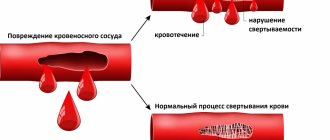
Detailed description of the study
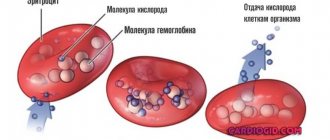
Iron is a trace element involved in many vital processes in the body. In its free form it has a toxic effect on tissues, so it circulates in the blood in combination with proteins. Iron enters the body with food, mainly meat products and some cereals. It is absorbed in the small intestine and from there enters the blood, where it combines with the carrier protein - transferrin.
The main amount of the microelement in combination with transferrin is transferred to the bone marrow. Here, iron is included in the hemoglobin inside red blood cells. Its function is to deliver oxygen to tissues. When red blood cells are destroyed, iron is released from them and again forms a compound with transferrin. The trace element is then sent to the bone marrow. This way it circulates in the body.
Some of the iron is used by the cells of internal organs for the synthesis of enzymes. Transferrin transports iron to the liver, kidneys, and muscles, where it is deposited in the form of ferritin. This form is the main reserve of the microelement.
Iron deficiency can negatively affect human health. This condition occurs against the background of insufficient intake of the microelement from food, impaired absorption in the intestines, increased consumption of its reserves - during the growth period in children, pregnancy and breastfeeding in women - or loss due to bleeding. With small losses of iron in the absence of compensation, latent, or hidden, iron deficiency is observed. In this case, there may be no disturbance in health, and the content of hemoglobin and red blood cells remains normal.
If iron levels continue to decrease, iron deficiency anemia develops. IDA is a condition in which hemoglobin synthesis is disrupted and oxygen delivery to cells is impaired. Symptoms of anemia include weakness, dizziness, and increased heart rate. Also characterized by pale skin, brittle hair and nails, inflammatory diseases of the oral cavity, changes in the senses of taste and smell.
To prevent these disorders, timely detection of iron deficiency in the body is necessary. Transferrin is one of the indicators that allows one to diagnose insufficient levels of a microelement even in the absence of iron deficiency anemia, that is, at the stage of latent iron deficiency. The less iron there is in the body, the more carrier protein molecules there will be in the blood; accordingly, the concentration of transferrin increases.
Not only a lack of iron, but also its excess leads to disturbances in the body. In this case, in a biochemical analysis, the level of transferrin in the blood serum will be reduced, which indicates an excess of iron in the tissues.
Excessive levels of microelements in the body are observed much less frequently than deficiency. This condition may be associated with mutations in genes responsible for iron metabolism. This disease is called primary hemochromatosis. Acquired, or secondary, hemochromatosis is also distinguished due to multiple blood transfusions, alcohol abuse, and hematological diseases. Symptoms appear slowly, predominantly in adulthood with feelings of weakness, joint pain, loss of weight and loss of appetite. Further developments occur: skin pigmentation, liver damage and diabetes mellitus.
Determining the amount of transferrin in the blood together with other indicators of iron saturation in the body makes it possible to diagnose iron metabolism disorders and prescribe treatment in a timely manner.
What is transferrin and where does it come from?
Iron entering the gastrointestinal tract with food, as a rule, is in the trivalent form (Fe+++), however, in order to be fully absorbed in the intestine, it must be reduced to the divalent form (Fe++), which occurs under the influence of numerous factors (vitamin C, enzymes, intestinal microflora, etc.). After ferric iron becomes divalent, in the cells of the mucous membrane of the duodenum it must again return to its original form (Fe+++), which allows it to combine with ferritin and, with the help of a specific protein transferrin, go to its destination (organs and tissues).
To saturate transferrin with iron, there are special areas (spaces) in the transport protein molecule that are ready to accept Fe ions. Depending on this, in the body the transport protein can be present and move in one of four different forms, each of which allocates its own place for iron:
- Apotransferrin;
- Monoglandular transferrin A (ferrum occupies exclusively the A-space);
- Monoglandular transferrin B (iron localization extends only to the B space;
- Diferric transferrin (both spaces are occupied by iron).
A transport protein molecule can accommodate 2 iron ions, and when transferrin, carrying these ions, encounters a cell on its way that has a butterfly-like transferrin receptor on its surface, it will certainly “notice” it, bind, penetrate inside the cell and give it iron , separating him from himself. It should be noted that the transport protein, having delivered this chemical element, does not give it (Fe) to everyone, each iron-binding space endows its specific tissue with iron: the erythron and placenta use the iron of the A-space, the liver and other organs receive Fe from the B space.
Transferrin is saturated with iron in the area responsible for the absorption of this chemical element in the body, that is, mainly in the duodenal mucosa, or in places where red blood cells die during their digestion by macrophages.
Determination of iron transport protein
Transferrin analysis is carried out in a plasma or serum sample taken, like all biochemical tests, in the morning on an empty stomach. Meanwhile, methods for studying transport proteins create certain difficulties, since they require the participation of special laboratory equipment and test kits that are not always available. However, the lack of equipment does not imply a refusal to test for Tf; in any case, the patient will not be left without examination.
An alternative way to solve this problem is to determine the coefficient of transferrin saturation with iron - an analysis that is better known as the total iron-binding capacity (TIBC) of blood serum (plasma), indicating the concentration of transferrin in the blood. In general, as much iron as transferrin binds, you become saturated with that amount of iron. In percentage terms, in healthy people this value is at least 25–30%. This means that in the normal state of the body, approximately 35% of Tf should be involved in binding and transporting iron to organs and tissues.
Most often, the need to determine transferrin arises in the differential diagnosis of various iron deficiency conditions, accompanied by:
- Reduced serum iron concentration;
- Increased content of transport protein;
- Reduced level of transferrin saturation with iron.
It is convenient to show the norms of transport protein and the degree of saturation of transferrin with iron in the table below. Meanwhile, the reader should keep in mind that the range of reference values, depending on the location of the analysis, may narrow or expand, so comparison of the results of determining a particular indicator must be made in accordance with the data of the laboratory conducting the study.
| Age | Transport protein content, g/l |
| Children under 10 years old | 2,03-3,60 |
| From 10 to 60 years | 2,00-4,00 |
| Adults over 60 years of age | 1,80-3,80 |
| Age | Transferrin saturation with iron, % |
| Children and adolescents under 14 years of age | 10-50 |
| 14-60 years | 15-50 |
| Adults over 60 years of age | 8-50 |
Females have a special relationship with both iron and its transport, so they have approximately 10% more Fe-transporting protein.
than their male peers. During pregnancy (third trimester), one can expect a 1.5-fold increase in the level of transferrin, while in old age its concentration, on the contrary, is reduced and no longer has any gender at all. During inflammatory processes, transferrin acts as a negative acute-phase protein; its level during inflammation is reduced.
In addition, the determination of transferrin in the blood can be carried out in other units - µmol/l, then its norm for adults will be within the range of 23 - 43 µmol/l (in males) and 21 - 46 µmol/l (in weak "half). The situation is similar with total transferrin (TGTS) in the blood, the norm of which, expressed in the same units as Tf, will be 26.85 - 41.17 µmol/l. The saturation of transferrin with iron in pregnant women increases consonant with the decrease in the content of iron itself in the blood.
Ways to reduce transferrin
Eating foods high in iron can help prevent iron deficiency. Iron-rich foods include red meat, liver, poultry and fish. []
Iron supplements will iron intake, but should only be taken if your iron deficiency cannot be corrected through diet. [, , ]
Reduce consumption of drinks such as coffee , cocoa , green and herbal tea , as they reduce the absorption of iron from food. [, , , , , ] Or postpone their consumption 1-2 hours before meals. alcohol consumption , which completely destroys heme iron in animal foods, but not in plant foods. []
Vitamin C increases iron absorption []. It is a good practice to add sources of vitamin C to iron-rich foods (dried rose hips, lemon juice, sauerkraut, etc.).
FOOD RICH IN IRON AND FOODS WITH VITAMIN C TO IMPROVE IRON ABSORPTION
It is worth noting that night shift work disrupts circadian rhythms and there is a disagreement between the “internal clocks” in the liver and brain. If, in the evening or at night, you eat food that contains a lot of iron (for example, sausage, hamburger, steak), then an even greater mismatch occurs between the circadian rhythms in the liver cells and in the brain. This disorder increases the risk of developing type 2 diabetes. []
Heme and non-heme iron
Heme is part of hemoglobin, myoglobin, and some enzymes (cytochromes, catalase, lactoperoxidase). The iron included in these compounds is commonly called heme iron .
Heme iron is much better absorbed by the body.
SOME FOOD PRODUCTS ARE SOURCES OF HEME AND NON-HEME IRON
Iron compounds found in plant foods are classified as non-heme iron . It is much less absorbed than heme iron, and its absorption is influenced by many additional factors.
Substances that reduce iron absorption
- Solubility of various non-heme iron compounds
- Integrity of the intestinal mucosa (healthy intestines absorb better)
- polyphenols in food – grains, vegetables, spices and drinks
- Calcium (cow's milk, calcium supplements)
- Phytic acid – bran, wheat, rice, legumes, nuts (can reduce absorption by 50-60%).
- Zinc supplements . It is better to take separately from food.
- Oxalic acid – found in spinach, cabbage, beets, nuts, chocolate and tea. Reduces the absorption of heme iron.
- Eggs contain a compound called phosvitin, which impairs iron absorption. One boiled egg can reduce the absorption of iron in food by 28%.
Substances that increase iron absorption
- Vitamin C – can increase absorption 4 times
- Beta-carotene – increases absorption and reduces the effect of phytic acid and tannins.
- Betaine hydrochloride is an acid that is present in the stomach and it helps with the breakdown of food and absorption of nutrients.
Table of examples of iron absorption from various foods
| Iron-rich foods | Iron type | Suction |
| Veal, beef | Heme | 22-25% |
| Fish, kidneys, liver | Heme | 11% |
| Eggs | Heme | 3% |
| Soya beans | Non-heme | 7% |
| Apples, pomegranates, buckwheat | Non-heme | 3% |
| Rice, spinach | Non-heme | 1% |
Definition of OZHSS
Increased or decreased values of the TfVS do not mean that in the same cases the Tf level will be increased or decreased. The presence of transferrin does not increase its binding to iron or, conversely, a low level of transport protein does not reduce its binding ability. The complex mechanism that occurs during the absorption, distribution and consumption of iron is difficult to present in a small article, so we will provide information informing about pathological conditions in which the level of TGSS is increased or decreased.
Increases overall binding capacity:
- IDA (iron deficiency anemia);
- Hormonal contraceptives;
- Damage to hepatocytes (liver cells) in acute inflammatory diseases (hepatitis) and cirrhosis;
- Excessive load of the body with iron (diet, ferrotherapy for a long time);
- Frequent blood transfusions;
- Hemochromatosis;
- Pregnancy (in later stages, closer to childbirth);
- Childhood.
A reduced CVSS indicator is observed in the following cases:
- A decrease in the concentration of total protein, which is often a consequence of starvation diets, malignant neoplasms, nephrotic syndrome;
- Chronic influence of some infectious agent that constantly “resides” in the body;
- Hemosiderosis, as a result of numerous blood transfusions;
- Iron deficiency conditions.
The coefficient of saturation of transferrin with iron depends on the concentration of Fe in the body: with an excess of iron, the TLC indicator will be increased both in numerical and percentage terms. This occurs in pathological conditions that involve increased breakdown of red blood cells and increased hemolysis, or in case of iron poisoning, if treatment with Fe preparations is overly active.
Additional examinations
Increased or decreased transferrin is an informative indicator; deviations indicate disorders: from liver pathologies to tumor processes.
However, it is impossible to say anything specific based on the level of siderophilin alone. We need auxiliary techniques.
- Oral interview and history taking. The very beginning of diagnosis. Based on the symptoms and lifestyle characteristics, one can draw conclusions about the origin and essence of the disorder.
- Ultrasound of the abdominal organs. The liver is closely examined. To a lesser extent the intestines.
- Hormone tests. Biochemical blood test. With determination of ALT and AST.
- If necessary, MRI or CT is prescribed. Tomographic techniques are especially important in diagnosing cancer and oncology in general.
- Cardiography. ECHO-KG.
- This is just a small part. What exactly and how to examine is decided by the doctor, at his own discretion.
Transferrin is a special transport protein. It transports iron and makes cellular respiration possible. Many other functions also depend on this substance.
All deviations are grounds for in-depth examination of the patient. Treatment is prescribed as needed, if indications exist. With timely treatment, there is every chance to forget about the problem and fully recover.
Transferrin in the analysis
Elevated transferrin levels
can be expected in the following cases:
- Iron deficiency conditions caused by a lack of iron in consumed foods or chronic blood loss (heavy periods, hemorrhoids, gum and nosebleeds);
- Pregnancy (iron concentration decreases, transferrin content in the blood increases);
- The use of estrogens, the use of hormonal drugs as contraceptives.
Decreased transferrin
found in the following pathological conditions:
- Various inflammatory processes with a chronic course;
- Malignant neoplasms;
- Liver diseases (cirrhosis, hepatitis), which is quite natural, because this organ is the main producer of transferrin;
- Loss of protein by the body (extensive thermal and chemical burns, nephrotic syndrome);
- Use of androgen drugs and glucocorticoids;
- Hereditary anomalies (atranferrinemia);
- Excessive intake of iron into the body (massive blood transfusions);
- Diseases leading to an increase in oncotic pressure (multiple myeloma, hepatocellular pathology);
- Hemochromatosis (a genetic pathology that results in a triad of syndromes caused by excessive absorption of iron in the gastrointestinal tract - unusual coloring of the skin, mucous membranes and internal organs, cirrhosis of the liver, diabetes);
- Hyperchromic anemia;
- Thalassemia.