoretically, killer T cells of the required tumor antigen specificity, activated in vitro, should kill the patient's tumor cells. In practice, such immunotherapy is complicated by the fact that tumor cells are able to suppress immune responses and render T-killer cells approaching the tumor into an inactive state of anergy. Often, anergic T-lymphocytes, primarily the TRM of a given tissue, accumulate in and around a growing tumor. Of the many active tumor-specific T cells injected into a patient, few will reach the target, and even these may be virtually useless in the immunosuppressive tumor microenvironment.
Deciphering the mechanisms that drive specific T cell clones to specific tissues could allow laboratory-engineered T cells to be targeted more effectively to tumors and usher in the era of accessible, personalized immunotherapy.
Human immune system
Ph.D. Goldinberg B.M., Vasyuk Ya.V.
City Center for Transfusiology of the health care institution "6th City Clinical Hospital", Minsk,
healthcare institution "7th city children's clinic", Minsk
HUMAN IMMUNE SYSTEM
Introduction
A group of organs that have a common origin, a common structural plan and perform a common function is called an organ system. Five of all ten organ systems are regulatory (control): nervous, circulatory, endocrine, lymphatic and immune. Let us clarify that the lymphatic organs and lymph nodes, of which there are about 600, are functionally part of the immune system, and the lymphatic system itself includes an extensive network of vessels that passes through almost all of our tissues, ensuring the movement of a fluid called lymph.
The word “immunity” comes from the Latin “immunis” (in English – immunity), which means “clean from anything”, immune to anything. The immune system appeared along with multicellular organisms and developed as an assistant to their survival. It unites organs and tissues that guarantee the body’s protection from genetically foreign cells and substances coming from the environment.
The immune system is represented at three levels: organ, cellular and molecular.
Organs of the human immune system
The immune system includes central and peripheral organs.
The central organs of the immune system are the red bone marrow and the thymus.
The bone marrow is the repository of stem cells from which blood cells are formed (Fig. 1). Depending on the situation, stem cells are transformed into immune B lymphocytes. If necessary, a certain part of B-lymphocytes turns into plasma cells, which are capable of producing antibodies.
Fig.1. Bone marrow contains stem cells
The thymus (or thymus gland) is one of the main organs of the immune system, located in a person behind the sternum below the collarbone, which is responsible for the formation of T-cells of the immune system in the lymphoid tissues of the body (Fig. 2).
Fig.2. Thymus
Peripheral organs include the spleen, tonsils and lymph nodes, which contain areas of maturation of immune cells.
Tonsils, which got their name because of their external resemblance to almonds, are a collection of lymphoid tissue in the upper part of the nasopharynx. A person has six tonsils: two palatine, two thoracic, and one each nasopharyngeal and lingual.
The largest of them are the palatine tonsils, or tonsils, which can be easily examined independently in the mirror if you open your mouth wide enough (Fig. 3).
Rice. 3. Palatine tonsils
The spleen is the largest lymphoid organ (Fig. 4). In addition, it can accumulate some blood. In emergency situations, the spleen is able to send its reserves into the general bloodstream. This allows you to improve the quality and speed of the body's immune reactions. The spleen cleanses the blood of bacteria and processes all kinds of harmful substances. It completely destroys endotoxins, as well as the remains of dead cells from burns, injuries or other tissue damage. In people who are left without a spleen for any reason, their immunity deteriorates.
Rice. 4. Spleen
Lymph nodes are small round-shaped formations (Fig. 5), located in the chest cavity (bronchopulmonary, bronchotracheal) and abdominal cavity (Peyer's patches, appendix and others), perithoracic on the surface of the chest, on the neck and on the limbs. The lymph node is one of the barriers to infections and cancer cells, playing the role of a kind of customs (Fig. 5). It produces lymphocytes - special cells that take an active part in the destruction of harmful substances.
Rice. 5. Lymph node
The central organs of the immune system are responsible for the formation and maturation of cells, and the peripheral organs provide protection, that is, the immune response. The peripheral and central organs of the immune system perform their work only together, and if any one of these organs fails, the body will lose its protective barrier.
Components of the immune system
Modern immunology distinguishes between two interacting components of the immune system - innate and acquired types of immunity , which ensure the development of an immune response to genetically foreign substances (entities).
Innate (species) immunity is a hereditarily fixed system of protecting the human body from pathogenic and non-pathogenic microorganisms, as well as products of tissue decay. Innate immune cells recognize pathogens by molecular markers specific to them—the so-called “pathogenicity patterns.” These markers do not allow one to accurately determine whether a pathogen belongs to one species or another, but only signal that the immune system has encountered troublemakers: a stranger or one’s own, but who has become a traitor to the body (Fig. 6).
Fig.6. Innate immunity: the main thing is calm!
Innate immunity at the cellular level is represented by:
- monocytes are the precursors of macrophages (cells that devour foreign particles). They are formed in the bone marrow, then enter the blood, but quickly leave it, turning into tissue macrophages and dendritic cells (Fig. 7);
Fig.7. Monocyte
- macrophages and dendritic cells are located in the skin and mucous membranes. They have mobility and are transported through the blood and lymph. They absorb (phagocytose) the pathogen, and within themselves, using the contents of the vacuoles, dissolve it. Dendritic cells branch like a tree. Thanks to the antenna branches, they work as communicators between the innate and acquired types of immunity (Fig. 8);
Fig.8. dendritic cell and
and macrophage
- blood cells containing granules (granulocytes) in the cytoplasm: neutrophils, eosinophils and basophils (Fig. 9);
Fig.9. Granulocytes
Neutrophils are the most numerous immune cells in human blood. They circulate in the blood for only 8-10 hours and spend most of their lives traveling through body tissues. When they encounter a pathogen, they capture it and digest it, after which they usually die themselves. Granules containing antibiotic substances are released from the destroyed neutrophils.
Granules of eosinophils and basophils provide chemical defense of the body against large pathogens, for example, parasitic worms, fungi, and extracellular bacteria. However, with excessive activity they can also participate in the development of an allergic reaction;
- natural killer cells or NK cells (English Natural killer cells, NK cells) are a type of cytotoxic lymphocytes involved in the functioning of innate immunity. They destroy cells infected with bacteria and viruses, as well as tumor cells, at high speed.
Fig. 10. Natural killer
Natural killers act with the help of aggressive substances perforin and granzyme, which, like gimlets, “bite” and destroy the affected cell that has become their target (Fig. 11)
Fig. 11. Penetration of perforin and granzyme into a cancer cell and its destruction
The molecular (humoral) factors of innate immunity are (Fig. 12):
- proteins that bind metal ions (iron, zinc) necessary for the life and reproduction of pathogens - lactoferrin, calprotectin, membrane protein and others;
- enzymes that generate oxidizing agents - oxygen and nitric oxide:
- enzymes capable of breaking down the cell membrane of pathogens - lysozyme, chitinase, phospholipase A2;
- proteins and peptides that violate the integrity of the cell membrane of a microorganism - complement, eosinophilic protein, defensins and others.
Fig. 12. Humoral factors of innate immunity
The complement system is a multicomponent, self-assembled system of more than 20 serum proteins that are normally inactive.
After activation, the biological effects of complement appear: the formation of a membrane attack complex for the lysis of pathogens, the release of inflammatory mediators to attract phagocytes and enhance their absorption capacity.
Cytokines are a system of low molecular weight proteins of the body, synthesized mainly by active cells of the immune and hematopoietic systems, regulating intercellular interactions (a “universal” language for all cells), presented in Fig. 13 and 14.
Rice. 13. Cytokines: IL – interleukins, of which there are currently 34 varieties;
Rice. 14. Multidirectional action of cytokines using the example of interferon gamma
As a result of activation of humoral and cellular factors of innate immunity, a basic reaction of infectious inflammation is formed within several hours after the introduction of a pathogen into the internal environment of the body (Fig. 15)
Rice. 15. Infectious inflammation of tissue at the site of foreign body insertion for the purpose of its removal
Acquired immunity (or adaptive – from the French adapter “ to adapt ” ) is formed individually during life under the influence of antigenic stimulation and, in turn, is divided into natural and artificial (Fig. 16).
Fig. 16. Adaptive
immunity
Natural immunity is formed when encountering a pathogen, as a result of which protective immune factors are produced in the body (active natural immunity), or they come ready-made from maternal orgasm during fetal development or during breastfeeding (passive natural immunity).
Artificial immunity is created by administering vaccines or toxoids that stimulate the production of antibodies against specific pathogens or their poisons. At the same time, for preventive purposes, the process of the patient’s immune system’s reaction to the pathogen is reproduced, but in an asymptomatic or mild clinical form, while maintaining their protective immune power for several months, years, or even for life (artificial active immunity). When it is necessary to quickly and for a short time protect a patient from the real risk of encountering a pathogen during an epidemic or neutralize a pathogen that has already entered his body, immunoglobulins (antibodies) are used both in purified form and in dosed volumes of plasma or serum obtained from the blood of a donor ( person or animal). The use of ready-made antibodies forms passive artificial immunity that lasts 2-3 weeks.
Adaptive immunity is based on three main processes:
- recognition of antigens (usually foreign to the body) using receptors;
- removal (elimination) of recognized foreign agents (Fig. 17);
- the formation of an immunological memory of contact with an antigen, allowing for faster and more efficient removal of this antigen when it is recognized again.
Fig. 17. Options for the response of the immune system to organ or tissue transplantation, the occurrence of malignant neoplasms and infections
The immunocompetent cells of adaptive immunity are lymphocytes that live in the human body from several months to several years. According to their functions, cells are divided into T-lymphocytes - 80% and B-lymphocytes - 20%.
The fact that the T lymphocyte recognizes only foreign antigens, and not molecules from its own body, is a consequence of a process called selection, which occurs in the thymus, where T cells complete their development. The essence of selection is this: the cells surrounding the young, or naive, lymphocyte show (present) to it the peptides of their own proteins. The lymphocyte that recognizes these protein fragments too well or too poorly is destroyed. The surviving cells (and this is less than 1% of all T-lymphocyte precursors that came to the thymus) have an intermediate affinity for the antigen, therefore, they, as a rule, do not consider their own cells to be targets for attack, but have the ability to react to a suitable foreign peptide.
To activate a T-lymphocyte, it needs to receive special signals from the receptors of the leukocyte antigen system and a cocktail of many pro-inflammatory cytokines.
Using special reagents, markers of surface proteins of leukocytes of a certain type are determined, which are called clusters of differentiation (CD). Currently, 350 CD antigens and their subtypes are known (Table 1).
Table 1. Main identification CD markers of cells
| Cluster designation | Cells |
| CD 10, CD34 | Lymphoid stem cell |
| CD3 | T lymphocyte |
| CD4 | T helper |
| CD8 | T-killer |
| CD19, CD72, CD79, etc. | B lymphocyte |
| CD16/ CD56 | NK cells |
| CD14, CD64 | Monocyte/macrophage |
T lymphocytes recognize cells carrying foreign antigens and destroy them after direct contact (attack), and also perform the function of regulating the immune response.
T lymphocytes have subtypes (Fig. 18):
Rice. 18. Subtypes of T-lymphocytes and their functions
- Killer T cells (also called CD8+ T lymphocytes), which, like an NK cell (natural killer), secrete the proteins perforin and granzyme, which leads to lysis of the target cell;
- T-helpers (from English helper - assistant). They are also referred to as Th cells, CD4+ T lymphocytes. Activated T helper cells produce chemokines and cytokines involved in the immune process (Fig. 19);
Rice. 19. Activation of different subpopulations of T-helper cells by cytokines
- Suppressor T cells (Ts) inhibit (suppress) B cell responses and block helper T cells. Moreover, these cells do not sabotage immune processes and do not harm health. They simply regulate the strength of the immune response, which allows the immune system to respond to stimuli with restraint and with moderate force (putting out a fire, not a fire);
- T-regulatory cells (Tr1) influence the formation of granular leukocytes (granulocytes), which we have already introduced as macrophages.
The ratio of CD4/CD8 cells is called the immunoregulatory index (IRI). If the patient’s IRI is increased (more than 2.2), this indicates excessive activity of T-helper cells and a weakening of the regulatory function of T-killer cells. At this rate, immune cells can destroy the body’s own tissues. Increased IRI is most often observed in patients with autoimmune diseases (systemic lupus erythematosus, scleroderma, rheumatoid arthritis, etc.). The cause of excessive activity of T-helper cells can also be a tumor of the thymus gland. With this pathology, an excessive number of lymphocytes are produced. High rates of IRI are observed in acute lymphoblastic leukemia. This severe oncological disease is accompanied by an uncontrolled increase in the number of immature lymphocytes.
If the immunoregulatory index is reduced (less than 1.6), then this indicates a serious deterioration in the functioning of the immune system. Low levels of IRI indicate that the function of protective cells in the body is weakened, and regulation by T-killers is excessive. This is usually observed in the following pathologies accompanied by immunodeficiency: infectious diseases (including HIV infection); congenital immunodeficiency; any protracted and chronic diseases; bone marrow tumors.
B lymphocytes are responsible for the humoral component of immunity - the production of antibodies. After an antigenic stimulus, the B lymphocyte turns into a lymphoblast, a cell capable of dividing. Some lymphoblasts differentiate into memory B lymphocytes, the other part turns into plasma cells that produce antibodies.
B lymphocytes carry the B cell receptor on their surface. Upon contact with an antigen, these cells are activated and turn into a special cell subtype - plasma cells , which live up to three weeks and have the unique ability to secrete thousands of antibodies .
The antibody has an affinity for the antigen it recognizes and, as it were, “sticks” to it. This allows antibodies to envelop (opsonize) cells and viral particles coated with antigen molecules, attracting macrophages and other immune cells to destroy the pathogen. Antibodies are also able to activate a special cascade of immunological reactions called the complement system, which leads to perforation of the pathogen’s cell membrane and its death.
Rice. 20. Antibody production and pathogen marking
There are several classes of antibodies (immunoglobulins). The first to appear after antigenic irritation, causing agglutination of bacteria and neutralization of viruses, are immunoglobulins M (IgM). Immunoglobulins G (IgG) are involved in long-term immunity.
Table 2 provides an interpretation of laboratory tests for the presence of a pathogen at the molecular level and using tests for immunoglobulins M and G.
Table 2. Interpretation of laboratory tests for the presence of a pathogen at the molecular level
| Result of molecular research | Antibody test | Interpretation | |
| IgM | IgG | ||
| Positive | Negative | Negative | Acute infection |
| Positive | Positive | Negative | Acute infection |
| Positive | Positive | Positive | Infected patient |
| Positive | Negative | Positive | Infected or re-infected patient |
| Negative | Positive | Negative | Early stages of infection. More research needed |
| Negative | Positive | Positive | Infection. More research needed |
| Negative | Negative | Positive | Post-infectious period |
| Negative | Negative | Negative | Uninfected patient |
Innate and acquired types of immunity have points of contact, which represent two triads (Fig. 21)
Rice. 21. Two triads combining innate and acquired types of immunity
The development of an adaptive immune response takes quite a long time (from several days to two weeks), and in order for the body to defend itself against an already familiar infection faster, so-called memory cells . They, like veterans, are present in the body in small quantities, and if a pathogen familiar to them appears, they are reactivated, quickly divide and come out as an entire army to defend the borders (Fig. 22).
Fig.22. Memory T cells quickly form a secondary immune response
Immunological tolerance
Immunological tolerance (tolerance, arereactivity) is understood as the absence of an immune response to a specific antigen. The list of antigens to which tolerance can develop is practically indistinguishable from the set of antigens against which a specific immune response develops (Fig. 23).
Rice. 23. Immune tolerance
Tolerance mechanisms are necessary because the immune system produces a huge variety of antigen-specific receptors, and some of them are specific to the body's own antigens; Tolerance prevents unwanted reactions against one’s own organs and tissues, also for the normal course of pregnancy.
Immune system disorders in humans
Immune system disorders can be divided into three categories: immunodeficiencies, autoimmune diseases, and hypersensitivity reactions.
Immunodeficiencies
Immunodeficiency is a decrease in the quantitative indicators and/or functional activity of the main components of the immune system, leading to a disruption of the body’s defense against pathogenic microorganisms and manifested by an increased incidence of infectious diseases.
Primary immunodeficiencies (PIDs) are hereditary diseases caused by defects in genes that control the immune response. In general, PIDs manifest themselves already in early childhood, but sometimes only by the age of 30-40.
- symptoms that may be signs of primary immunodeficiencies:
- 4 or more cases of otitis during the year;
- 2 or more sinusitis during the year;
- low effectiveness of antibiotics for two or more months of use;
- 2 or more cases of pneumonia during the year;
- the child’s inability to gain weight and grow normally;
- frequent and deep abscesses of the skin and internal organs
- constant candidiasis of the oral cavity and skin;
- the need for intravenous antibiotics to resolve the infection;
- two or more systemic infections, including sepsis;
- hereditary predisposition.
According to development mechanisms, there are 4 main groups of PIDs (Table 3):
- Group 1 – predominantly humoral or B-cell PIDs;
- Group 2 – combined PID (all T-cell immunodeficiencies have impaired B-cell function);
- Group 3 – PID caused by defects in phagocytosis;
- Group 4 – PID caused by defects in the complement system.
Table 3. Some primary immunodeficiencies
| Pathology | Symptoms | Diagnostics | Treatment |
| Defects in antibody formation | |||
| Agammaglobulinemia | Frequent bacterial infections | Deficiency or complete absence of B lymphocytes | Antibiotics, lifelong administration of IgG |
| General variable immune deficiency | Frequent respiratory infections, otitis | Defects of T- and B-lymphocytes | Antibiotics, lifelong administration of IgG |
| Combined PIDs | |||
| Ataxia-telangiectasia (Louis-Bar syndrome) | Abnormal motor function, muscle weakness, speech impairment | Deficiency of T- and B-lymphocytes | Symptomatic |
| PID caused by defects in phagocytosis | |||
| Chronic granulomatous disease | Frequent pneumonia, purulent infections | Genetic defect | Lifelong antibacterial and antifungal therapy, interferon gamma |
| PID caused by defects in the complement system | |||
| Hereditary angioedema | Swelling of the lips and eyelids without itching. Swelling of the larynx, nose, tongue is life-threatening | Low concentration of C1 esterase inhibitor | Administration of C1 esterase inhibitor concentrate |
As follows from Table 3, the main and often the only treatment method for most patients with primary B-cell immunodeficiencies are immunoglobulins. These are medicines obtained from human blood plasma. They are designed to replace protective antibodies missing in the immune system in order to prevent or stop the development of severe infectious diseases. Today, a doctor’s arsenal includes immunoglobulins that differ in the concentration of the active substance (5 and 10%), as well as in the method of administration (intravenous and subcutaneous).
PID can appear at any age. Depending on this, the patient has unique problems that require certain types of support throughout his life (Table 4).
Table 4. Need for types of support for patients with PID in different age groups
| Age, years | Types of support | |||
| families | doctor | psychologist | society | |
| 0-14 | +++ | + | + | +++ |
| 14-18 | +++ | + | +++ | +++ |
| 18-65 | + | ++ | + | +++ |
| Over 65 | + | ++ | ++ | +++ |
Between the ages of 0 and 14 years, parental care is required to prevent infections and during the treatment period. May require: home training; providing psychological assistance; social support in purchasing medicines.
In adolescence (14-18 years), additional needs may arise for continued continuous education, vocational guidance, establishing relationships with peers, and organizing leisure time.
At the age of 18 to 65 years, patients more often experience infectious complications, and with them the costs of purchasing medications that are not subject to replenishment, as well as problems with employment.
In old age (over 65 years), there are needs for material, social and psychological support for a patient with PID.
Autoimmune pathology
Damage to the body's own organs and tissues by the immune system is called an autoimmune process . About 5% of humanity suffers from diseases of this type. The patient’s body develops hostilities reminiscent of a civil war: “friends against friends” go on the attack. There are no winners in this struggle - only suffering.
The selection of T lymphocytes in the thymus, as well as the removal of autoreactive cells in the periphery (central and peripheral immunological tolerance), which we discussed earlier, cannot completely rid the body of autoreactive T lymphocytes. As for B lymphocytes, the question of how strictly their selection is carried out still remains open. Therefore, in the body of every person there are necessarily many autoreactive lymphocytes, which, if an autoimmune reaction develops, can damage their own organs and tissues.
As an analogue, we can cite the Janissary infantry created by the Turks in the 14th century, which recruited Christian youths aged 8-16 who fought against their relatives.
T-cell autoimmune aggression has been well studied in rheumatoid arthritis, type 1 diabetes, multiple sclerosis and many other diseases.
The same Janissary cells, which do not remember their kinship, can be traced among B-lymphocytes:
- autoantibodies can cause cell death by activating the complement system on their surface or attracting macrophages;
- Receptors on the cell surface can become targets for antibodies.
For example, due to a breakdown of immunological tolerance, B-lymphocytes that produce antibodies are activated. This leads to a marked increase in the production of thyroid hormones (T4 and T3), as well as an increase in the size of the thyroid gland (hypertrophy). The pathology is called Graves' disease.
Another example would be myasthenia gravis, which is characterized by skeletal muscle weakness due to the formation of autoantibodies against structures responsible for cholinergic transmission and muscle fiber contraction;
- autoantibodies, together with soluble antigens, can form immune complexes that settle in various organs and tissues (for example, in the renal glomeruli, joints, on the vascular endothelium), disrupting their function and causing inflammatory processes.
Typically, an autoimmune disease occurs suddenly, and it is impossible to determine exactly what caused it. It is believed that almost any stressful situation can serve as a trigger, be it an infection, injury or hypothermia. A significant contribution to the likelihood of an autoimmune disease is made by both a person’s lifestyle and genetic predisposition - the presence of a certain variant of a gene.
Hypersensitivity
Hypersensitivity refers to an excessive immune response to an antigen. Hypersensitivity reactions are divided into several types depending on their duration and the mechanisms underlying them:
- Type I hypersensitivity involves immediate anaphylactic reactions, often associated with allergies. This type of reaction can range from mild discomfort to death. The basis of type I hypersensitivity is immunoglobulin E (IgE), which causes degranulation of basophils and mast cells;
- Type II hypersensitivity is characterized by the presence of antibodies that recognize its own proteins and mark the cells that express them for destruction. Type II hypersensitivity is also called antibody-dependent or cytotoxic hypersensitivity and is based on immunoglobulins G (IgG) and (IgM);
- type III hypersensitivity is caused by immune complexes consisting of antigens, complement proteins, IgG and IgM;
- Type IV hypersensitivity, also known as delayed hypersensitivity, develops over 2-3 days. Type IV hypersensitivity reactions are observed in many autoimmune and infectious diseases and are based on T cells, monocytes and macrophages.
Effective methods of influencing the immune system:
- Regular vaccination in terms of speed and quality of reaction exceeds the natural process of developing immunity to a specific infection;
- a balanced diet that ensures the maintenance of normal metabolism;
- regular physical activity ensuring the physiological functioning of all body systems and maintaining optimal body weight;
- giving up bad habits that lead to addiction (alcohol, nicotine, drugs, toxic, computer);
- daily routine, especially the influence of circadian rhythms (day and night): during wakefulness, the number of T-killer and NK cells peaks, as well as the concentration of anti-inflammatory substances such as cortisol and catecholamines; During sleep, the formation of memory T cells reaches its peak.
Speculative methods around immunity:
- taking immunostimulants is not clinically justified. If you constantly stimulate the production of leukocytes with drugs, the immune system will begin to lose its direct functions. This is when the moment of serious problems with immunity begins. Natural adaptogens do not affect the immune system at all: Schisandra chinensis, ginseng, eleutherococcus, radiola rosea. They act as amplifiers of RNA and protein synthesis (the basis of human cells), activate metabolic enzymes and the work of the endocrine and vegetative systems;
- Taking vitamins is clearly overrated. Vitamin D has a positive effect on the immune system, which stimulates the formation of T-killer cells. All other groups of vitamins do not directly participate in the functioning of the immune system;
- bath procedures and sauna do not affect the immune system;
- folk remedies such as honey and garlic have a mild bactericidal but not immunogenic effect.
Conclusion
The immune system is represented by three levels: organ, cellular and molecular with complex interactions between them.
Modern immunology distinguishes two interacting components of the immune system - innate and acquired (adaptive) types of immunity, which ensure the development of an immune response to genetically foreign substances, which are microorganisms, malignant tumor cells, transplanted organs and tissues.
Adaptive immunity is based on three main processes: recognition of antigens, their removal (elimination) and the formation of immunological memory.
Failures in the structure of the immune system lead to the development of immunodeficiencies, autoimmune diseases or hypersensitivity reactions.
Immunodeficiency at the genetic level (primary) or acquired (secondary) can appear at any age and lead to increased infectious morbidity. In recent years, replacement therapy has become available to prolong the lives of these patients. To improve their quality of life, it is necessary not only to provide expensive treatment, but also to organize support from family, psychologists and social institutions.
Autoimmune diseases and hypersensitivity are the body’s inability to resist a raging immune system that has confused its own and someone else’s.
Unfortunately, medicine has not yet learned to cure any of the diseases of the immune system, but only to use replacement therapy.
Effective preventive methods of influencing the immune system are vaccination and a healthy lifestyle. No one has yet been able to buy immunity at the pharmacy.
Literature
- Clark RA (2010). Skin resident T cells: the ups and downs of on site immunity. J. Invest. Dermatol. 130 (2), 362–370;
- Husband and wife, one of Satan?;
- Iijima N. and Iwasaki A. (2015). Tissue instruction for migration and retention of TRM cells. Trends Immunol. 36, 556–564;
- Schenkel J. M. and Masopust D. (2014). Tissue-resident memory T cells. Immunity. 41, 885–897;
- Thome JJ, Yudanin N., Ohmura Y., Kubota M., Grinshpun B., Sathaliyawala T. et al. (2014). Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 159, 814–828;
- Where did vision come from?
- Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyártó BZ et al. (2015). Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell. 161, 737–749;
- Frost EL, Kersh AE, Evavold BD, Lukacher AE (2015). Cutting edge: resident memory CD8 T cells express high-affinity TCRs. J. Immunol. 195, 3520–3524;
- Park CO and Kupper T S (2015). The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat. Med. 21, 688–697;
- Schluns KS and Klonowski K. Diverse functions of mucosal resident memory T cells. E-book, 2015;
- Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB (2015). The burgeoning family of unconventional T cells. Nat. Immunol. 16, 1114–1123;
- Castellana D., Paus R., Perez-Moreno M. (2014). Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLoS Biol. 12 , e1002002;
- Rodero M. P. and Khosrotehrani K. (2010). Skin wound healing modulation by macrophages. Int. J. Clin. Exp. Pathol. 3 (7), 643–653;
- Farber D., Yudanin N., Restifo N. P. (2014). Human memory T cells: generation, compartmentalization and homeostasis. Nat. Rev. Immunol. 14, 24–35..